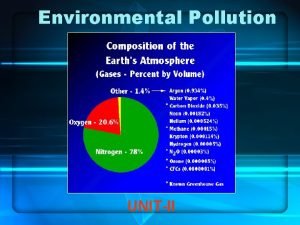
LECTURE 15 AOSC 434 AIR POLLUTION RUSSELL R








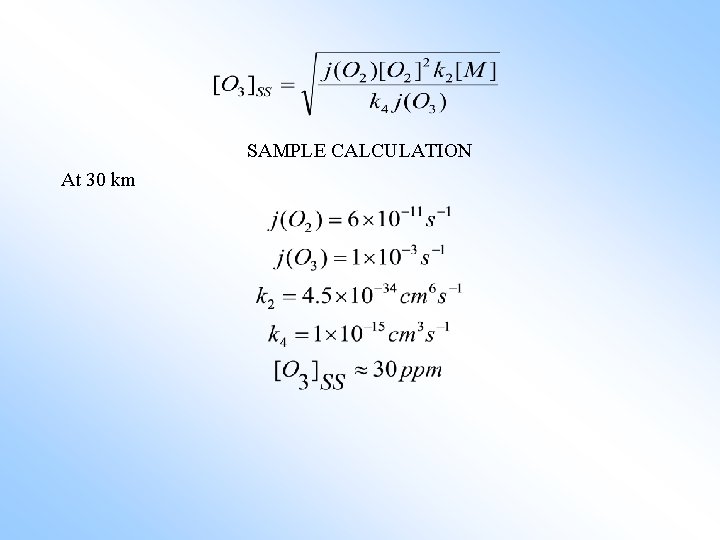


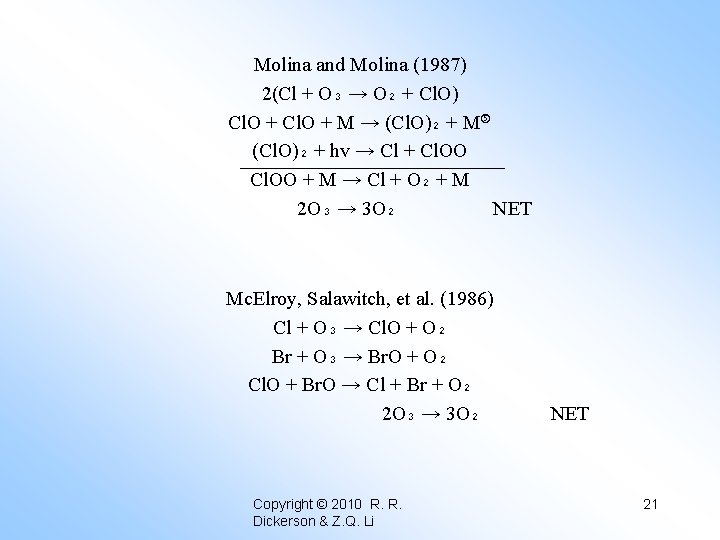




- Slides: 39

LECTURE 15 AOSC 434 AIR POLLUTION RUSSELL R. DICKERSON 2014

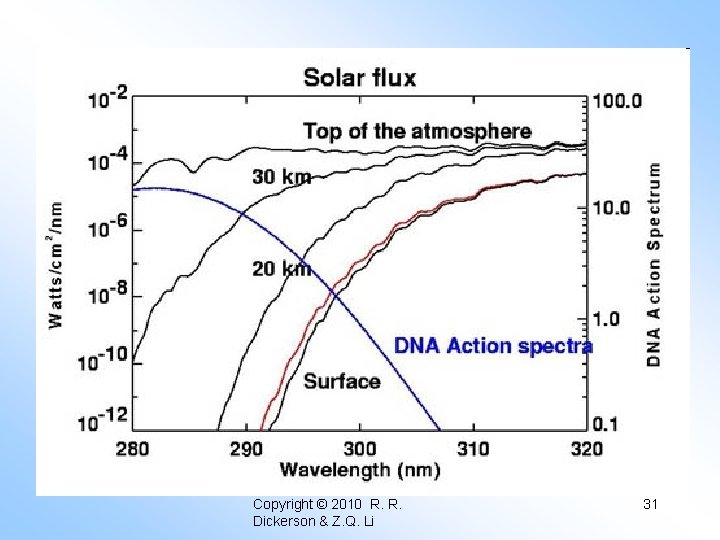
STRATOSPHERIC POLLUTION Without ozone in the atmosphere there could be no life as we know it on the surface of the Earth. All of the atmospheric ozone, that is the “ozone column” is only about 0. 3 atm cm. In other words, if all the air were squeezed out of the atmosphere, and the remaining ozone were brought to STP, it would be only 0. 3 cm thick. – Murphy’s Law is strictly obeyed by NOx pollution in the atmosphere. – Chemistry of the stratosphere different from troposphere. Table 15. 1 Solar intensity at the Earth’s surface assuming 0. 30 atm cm (300 D. U. ) ozone. Note that the maximum flux is about 7 x 10¹⁵ (photons/(cm²s)/10 nm).

