Lecture Presentation Chapter 17 Additional Aspects of Aqueous




- Slides: 31

Lecture Presentation Chapter 17 Additional Aspects of Aqueous Equilibria © 2015 Pearson Education, Inc. James F. Kirby Quinnipiac University Hamden, CT

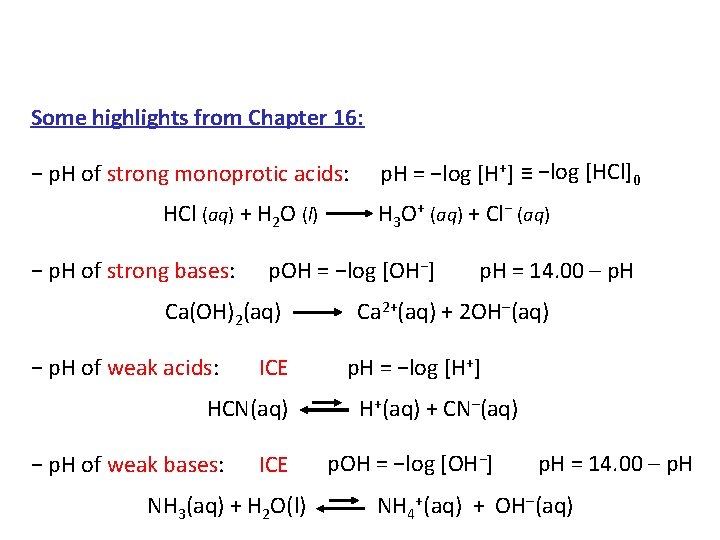
Some highlights from Chapter 16: − p. H of strong monoprotic acids: p. H = −log [H+] ≡ −log [HCl]0 HCl (aq) + H 2 O (l) H 3 O+ (aq) + Cl− (aq) − p. H of strong bases: p. OH = −log [OH−] p. H = 14. 00 – p. H Ca(OH)2(aq) Ca 2+(aq) + 2 OH (aq) − p. H of weak acids: ICE p. H = −log [H+] HCN(aq) H+(aq) + CN–(aq) − p. H of weak bases: ICE p. OH = −log [OH−] p. H = 14. 00 – p. H NH 3(aq) + H 2 O(l) NH 4+(aq) + OH (aq)

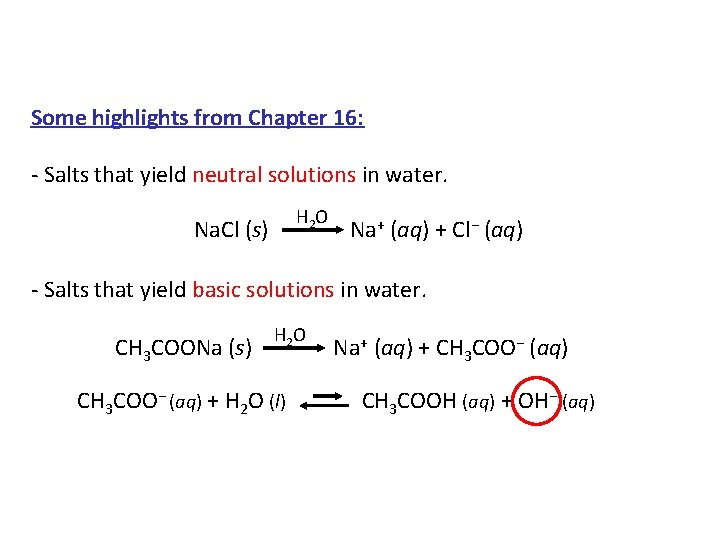
Some highlights from Chapter 16: - Salts that yield neutral solutions in water. H 2 O Na. Cl (s) Na+ (aq) + Cl− (aq) - Salts that yield basic solutions in water. HO 2 + (aq) + CH COO− (aq) CH 3 COONa (s) Na 3 CH 3 COO− (aq) + H 2 O (l) CH 3 COOH (aq) + OH− (aq)

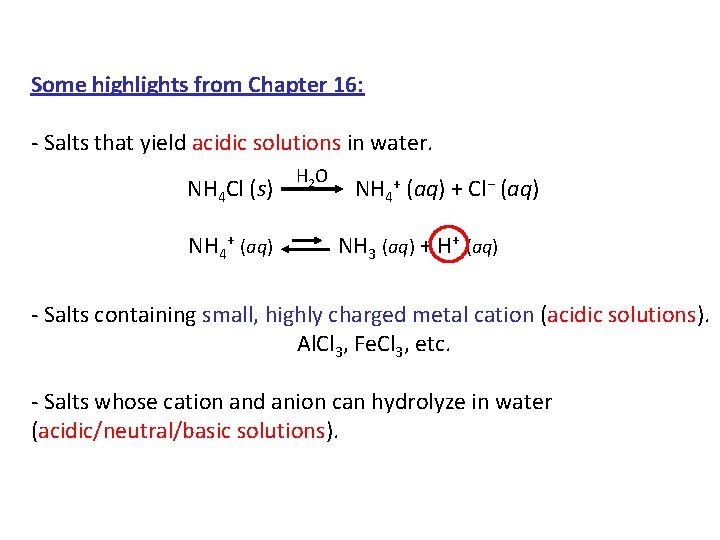
Some highlights from Chapter 16: - Salts that yield acidic solutions in water. H 2 O NH 4 Cl (s) NH 4+ (aq) + Cl− (aq) NH 4+ (aq) NH 3 (aq) + H+ (aq) - Salts containing small, highly charged metal cation (acidic solutions). Al. Cl 3, Fe. Cl 3, etc. - Salts whose cation and anion can hydrolyze in water (acidic/neutral/basic solutions).

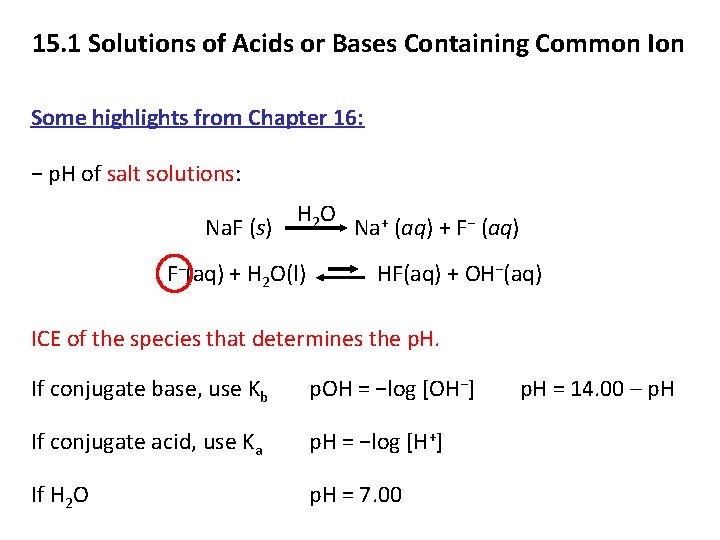
15. 1 Solutions of Acids or Bases Containing Common Ion Some highlights from Chapter 16: − p. H of salt solutions: H 2 O + (aq) + F− (aq) Na. F (s) Na F−(aq) + H 2 O(l) HF(aq) + OH−(aq) ICE of the species that determines the p. H. If conjugate base, use Kb p. OH = −log [OH−] If conjugate acid, use Ka p. H = −log [H+] If H 2 O p. H = 7. 00 p. H = 14. 00 – p. H

