Life Sciences QAD Life Sciences Industry Challenges Operational







![QAD Life Sciences Management Review • Procedures for management review have not been [adequately] QAD Life Sciences Management Review • Procedures for management review have not been [adequately]](https://slidetodoc.com/presentation_image_h2/ae6f587d381edb2f544592382a81f62c/image-13.jpg)


![QAD Life Sciences Audits • Quality audits were not performed [at defined intervals] [at QAD Life Sciences Audits • Quality audits were not performed [at defined intervals] [at](https://slidetodoc.com/presentation_image_h2/ae6f587d381edb2f544592382a81f62c/image-24.jpg)








- Slides: 34

Life Sciences

QAD Life Sciences Industry Challenges Operational Excellence Supply Chain Security Quality Management Regulatory Compliance Rapidly Advancing Technology

QAD Life Sciences Operational Excellence • Poor operational metrics industrywide • High levels of inventory • Long cash to cash cycles • Lagging delivery metrics • High SG&A costs

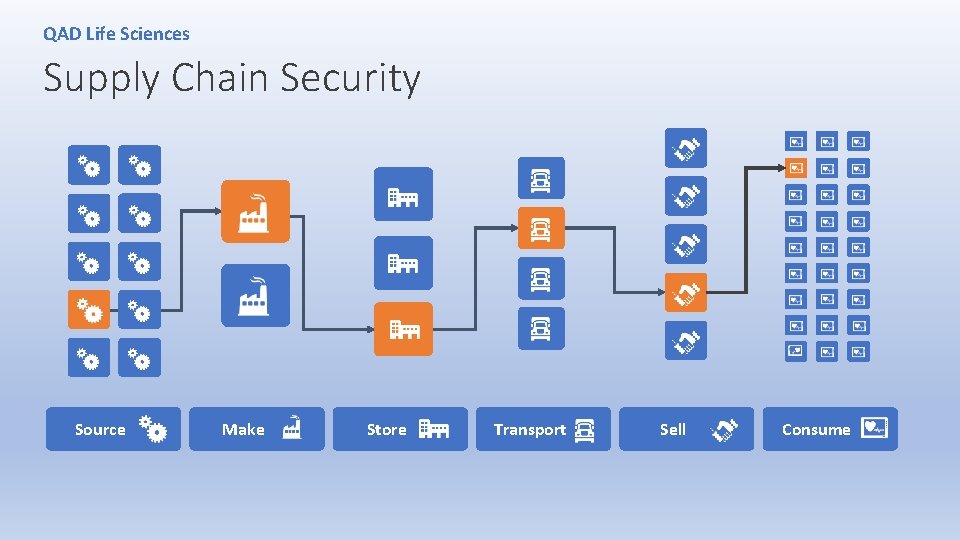
QAD Life Sciences Supply Chain Security Source Make Store Transport Sell Consume

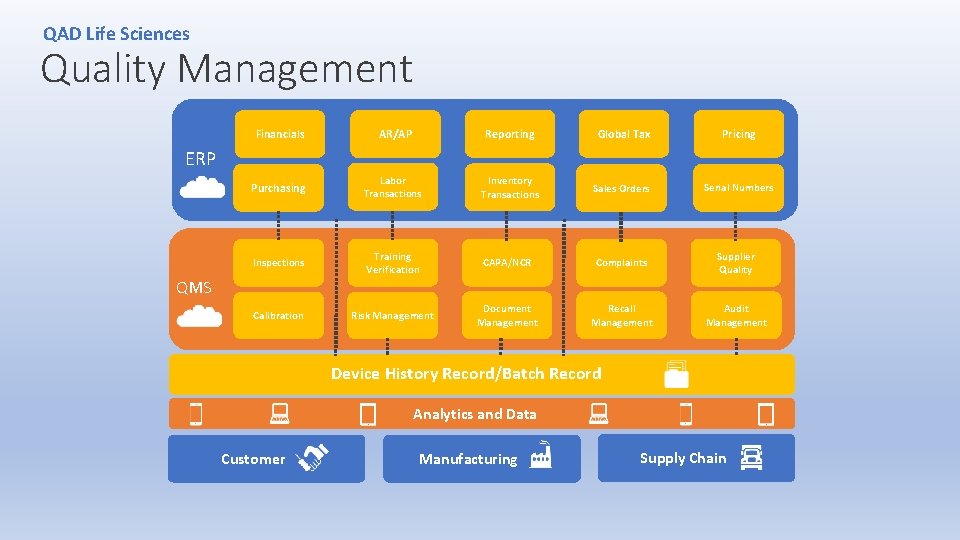
QAD Life Sciences Quality Management Financials AR/AP Reporting Global Tax Pricing Purchasing Labor Transactions Inventory Transactions Sales Orders Serial Numbers Inspections Training Verification CAPA/NCR Complaints Supplier Quality Calibration Risk Management Document Management Recall Management Audit Management ERP QMS Device History Record/Batch Record Analytics and Data Customer Manufacturing Supply Chain