Layers in the atmosphere Copyright © 2013 R. R. Dickerson & Z. Q. Li 3

https: //www. youtube. com/watch? v=f. Vs. ONlc 3 OUY

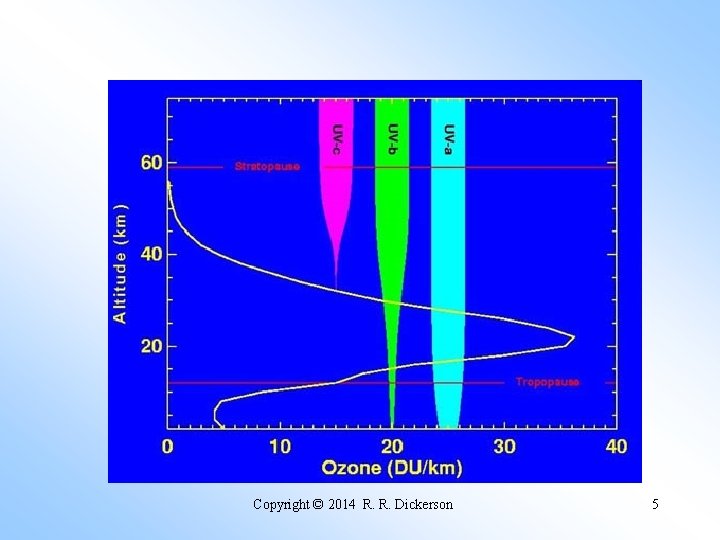
Copyright © 2014 R. R. Dickerson 5

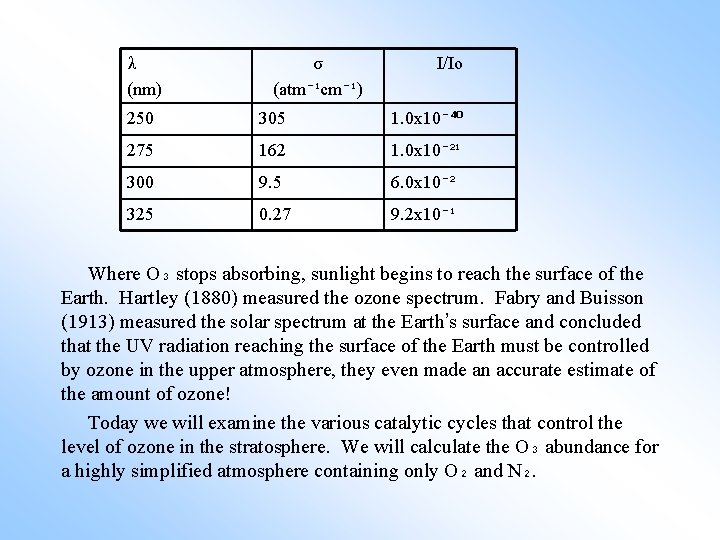
λ (nm) σ (atm⁻¹cm⁻¹) I/Io 250 305 1. 0 x 10⁻⁴⁰ 275 162 1. 0 x 10⁻²¹ 300 9. 5 6. 0 x 10⁻² 325 0. 27 9. 2 x 10⁻¹ Where O₃ stops absorbing, sunlight begins to reach the surface of the Earth. Hartley (1880) measured the ozone spectrum. Fabry and Buisson (1913) measured the solar spectrum at the Earth’s surface and concluded that the UV radiation reaching the surface of the Earth must be controlled by ozone in the upper atmosphere, they even made an accurate estimate of the amount of ozone! Today we will examine the various catalytic cycles that control the level of ozone in the stratosphere. We will calculate the O₃ abundance for a highly simplified atmosphere containing only O₂ and N₂.