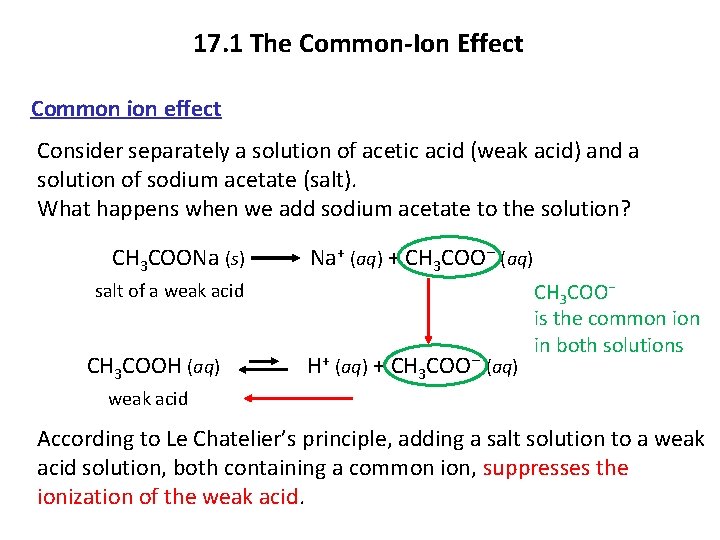
17. 1 The Common-Ion Effect Common ion effect Consider separately a solution of acetic acid (weak acid) and a solution of sodium acetate (salt). What happens when we add sodium acetate to the solution? CH 3 COONa (s) Na+ (aq) + CH 3 COO− (aq) salt of a weak acid CH 3 COOH (aq) H+ (aq) + CH 3 COO− (aq) CH 3 COO− is the common in both solutions weak acid According to Le Chatelier’s principle, adding a salt solution to a weak acid solution, both containing a common ion, suppresses the ionization of the weak acid.

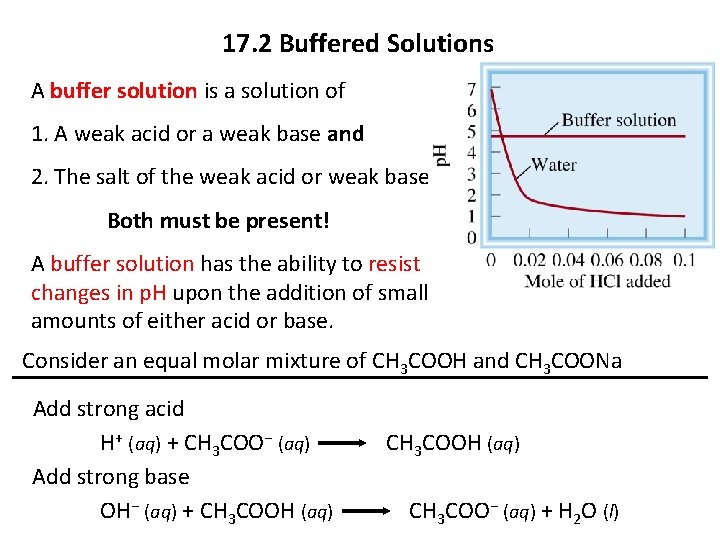
17. 2 Buffered Solutions A buffer solution is a solution of 1. A weak acid or a weak base and 2. The salt of the weak acid or weak base Both must be present! A buffer solution has the ability to resist changes in p. H upon the addition of small amounts of either acid or base. Consider an equal molar mixture of CH 3 COOH and CH 3 COONa Add strong acid H+ (aq) + CH 3 COO− (aq) CH 3 COOH (aq) Add strong base OH− (aq) + CH 3 COOH (aq) CH 3 COO− (aq) + H 2 O (l)

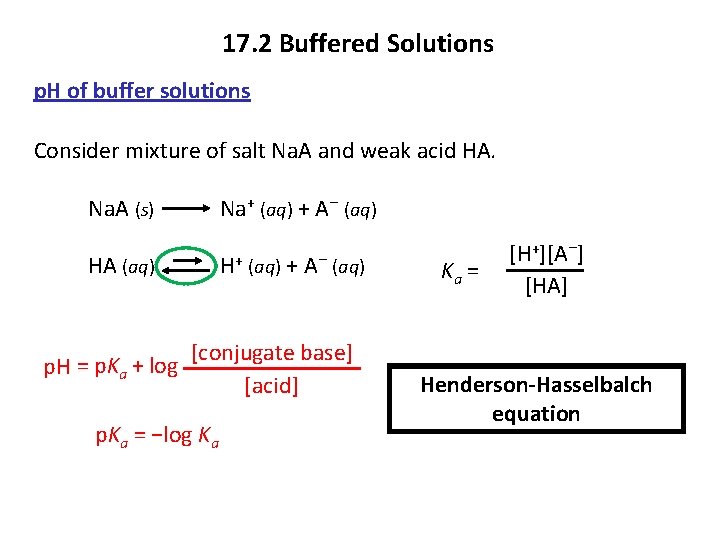
17. 2 Buffered Solutions p. H of buffer solutions Consider mixture of salt Na. A and weak acid HA. Na. A (s) Na+ (aq) + A− (aq) HA (aq) H+ (aq) + A− (aq) p. H = p. Ka + log [conjugate base] [acid] p. Ka = −log Ka Ka = [H+][A−] [HA] Henderson-Hasselbalch equation

EXERCISE #1 Which of the following solutions can be classified as a buffer? a) HCl/Na. Cl b) NH 3/NH 4 Br c) HCN/Na. CN d) CH 3 COOH e) Li. NO 2 Buffer: 1. weak acid (or base) 2. salt containing the conjugate base (or conjugate acid)