QAD Life Sciences 13485: 2016 • Updated standard published in March 2016 replaces 13485: 2003 • Places a greater emphasis on: • QMS throughout the supply chain • Product lifecycle • Device usability and post market surveillance requirements. • Over the next three years both will coexist, allowing time to transition • February 28 th, 2019 deadline

QAD Life Sciences New EU IVDR and MDR Regulations • Regulations entered into force on May 25 th 2017 • MDR replaces the Medical Devices Directive (93/42/EEC) and Active Implantable Medical Devices Directive (90/385/EEC) • Greater scrutiny of technical documentation • Stricter requirements on clinical evaluation and post-market clinical follow-up • Better traceability of devices through the supply chain. • MDR and IVDR deadlines are May 2020 and May 2022

QAD Life Sciences Advanced Technology • 3 D printed devices • Hearing aids & prosthetics • Io. T device and patient monitoring • Cardiac and glucose monitoring • Autonomous robotic surgery • Smart Tissue Autonomous Robot (STAR) faster and more accurate • Digital twin heart simulations • Internet for the body

QAD Life Sciences CDRH 2016 -2017 Strategic Priorities • Establish a National Evaluation System for medical devices • Partner with patients • Promote a culture of quality and organizational excellence • MDSAP • Voluntary Compliance Improvement Program

QAD Life Sciences MDSAP • The Medical Device Single Audit Program allows an MDSAP recognized Auditing Organization to conduct a single regulatory audit of a medical device manufacturer that satisfies the relevant requirements of the regulatory authorities participating in the program. • International partners that are participating in the MDSAP include: Therapeutic Goods Administration of Australia Brazil’s Agência Nacional de Vigilância Sanitária Health Canada Japan’s Ministry of Health, Labour and Welfare, and the Japanese Pharmaceuticals and Medical Devices Agency • The World Health Organization (WHO) and the European Union (EU) are Official Observers • •

QAD Life Sciences Voluntary Compliance Improvement Program • The CMMI (and SCAMPI method) are providing a full end-to-end view of quality in an continual improvement context (vs. compliance) • Allows firms to voluntarily self identify and correct possible regulatory violations instead of undergoing FDA inspection • Reduced inspections • Quicker PMA approvals

QAD Life Sciences Quality High Low Compliance High
![QAD Life Sciences Management Review Procedures for management review have not been adequately QAD Life Sciences Management Review • Procedures for management review have not been [adequately]](https://slidetodoc.com/presentation_image_h2/ae6f587d381edb2f544592382a81f62c/image-13.jpg)
QAD Life Sciences Management Review • Procedures for management review have not been [adequately] established. • Management with executive responsibility has not reviewed the suitability and effectiveness of the quality system [at defined intervals] [with sufficient frequency]. • List of inputs and outputs of management review has expanded and “documented planned intervals” (ISO 13485: 2016)