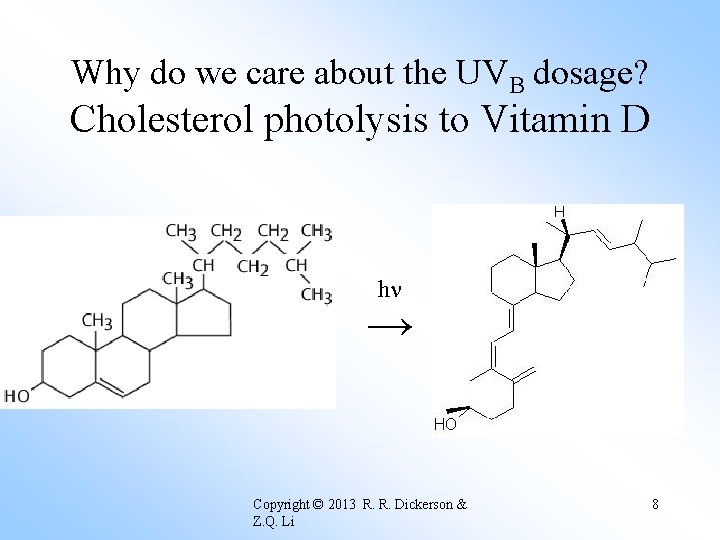
Why do we care about the UVB dosage? Cholesterol photolysis to Vitamin D h → Copyright © 2013 R. R. Dickerson & Z. Q. Li 8

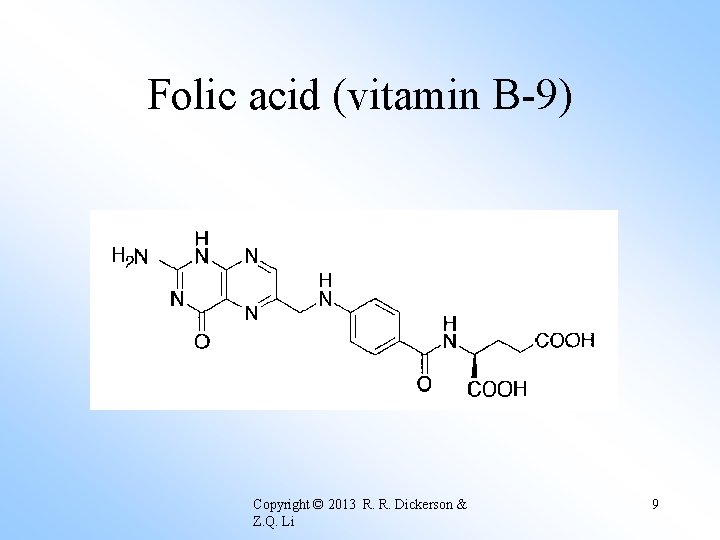
Folic acid (vitamin B-9) Copyright © 2013 R. R. Dickerson & Z. Q. Li 9

If you have a weak stomach Go get a cup of coffee for the next 3 min.