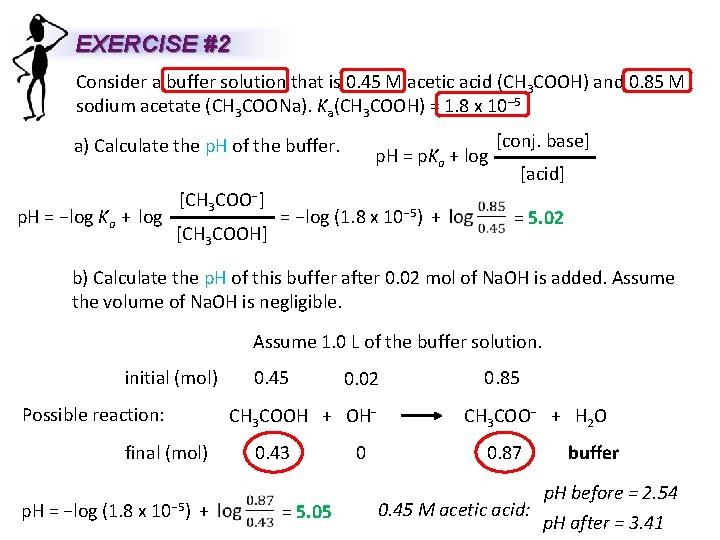
EXERCISE #2 Consider a buffer solution that is 0. 45 M acetic acid (CH 3 COOH) and 0. 85 M sodium acetate (CH 3 COONa). Ka(CH 3 COOH) = 1. 8 x 10– 5 a) Calculate the p. H of the buffer. p. H = −log Ka + log [CH 3 COO−] [CH 3 COOH] p. H = p. Ka + log = −log (1. 8 x 10− 5) + [conj. base] [acid] = 5. 02 b) Calculate the p. H of this buffer after 0. 02 mol of Na. OH is added. Assume the volume of Na. OH is negligible. Assume 1. 0 L of the buffer solution. initial (mol) Possible reaction: p. H = 0. 02 0. 85 CH 3 COOH + OH− CH 3 COO− + H 2 O final (mol) −log (1. 8 x 10− 5) 0. 45 + 0. 43 = 5. 05 0 0. 87 0. 45 M acetic acid: buffer p. H before = 2. 54 p. H after = 3. 41

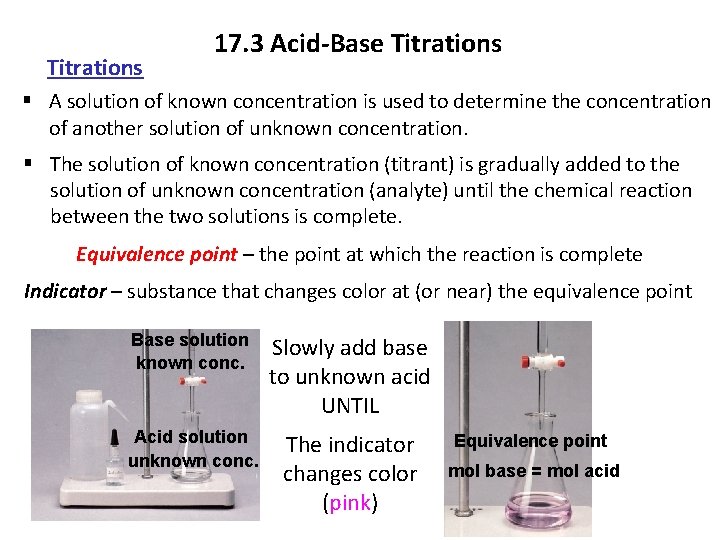
Titrations 17. 3 Acid-Base Titrations § A solution of known concentration is used to determine the concentration of another solution of unknown concentration. § The solution of known concentration (titrant) is gradually added to the solution of unknown concentration (analyte) until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at (or near) the equivalence point Base solution known conc. Slowly add base to unknown acid UNTIL Acid solution unknown conc. The indicator changes color (pink) Equivalence point mol base = mol acid

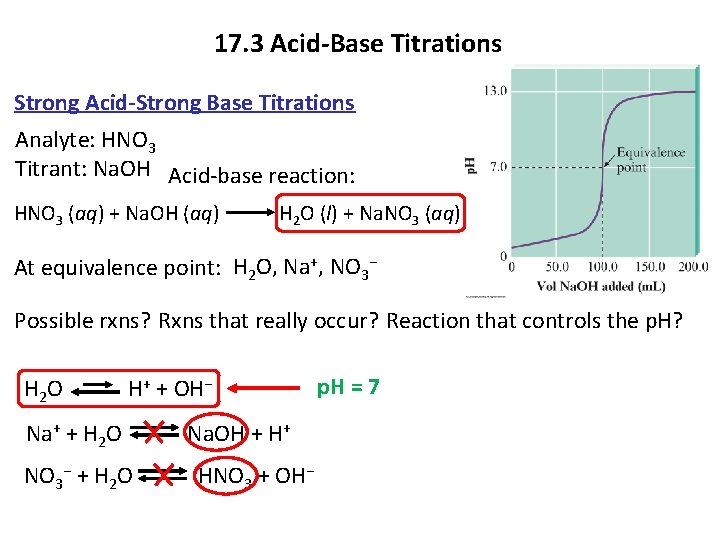
17. 3 Acid-Base Titrations Strong Acid-Strong Base Titrations Analyte: HNO 3 Titrant: Na. OH Acid-base reaction: HNO 3 (aq) + Na. OH (aq) H 2 O (l) + Na. NO 3 (aq) At equivalence point: H 2 O, Na+, NO 3− Possible rxns? Rxns that really occur? Reaction that controls the p. H? H 2 O H+ + OH− Na+ + H 2 O Na. OH + H+ NO 3− + H 2 O HNO 3 + OH− p. H = 7

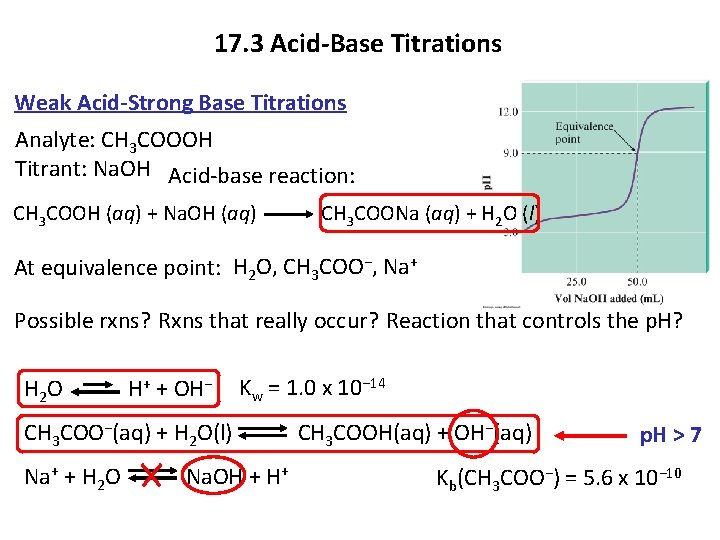
17. 3 Acid-Base Titrations Weak Acid-Strong Base Titrations Analyte: CH 3 COOOH Titrant: Na. OH Acid-base reaction: CH 3 COOH (aq) + Na. OH (aq) CH 3 COONa (aq) + H 2 O (l) At equivalence point: H 2 O, CH 3 COO−, Na+ Possible rxns? Rxns that really occur? Reaction that controls the p. H? H 2 O H+ + OH− Kw = 1. 0 x 10− 14 CH 3 COO−(aq) + H 2 O(l) CH 3 COOH(aq) + OH−(aq) Na+ + H 2 O Na. OH + H+ p. H > 7 Kb(CH 3 COO−) = 5. 6 x 10− 10