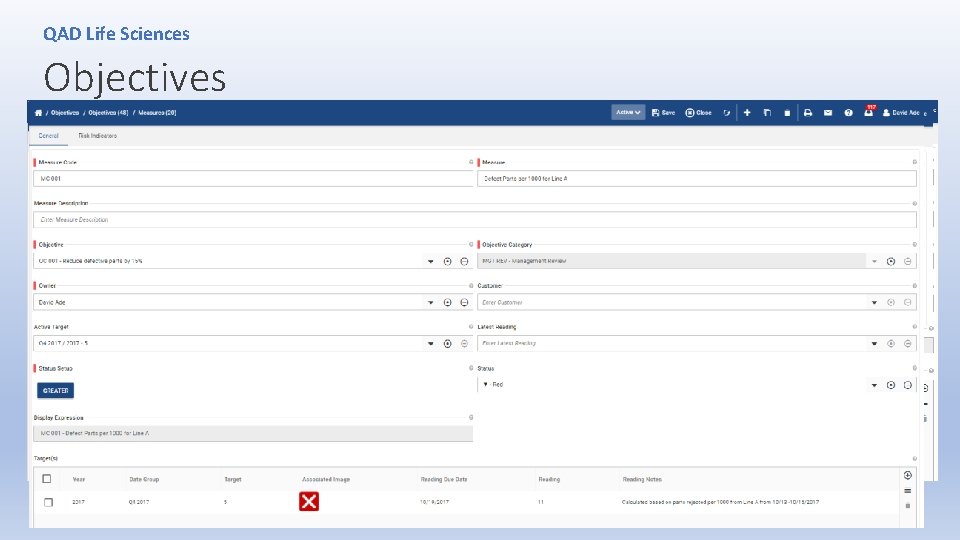
QAD Life Sciences Objectives

QAD Life Sciences Meetings

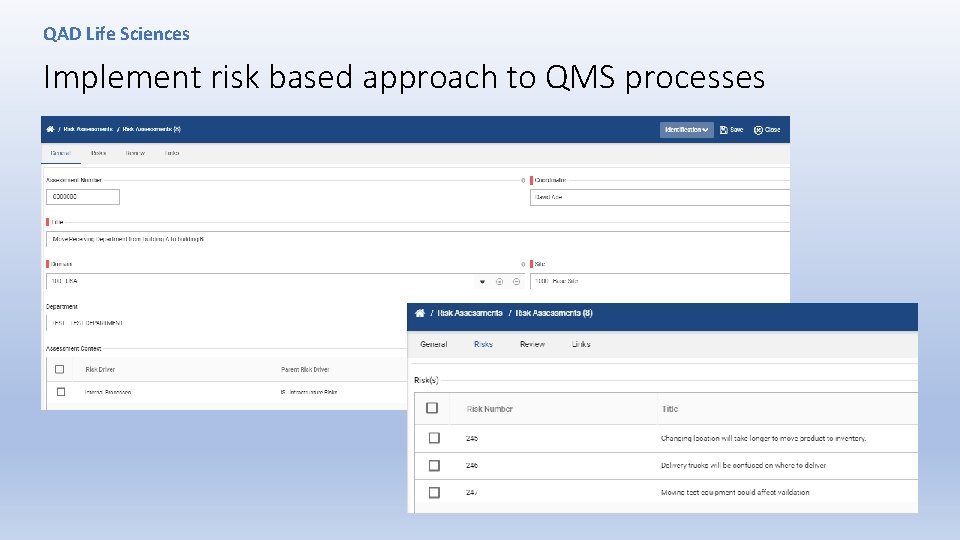
QAD Life Sciences Implement risk based approach to QMS processes