Too little UV radiation means rickets; UV converts cholesterol to Vitamin D. UVC - 100 to 290 nm UVB - 290 to 320 nm UVA - 320 to 400 nm Copyright © 2013 R. R. Dickerson & Z. Q. Li 11

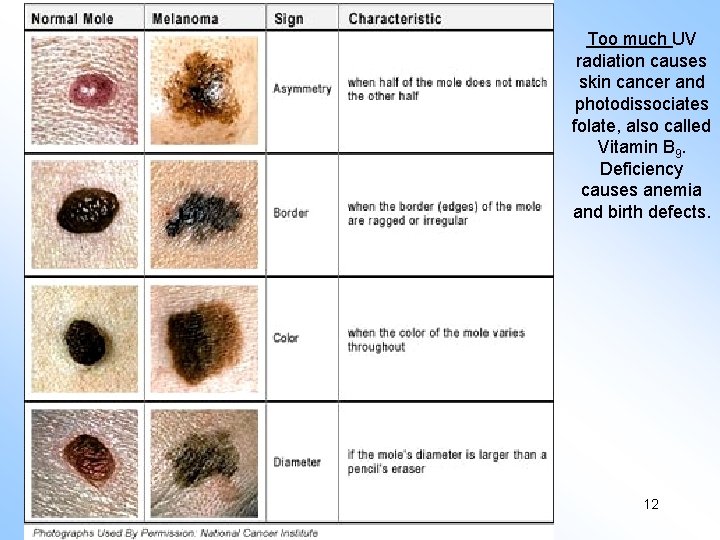
Too much UV radiation causes skin cancer and photodissociates folate, also called Vitamin B 9. Deficiency causes anemia and birth defects. Copyright © 2013 R. R. Dickerson & Z. Q. Li 12

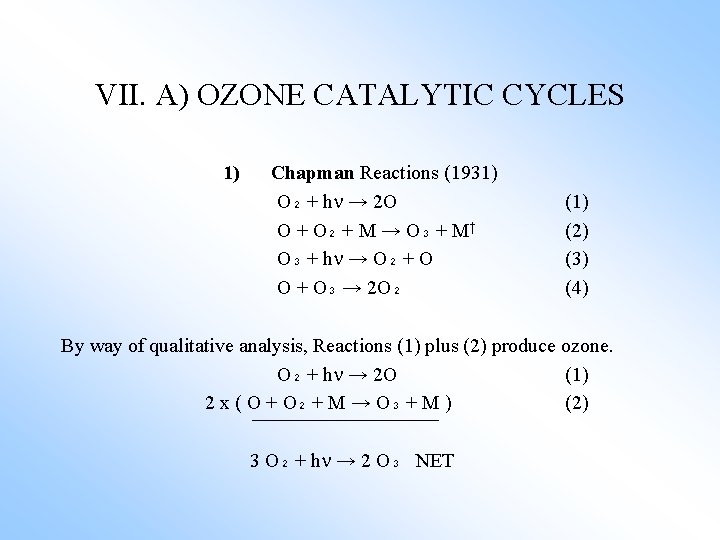
VII. A) OZONE CATALYTIC CYCLES 1) Chapman Reactions (1931) O₂ + h → 2 O O + O₂ + M → O₃ + M† O₃ + h → O₂ + O O + O₃ → 2 O₂ (1) (2) (3) (4) By way of qualitative analysis, Reactions (1) plus (2) produce ozone. O₂ + h → 2 O (1) 2 x ( O + O₂ + M → O₃ + M ) (2) 3 O₂ + h → 2 O₃ NET