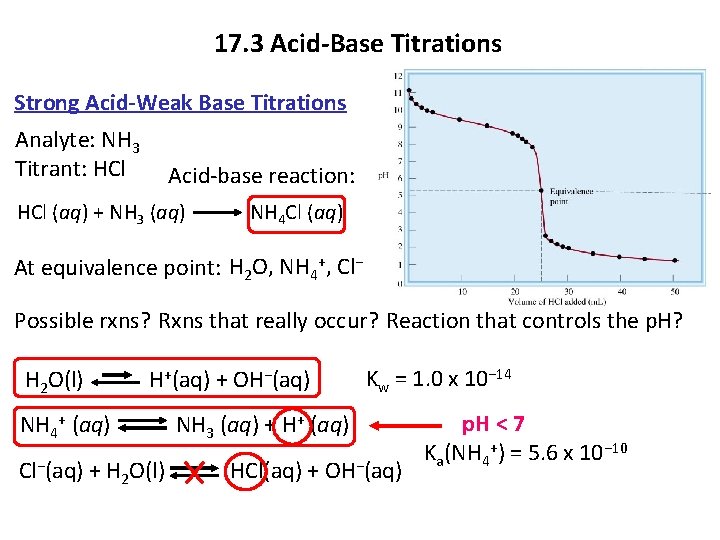
17. 3 Acid-Base Titrations Strong Acid-Weak Base Titrations Analyte: NH 3 Titrant: HCl Acid-base reaction: HCl (aq) + NH 3 (aq) NH 4 Cl (aq) At equivalence point: H 2 O, NH 4+, Cl− Possible rxns? Rxns that really occur? Reaction that controls the p. H? H 2 O(l) H+(aq) + OH−(aq) Kw = 1. 0 x 10− 14 NH 4+ (aq) NH 3 (aq) + H+ (aq) Cl−(aq) + H 2 O(l) HCl(aq) + OH−(aq) Copyright © Cengage Learning. All rights reserved p. H < 7 Ka(NH 4+) = 5. 6 x 10− 10 14

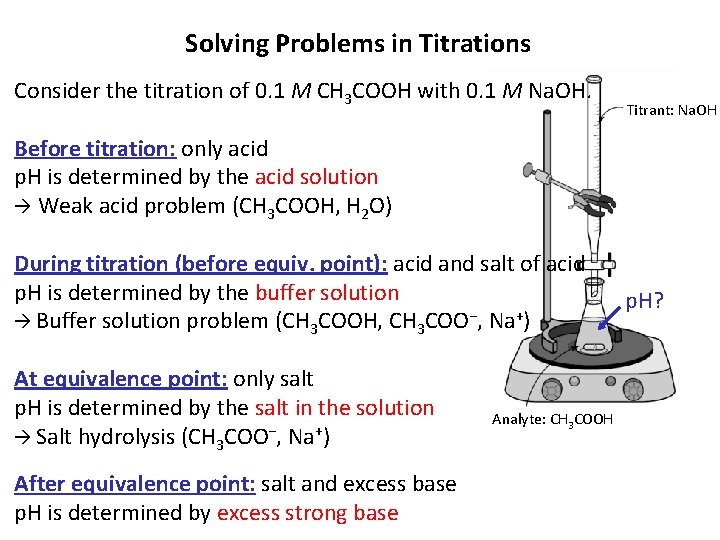
Solving Problems in Titrations Consider the titration of 0. 1 M CH 3 COOH with 0. 1 M Na. OH. Titrant: Na. OH Before titration: only acid p. H is determined by the acid solution Weak acid problem (CH 3 COOH, H 2 O) During titration (before equiv. point): acid and salt of acid p. H is determined by the buffer solution Buffer solution problem (CH 3 COOH, CH 3 COO−, Na+) At equivalence point: only salt p. H is determined by the salt in the solution Salt hydrolysis (CH 3 COO−, Na+) After equivalence point: salt and excess base p. H is determined by excess strong base Analyte: CH 3 COOH p. H?

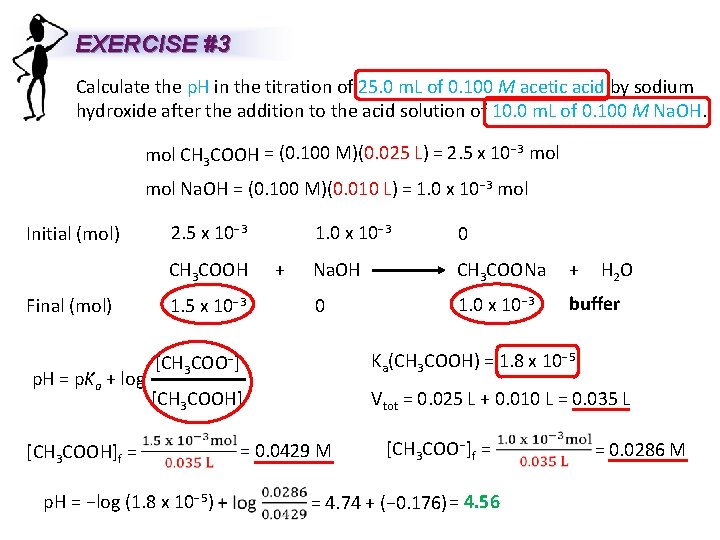
EXERCISE #3 Calculate the p. H in the titration of 25. 0 m. L of 0. 100 M acetic acid by sodium hydroxide after the addition to the acid solution of 10. 0 m. L of 0. 100 M Na. OH. mol CH 3 COOH = (0. 100 M)(0. 025 L) = 2. 5 x 10− 3 mol Na. OH == (0. 100 M)(0. 010 L) = 1. 0 x 10− 3 mol Initial (mol) Final (mol) p. H = p. Ka + log 2. 5 x 10− 3 1. 0 x 10− 3 0 CH 3 COOH + Na. OH CH 3 COONa + 1. 5 x 10− 3 0 1. 0 x 10− 3 H 2 O buffer [CH 3 COO−] Ka(CH 3 COOH) = 1. 8 x 10− 5 [CH 3 COOH] Vtot = 0. 025 L + 0. 010 L = 0. 035 L [CH 3 COOH]f = p. H = −log (1. 8 x 10− 5) = 0. 0429 M [CH 3 COO−]f = = 4. 74 + (− 0. 176) = 4. 56 = 0. 0286 M