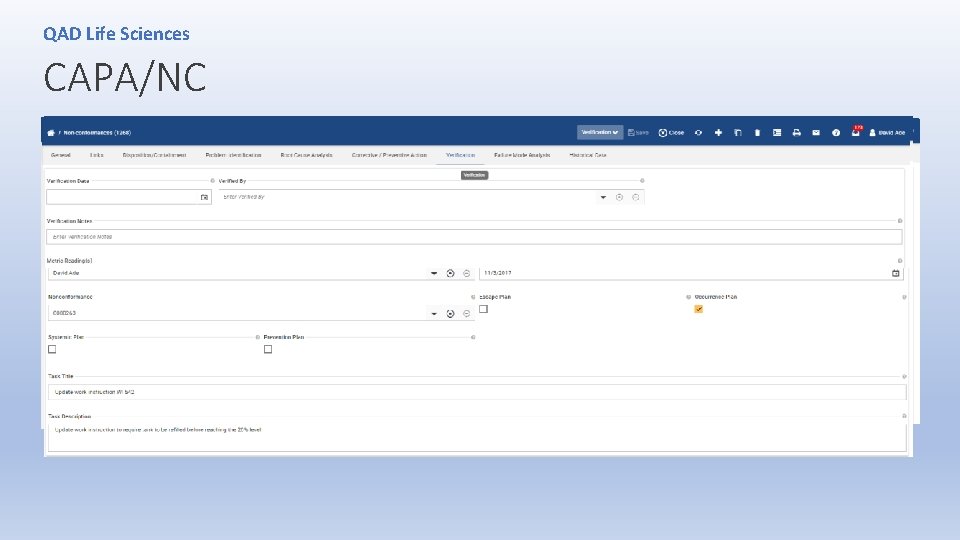
QAD Life Sciences Non-conformance/CAPA • Procedures for corrective and preventive action have not been [adequately] established. (344 observations from FDA 483) • Procedures have not been [adequately] established to control product that does not conform to specified requirements. • Corrective and preventive action activities and/or results have not been [adequately] documented. • Products that do not conform to specifications are not adequately controlled.

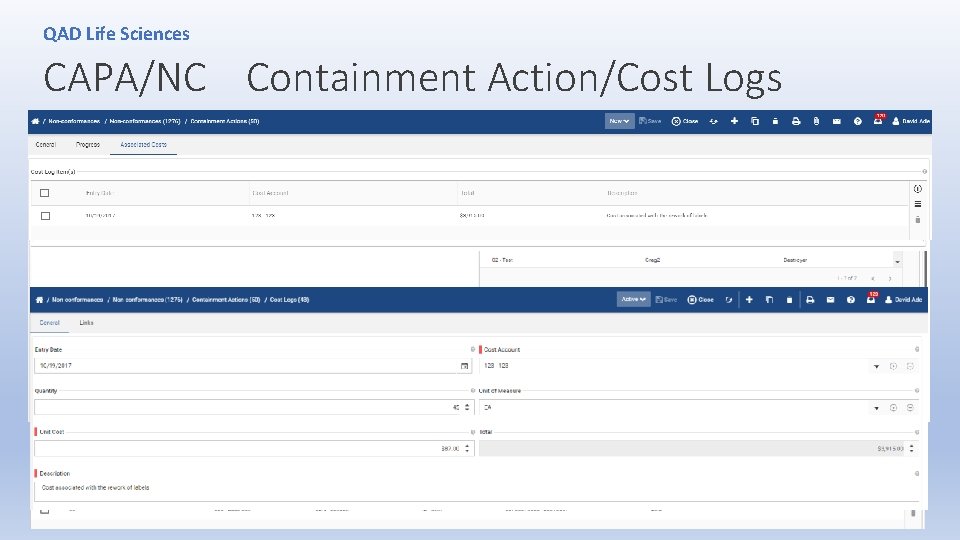
QAD Life Sciences CAPA/NC Containment Action/Cost Logs