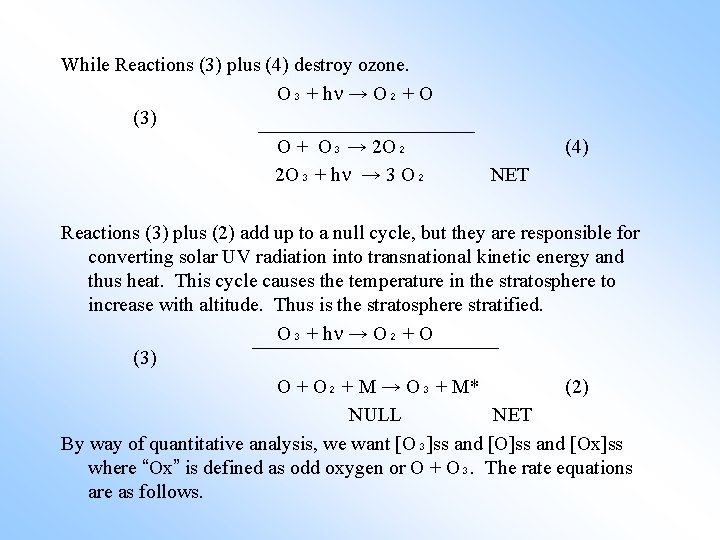
While Reactions (3) plus (4) destroy ozone. O₃ + h → O₂ + O (3) O + O₃ → 2 O₂ 2 O₃ + h → 3 O₂ (4) NET Reactions (3) plus (2) add up to a null cycle, but they are responsible for converting solar UV radiation into transnational kinetic energy and thus heat. This cycle causes the temperature in the stratosphere to increase with altitude. Thus is the stratosphere stratified. O₃ + h → O₂ + O (3) O + O₂ + M → O₃ + M* (2) NULL NET By way of quantitative analysis, we want [O₃]ss and [Ox]ss where “Ox” is defined as odd oxygen or O + O₃. The rate equations are as follows.

(a) (b) (a+b) From the representation for O atom chemistry: In the middle of the stratosphere, however, R₃ >>2 R₁ and R₂ >> R₄ thus: (I) This does not mean that R₄ is unimportant, but it can be ignored in an approximation of [O]ss at the altitude of the ozone layer. The ratio of [O] to [O₃] can also be useful:

(II) Reactions 2 and 3 set the ratio of O to O₃, while Reactions 1 and 4 set the absolute concentrations. Now we will derive the steady state ozone concentration fro the stratosphere. From the assumption that Ox is in ready state we know: R₁ = R₄ Thus j(O₂)[O₂] = k₄[O][O₃] Substituting from (I), the steady state O atom concentration: or
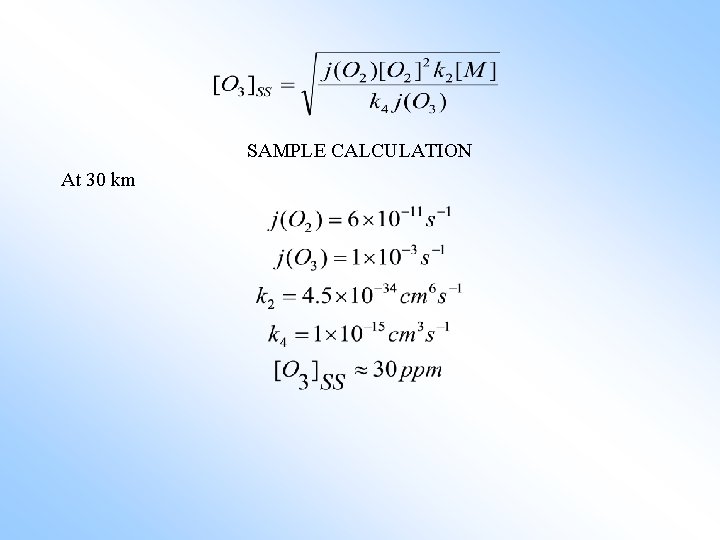
SAMPLE CALCULATION At 30 km

This is almost a factor of ten above the true concentration! What is wrong? There must be ozone sinks missing. 2) Bates and Nicolet (1950) “HOx” Odd hydrogen “HOx” is the sum of OH and HO₂ (sometimes H and H₂O₂ are included as well). HO₂ + O₃ → OH + 2 O₂ OH + O₃ → HO₂ + O₂ 2 O₃ → 3 O₂ The following catalytic also destroys ozone. OH + O₃ → HO₂ + O₂ HO₂ + O → OH + O₂ O + O₃ → 2 O₂ (5) (6) NET (6) (7) NET