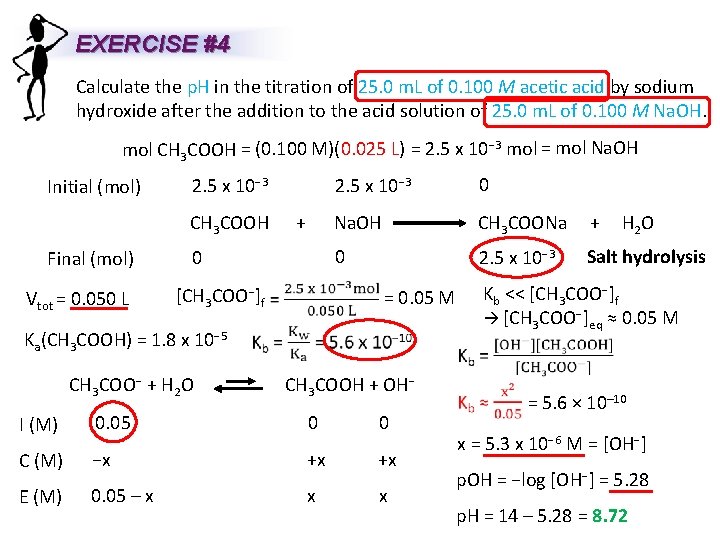
EXERCISE #4 Calculate the p. H in the titration of 25. 0 m. L of 0. 100 M acetic acid by sodium hydroxide after the addition to the acid solution of 25. 0 m. L of 0. 100 M Na. OH. mol CH 3 COOH = (0. 100 M)(0. 025 L) = 2. 5 x 10− 3 mol = mol Na. OH Initial (mol) Final (mol) Vtot = 0. 050 L 2. 5 x 10− 3 0 CH 3 COOH + Na. OH CH 3 COONa + 0 0 2. 5 x 10− 3 [CH 3 COO−]f Ka(CH 3 COOH) = 1. 8 x 10− 5 CH 3 COO− + H 2 O Salt hydrolysis Kb << [CH 3 COO−]f [CH 3 COO−]eq ≈ 0. 05 M = 0. 05 M H 2 O CH 3 COOH + OH− I (M) 0. 05 0 0 C (M) −x +x +x E (M) 0. 05 – x x x = 5. 6 × 10– 10 x = 5. 3 x 10− 6 M = [OH−] p. OH = −log [OH−] = 5. 28 p. H = 14 – 5. 28 = 8. 72

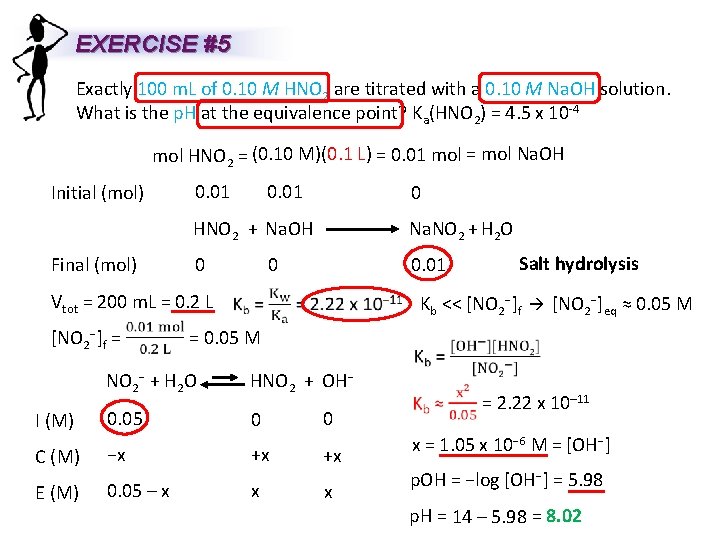
EXERCISE #5 Exactly 100 m. L of 0. 10 M HNO 2 are titrated with a 0. 10 M Na. OH solution. What is the p. H at the equivalence point? Ka(HNO 2) = 4. 5 x 10 -4 mol HNO 2 = (0. 10 M)(0. 1 L) = 0. 01 mol = mol Na. OH Initial (mol) Final (mol) 0. 01 0 HNO 2 + Na. OH Na. NO 2 + H 2 O 0 0. 01 Vtot = 200 m. L = 0. 2 L [NO 2−]f = 0. 01 0 Salt hydrolysis Kb << [NO 2−]f [NO 2−]eq ≈ 0. 05 M = 0. 05 M NO 2− + H 2 O HNO 2 + OH− I (M) 0. 05 0 C (M) −x E (M) 0. 05 – x +x x 0 +x x = 2. 22 x 10– 11 x = 1. 05 x 10− 6 M = [OH−] p. OH = −log [OH−] = 5. 98 p. H = 14 – 5. 98 = 8. 02

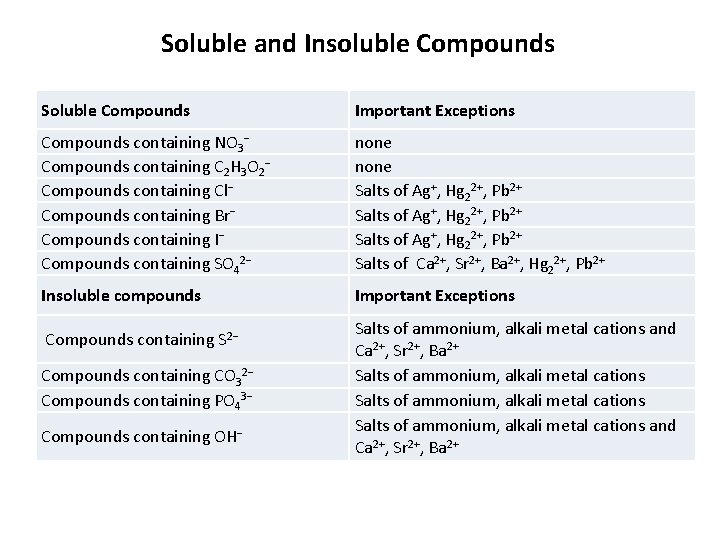
Soluble and Insoluble Compounds Soluble Compounds Important Exceptions Compounds containing NO 3 Compounds containing C 2 H 3 O 2 Compounds containing Cl Compounds containing Br Compounds containing I Compounds containing SO 42 none Salts of Ag+, Hg 22+, Pb 2+ Salts of Ca 2+, Sr 2+, Ba 2+, Hg 22+, Pb 2+ Insoluble compounds Important Exceptions Compounds containing S 2 Compounds containing CO 32 Compounds containing PO 43 Compounds containing OH Salts of ammonium, alkali metal cations and Ca 2+, Sr 2+, Ba 2+ Salts of ammonium, alkali metal cations and Ca 2+, Sr 2+, Ba 2+