QAD Life Sciences CAPA/NC

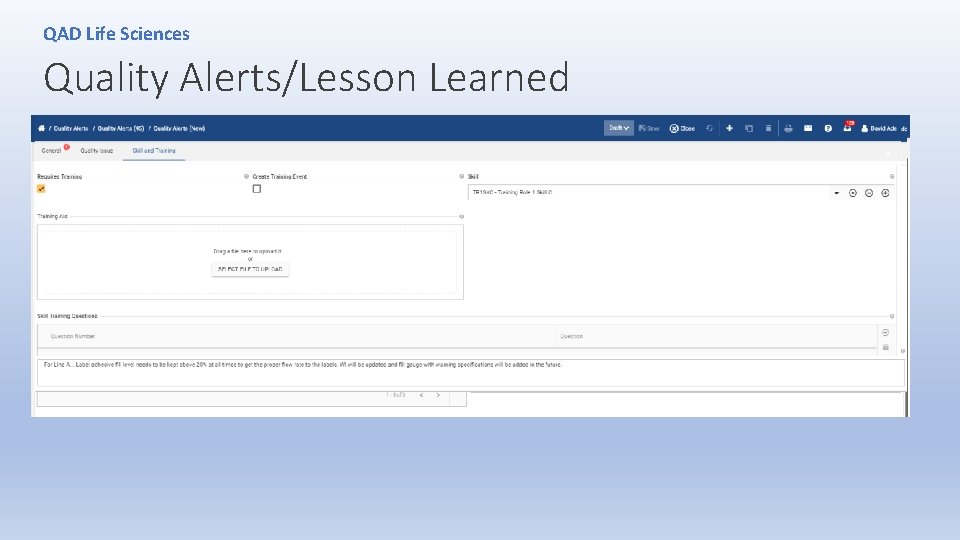
QAD Life Sciences Quality Alerts/Lesson Learned

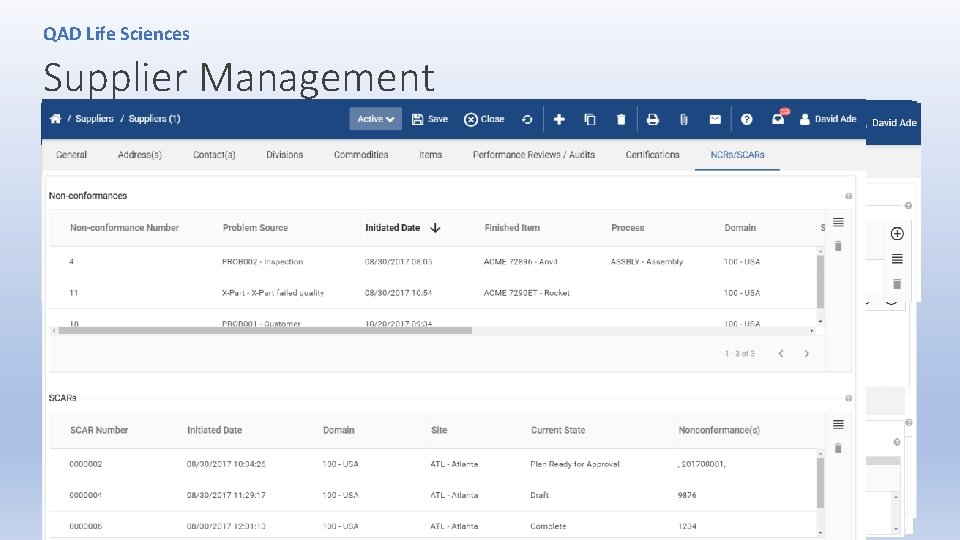
QAD Life Sciences Supplier Management • The FDA inspector noted that the company didn’t include a risk-based approach to its annual evaluation of suppliers since suppliers listed as critical are not reviewed or monitored. Moreover, the firm’s annual review of suppliers only rates suppliers on their late deliveries, dollars rejected, and responsiveness to corrective actions. (from FDA Warning Letter) • Warning letter dings the firm for failing to ensure that all suppliers conform to specified requirements. (from FDA Warning Letter) • Tate’s procedures for supplier selection and evaluation did not feature requirements for suppliers, contractors and consultants regarding medical devices, and the company failed to document its evaluation of potential suppliers. (from FDA Warning Letter)