The second catalytic cycle speeds up Reaction 4, that is it effectively increases k₄. Note that any loss of odd oxygen is the same as loss of ozone. These catalytic losses are still insufficient to explain the observed ozone concentration. 3) Crutzen (1970); Johnston (1971) “NOx” Odd nitrogen or “NOx” is the sum of NO and NO₂. Often “NOx” is used as “odd nitrogen” which includes NO₃, HNO₃, 2 N₂O₅, HONO, PAN and other species. This total of “odd nitrogen” is better called “NOy” or “total reactive nitrogen. ” N₂ and N₂O are unreactive. NO + O₃ → NO₂ + O₂ O + NO₂ → NO + O₂ O + O₃ → 2 O₂ NET This is the major means of destruction of stratospheric ozone. The NOx cycle accounts for about 70% of the ozone loss at 30 km. We will calculate the implied steady ozone concentration later.

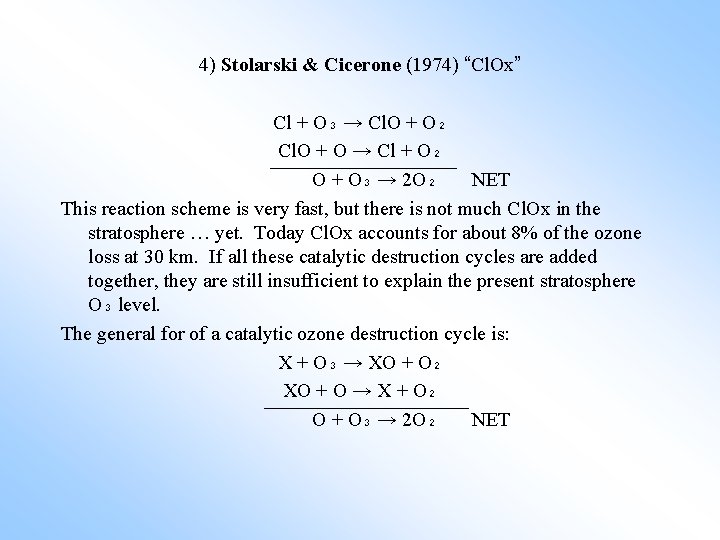
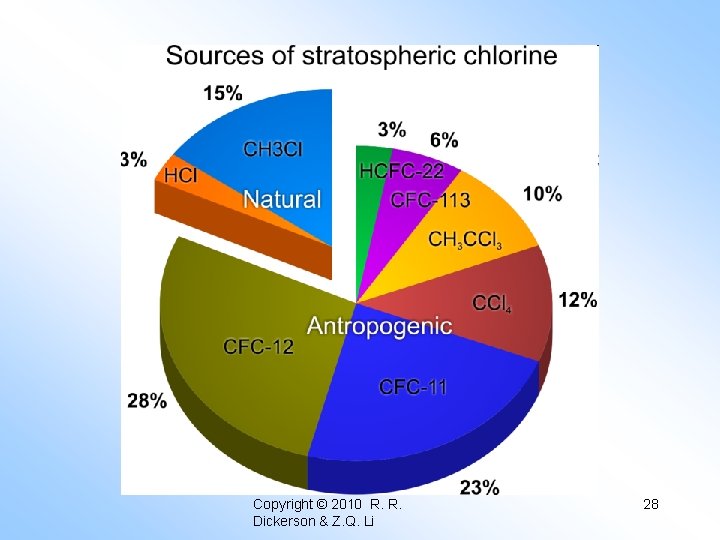
4) Stolarski & Cicerone (1974) “Cl. Ox” Cl + O₃ → Cl. O + O₂ Cl. O + O → Cl + O₂ O + O₃ → 2 O₂ NET This reaction scheme is very fast, but there is not much Cl. Ox in the stratosphere … yet. Today Cl. Ox accounts for about 8% of the ozone loss at 30 km. If all these catalytic destruction cycles are added together, they are still insufficient to explain the present stratosphere O₃ level. The general for of a catalytic ozone destruction cycle is: X + O₃ → XO + O₂ XO + O → X + O₂ O + O₃ → 2 O₂ NET
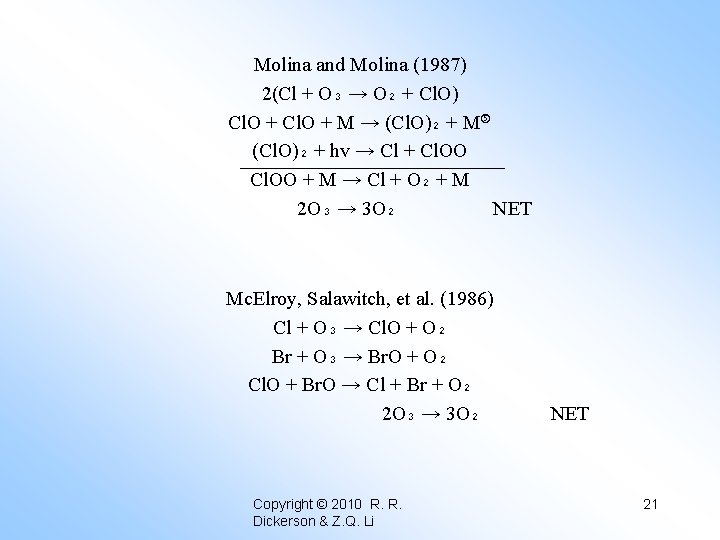
Molina and Molina (1987) 2(Cl + O₃ → O₂ + Cl. O) Cl. O + M → (Cl. O)₂ + M (Cl. O)₂ + hv → Cl + Cl. OO + M → Cl + O₂ + M 2 O₃ → 3 O₂ NET Mc. Elroy, Salawitch, et al. (1986) Cl + O₃ → Cl. O + O₂ Br + O₃ → Br. O + O₂ Cl. O + Br. O → Cl + Br + O₂ 2 O₃ → 3 O₂ Copyright © 2010 R. R. Dickerson & Z. Q. Li NET 21