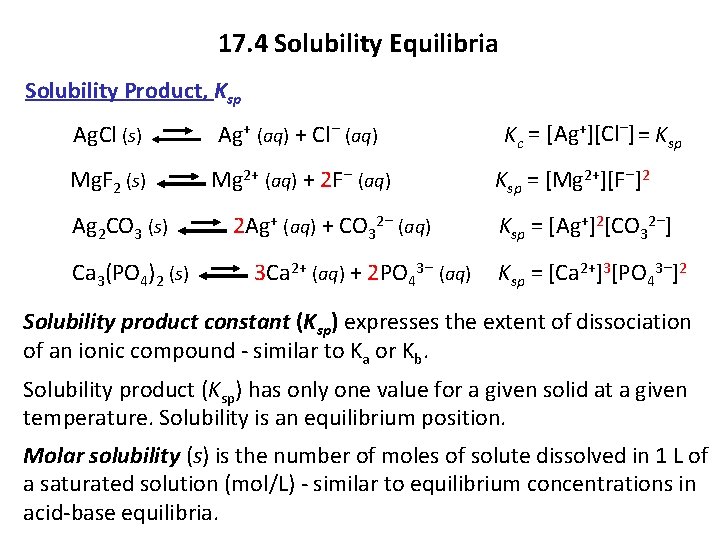
17. 4 Solubility Equilibria Solubility Product, Ksp Ag. Cl (s) Ag+ (aq) + Cl− (aq) Kc = [Ag+][Cl−] = Ksp Mg. F 2 (s) Mg 2+ (aq) + 2 F− (aq) Ksp = [Mg 2+][F−]2 Ag 2 CO 3 (s) 2 Ag+ (aq) + CO 32− (aq) Ksp = [Ag+]2[CO 32−] Ca 3(PO 4)2 (s) 3 Ca 2+ (aq) + 2 PO 43− (aq) Ksp = [Ca 2+]3[PO 43−]2 Solubility product constant (Ksp) expresses the extent of dissociation of an ionic compound - similar to Ka or Kb. Solubility product (Ksp) has only one value for a given solid at a given temperature. Solubility is an equilibrium position. Molar solubility (s) is the number of moles of solute dissolved in 1 L of a saturated solution (mol/L) - similar to equilibrium concentrations in acid-base equilibria.


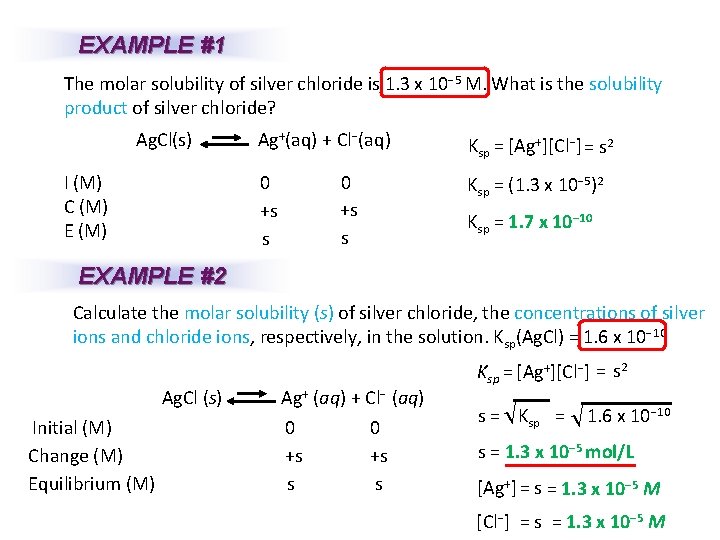
EXAMPLE #1 The molar solubility of silver chloride is 1. 3 x 10− 5 M. What is the solubility product of silver chloride? Ag. Cl(s) Ag+(aq) + Cl−(aq) I (M) C (M) E (M) 0 +s s Ksp = [Ag+][Cl−]= s 2 Ksp = (1. 3 x 10− 5)2 Ksp = 1. 7 x 10− 10 EXAMPLE #2 Calculate the molar solubility (s) of silver chloride, the concentrations of silver ions and chloride ions, respectively, in the solution. Ksp(Ag. Cl) = 1. 6 x 10− 10 Ag. Cl (s) Ag+ (aq) + Cl− (aq) 0 0 Initial (M) +s +s Change (M) s s Equilibrium (M) Ksp = [Ag+][Cl−] = s 2 − 10 s = K sp = 1. 6 x 10 s = 1. 3 x 10− 5 mol/L [Ag+] = s = 1. 3 x 10− 5 M [Cl−] = s = 1. 3 x 10− 5 M

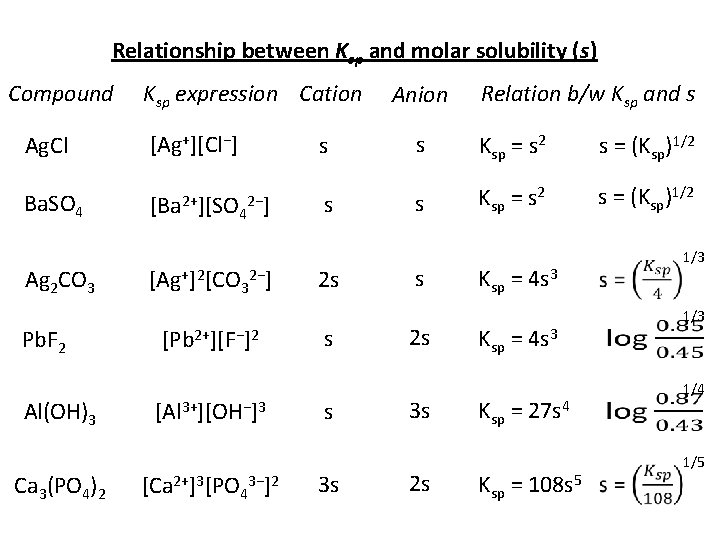
Relationship between Ksp and molar solubility (s) Compound Ksp expression Cation Anion Relation b/w Ksp and s Ag. Cl [Ag+][Cl−] s s Ksp = s 2 s = (Ksp)1/2 Ba. SO 4 [Ba 2+][SO 42−] s s Ksp = s 2 s = (Ksp)1/2 Ag 2 CO 3 Pb. F 2 Al(OH)3 Ca 3(PO 4)2 [Ag+]2[CO 32−] [Pb 2+][F−]2 [Al 3+][OH−]3 [Ca 2+]3[PO 43−]2 2 s s s 3 s s 2 s 3 s 2 s Ksp = 4 s 3 Ksp = 27 s 4 Ksp = 108 s 5 1/3 1/4 1/5