QAD Life Sciences Supplier Management

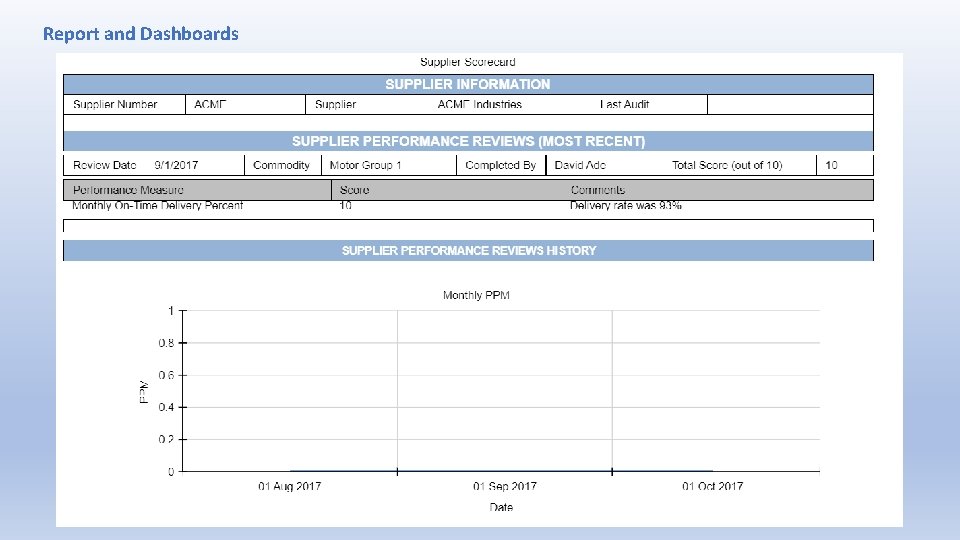
Report and Dashboards
![QAD Life Sciences Audits Quality audits were not performed at defined intervals at QAD Life Sciences Audits • Quality audits were not performed [at defined intervals] [at](https://slidetodoc.com/presentation_image_h2/ae6f587d381edb2f544592382a81f62c/image-24.jpg)
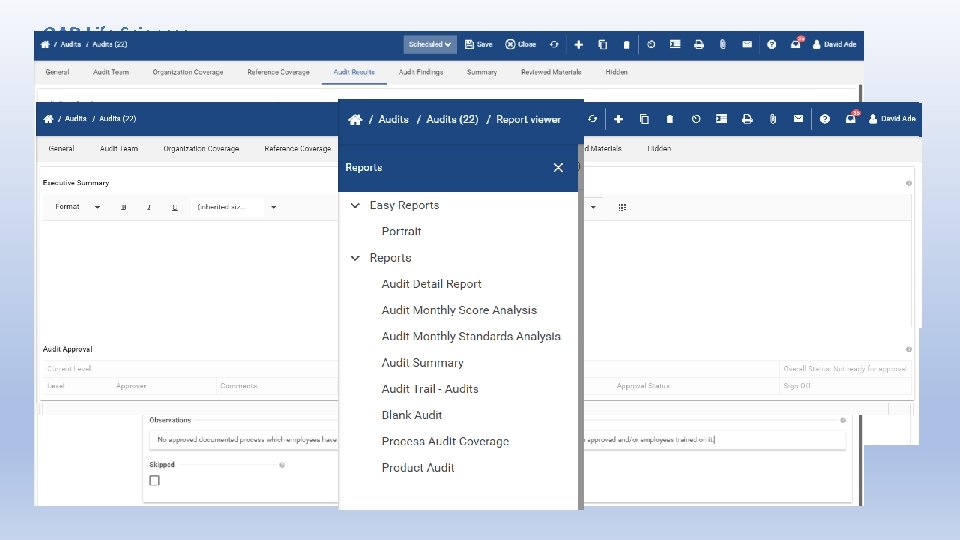
QAD Life Sciences Audits • Quality audits were not performed [at defined intervals] [at sufficient frequency] to determine whether the quality system activities and results comply with quality system procedures. • Quality [audits] [reaudits] have not been performed. • Audit reports were not reviewed by management having responsibility for the matters audited. (from FDA Warning Letter) • Inspectors further noted that the firm had not established a required audit schedule or conducted an internal audit as of April 11, 2017. (from FDA Warning Letter)