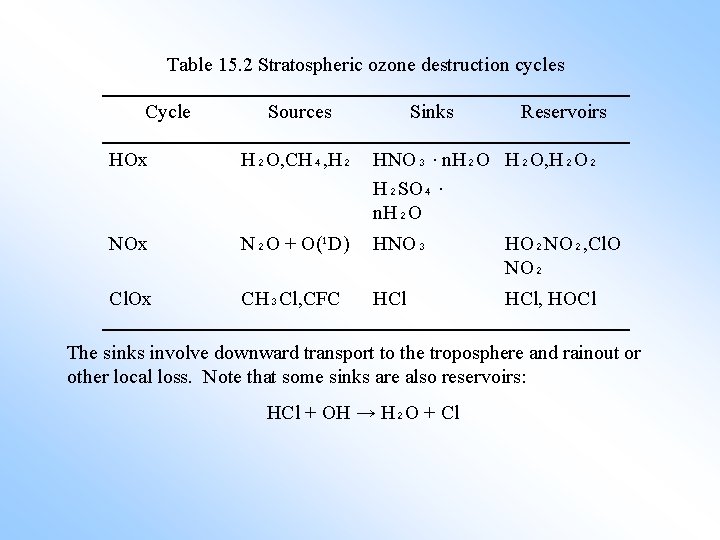
Table 15. 2 Stratospheric ozone destruction cycles Cycle Sources Sinks Reservoirs HOx H₂O, CH₄, H₂ HNO₃ · n. H₂O, H₂O₂ H₂SO₄ · n. H₂O NOx N₂O + O(¹D) HNO₃ HO₂NO₂, Cl. O NO₂ Cl. Ox CH₃Cl, CFC HCl, HOCl The sinks involve downward transport to the troposphere and rainout or other local loss. Note that some sinks are also reservoirs: HCl + OH → H₂O + Cl