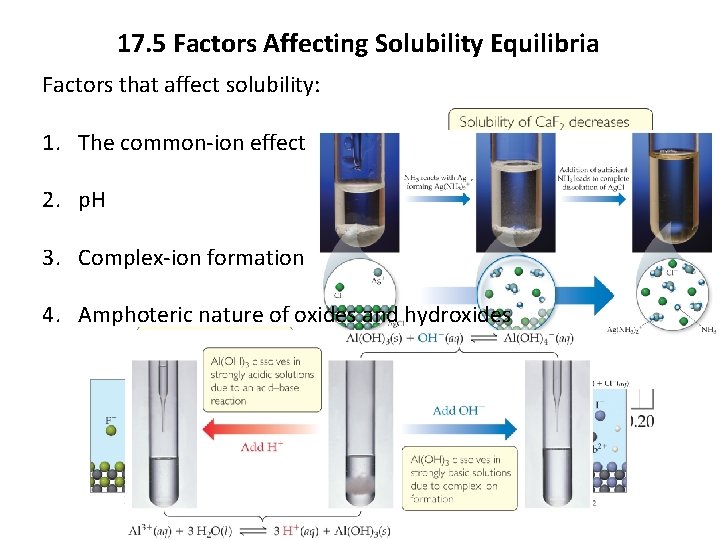
17. 5 Factors Affecting Solubility Equilibria Factors that affect solubility: 1. The common-ion effect 2. p. H 3. Complex-ion formation 4. Amphoteric nature of oxides and hydroxides

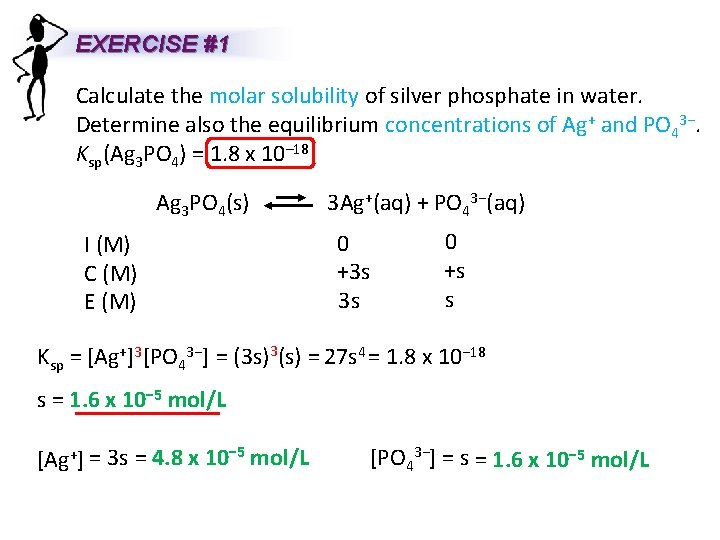
EXERCISE #1 Calculate the molar solubility of silver phosphate in water. Determine also the equilibrium concentrations of Ag+ and PO 43−. Ksp(Ag 3 PO 4) = 1. 8 x 10– 18 Ag 3 PO 4(s) 3 Ag+(aq) + PO 43−(aq) I (M) C (M) E (M) 0 +3 s 3 s 0 +s s Ksp = [Ag+]3[PO 43−] = (3 s)3(s) = 27 s 4 = 1. 8 x 10− 18 s = 1. 6 x 10− 5 mol/L [Ag+] = 3 s = 4. 8 x 10− 5 mol/L [PO 43−] = s = 1. 6 x 10− 5 mol/L

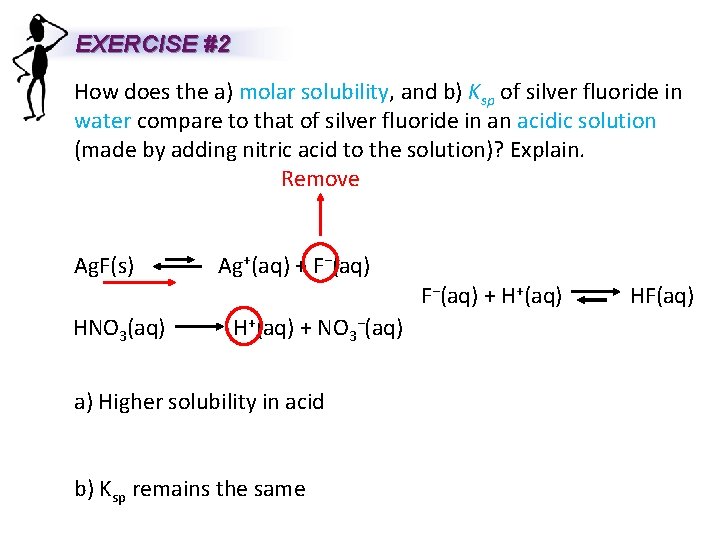
EXERCISE #2 How does the a) molar solubility, and b) Ksp of silver fluoride in water compare to that of silver fluoride in an acidic solution (made by adding nitric acid to the solution)? Explain. Remove Ag. F(s) Ag+(aq) + F−(aq) + H+(aq) HF(aq) HNO 3(aq) H+(aq) + NO 3−(aq) a) Higher solubility in acid b) Ksp remains the same

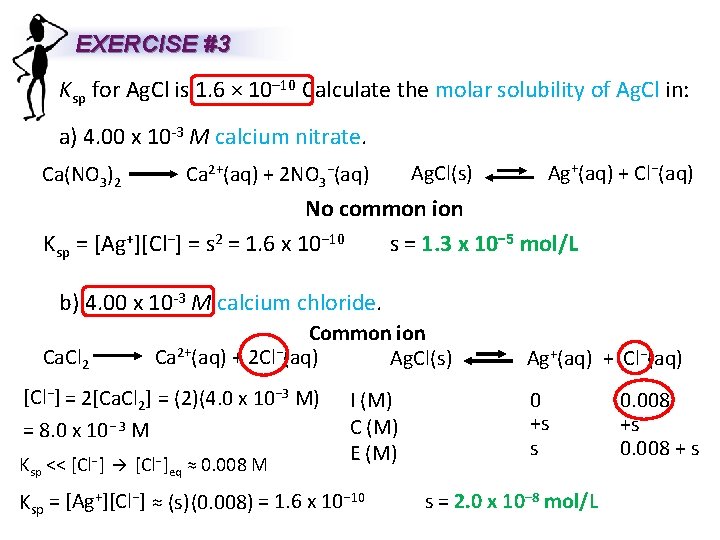
EXERCISE #3 Ksp for Ag. Cl is 1. 6 × 10– 10 Calculate the molar solubility of Ag. Cl in: a) 4. 00 x 10 -3 M calcium nitrate. Ca(NO 3)2 Ca 2+(aq) + 2 NO 3−(aq) Ag. Cl(s) Ag+(aq) + Cl−(aq) No common ion s = 1. 3 x 10− 5 mol/L Ksp = [Ag+][Cl−] = s 2 = 1. 6 x 10− 10 b) 4. 00 x 10 -3 M calcium chloride. Ca. Cl 2 Common ion Ca 2+(aq) + 2 Cl−(aq) Ag. Cl(s) Ag+(aq) + Cl−(aq) [Cl−] = 2[Ca. Cl 2] = (2)(4. 0 x 10− 3 M) = 8. 0 x 10− 3 M Ksp << [Cl−]eq ≈ 0. 008 M I (M) C (M) E (M) Ksp = [Ag+][Cl−] ≈ (s) (0. 008) = 1. 6 x 10− 10 0 +s s s = 2. 0 x 10− 8 mol/L 0. 008 +s 0. 008 + s