QAD Life Sciences Audits

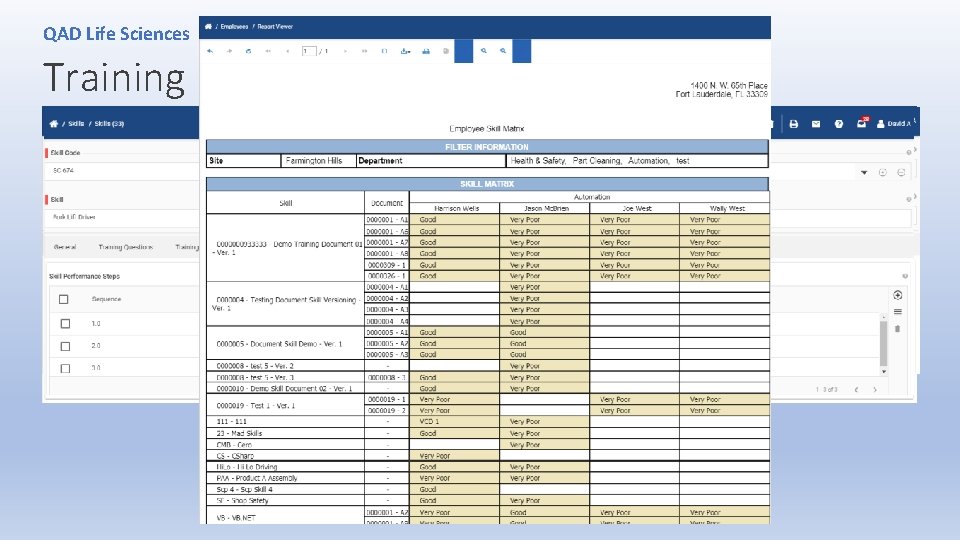
QAD Life Sciences Training • Procedures for training and identifying training needs have not been [adequately] established. • Personnel training is not documented. • “Personnel performing work affecting product quality shall be competent on the basis of appropriate, education, training, skills and experience. ” (ISO 13485: 2016)

QAD Life Sciences Training The organization shall: • a) determine the necessary competence for personnel performing work affecting product quality; • b) provide training or take other actions to achieve or maintain the necessary competence; • c) evaluate the effectiveness of the actions taken; • d) ensure that its personnel are aware of the relevance and importance of their activities and how • they contribute to the achievement of the quality objectives; • e) maintain appropriate records of education, training, skills and experience (see 4. 2. 5). (ISO 13485: 2016)

QAD Life Sciences Training

Case Study

QAD Life Sciences Heartware Background • Global medical device company • Class III Ventricular Assist Devices (VADs) • Over 10, 000 implanted worldwide • Rapid growth in recent years • Manual complaint handling process

QAD Life Sciences Industry Common Complaint Situation • Companies use inefficient paper-based system for complaints • Unstructured complaint information from disparate sources • Complaints processed by different departmental personnel • Complaint procedure in place, but • Not all employees sufficiently trained and complaint is neglected • Data lost during transfer to the responsible party • Complaint left open because no decision made to close the complaint • Challenges handling complaint volume

QAD Life Sciences Heartware Challenges • • • Manual input, tracking and investigation for each complaint Home-grown system with multiple databases Frequent and multiple iterations to correct errors Resources stressed and cannot support volume No integration among design teams, manufacturing, distribution and quality assurance Result: Backlog of complaints, late and/or missed reports

QAD Life Sciences Heartware Results • 54% improvement in complaint closure rate • 53% reduction in open complaints • 62% reduction in late report submissions

QAD Life Sciences Explore 2018: Save the Date