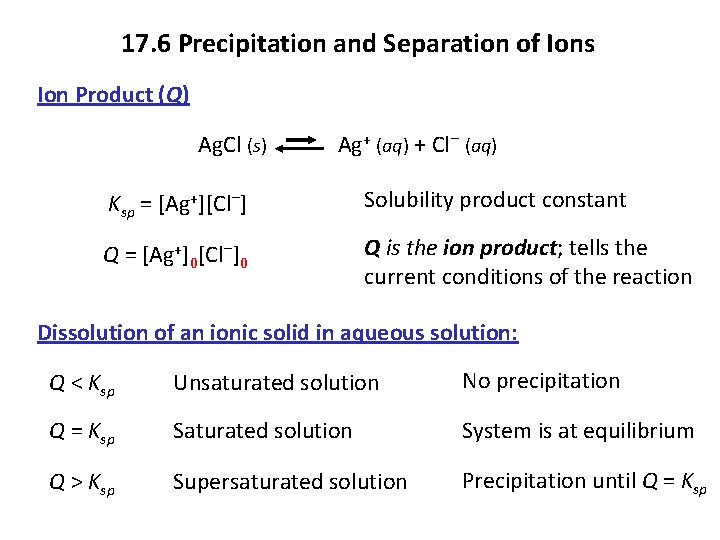
17. 6 Precipitation and Separation of Ions Ion Product (Q) Ag. Cl (s) Ag+ (aq) + Cl− (aq) Ksp = [Ag+][Cl−] Solubility product constant Q = [Ag+]0[Cl−]0 Q is the ion product; tells the current conditions of the reaction Dissolution of an ionic solid in aqueous solution: Q < Ksp Unsaturated solution No precipitation Q = Ksp Saturated solution System is at equilibrium Q > Ksp Supersaturated solution Precipitation until Q = Ksp

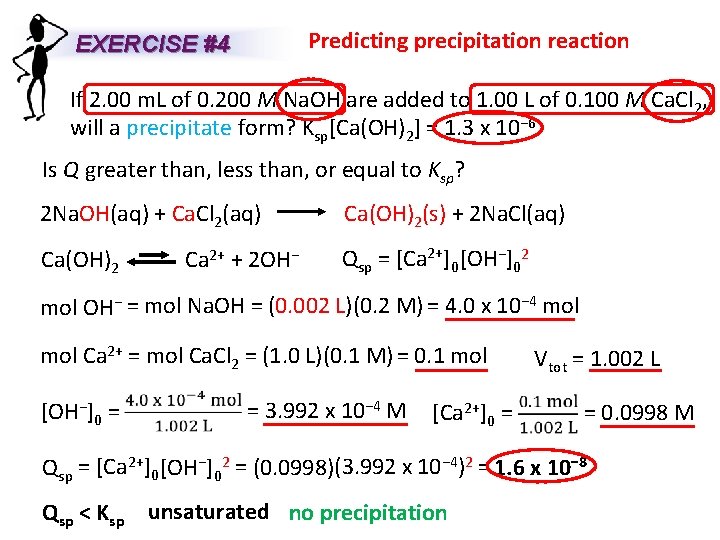
Predicting precipitation reaction EXERCISE #4 If 2. 00 m. L of 0. 200 M Na. OH are added to 1. 00 L of 0. 100 M Ca. Cl 2, will a precipitate form? Ksp[Ca(OH)2] = 1. 3 x 10− 6 Is Q greater than, less than, or equal to Ksp? 2 Na. OH(aq) + Ca. Cl 2(aq) Ca(OH)2 Ca 2+ + 2 OH− Ca(OH)2(s) + 2 Na. Cl(aq) Qsp = [Ca 2+]0 [OH−]02 mol OH− = mol Na. OH = (0. 002 L)(0. 2 M) = 4. 0 x 10− 4 mol Ca 2+ = mol Ca. Cl 2 = (1. 0 L)(0. 1 M) = 0. 1 mol [OH−]0 = = 3. 992 x 10− 4 M [Ca 2+]0 = Vtot = 1. 002 L = 0. 0998 M Qsp = [Ca 2+]0 [OH−]02 = (0. 0998)(3. 992 x 10− 4)2 = 1. 6 x 10− 8 Qsp < Ksp unsaturated no precipitation

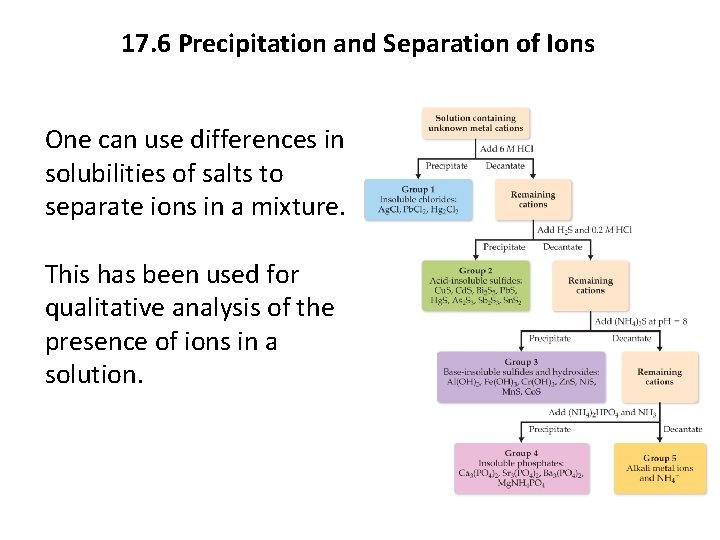
17. 6 Precipitation and Separation of Ions One can use differences in solubilities of salts to separate ions in a mixture. This has been used for qualitative analysis of the presence of ions in a solution.

END OF CHAPTER 17