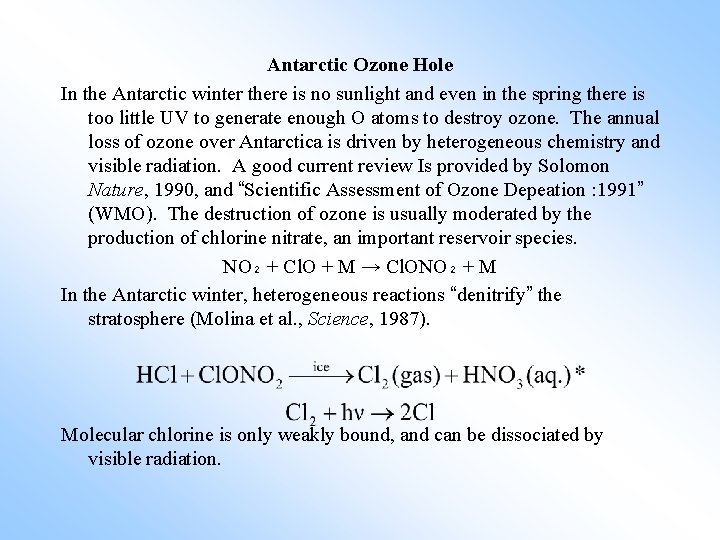
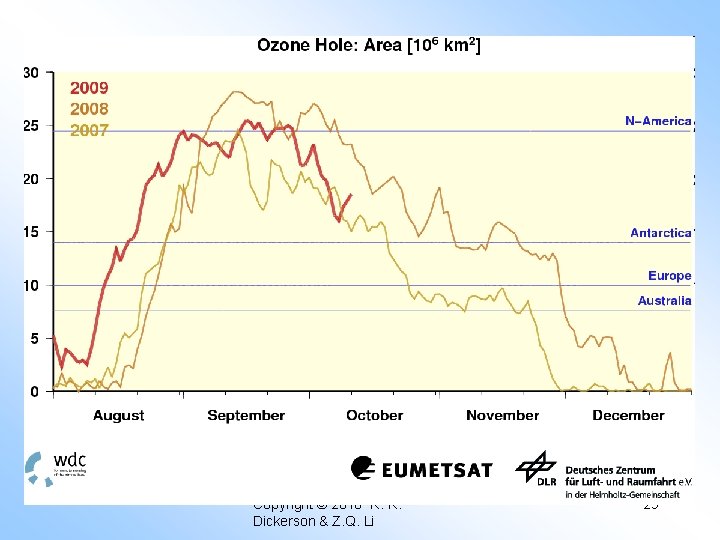
Antarctic Ozone Hole In the Antarctic winter there is no sunlight and even in the spring there is too little UV to generate enough O atoms to destroy ozone. The annual loss of ozone over Antarctica is driven by heterogeneous chemistry and visible radiation. A good current review Is provided by Solomon Nature, 1990, and “Scientific Assessment of Ozone Depeation : 1991” (WMO). The destruction of ozone is usually moderated by the production of chlorine nitrate, an important reservoir species. NO₂ + Cl. O + M → Cl. ONO₂ + M In the Antarctic winter, heterogeneous reactions “denitrify” the stratosphere (Molina et al. , Science, 1987). Molecular chlorine is only weakly bound, and can be dissociated by visible radiation.

Cl + O₃ → O₂ + Cl. O + M → (Cl. O)₂ + M (Cl. O)₂ + hv → Cl + Cl. OO + M → Cl + O₂ + M 2 O₃ → 3 O₂ NET Two types of Polar Stratospheric Clouds (PSC’s) exist. Type I = HNO₃ · 3 H₂O Nitric acid trihydrate, formed at T ≤ 195 K Type II = H₂O Ice formed at T ≤ 190 K • They move NOy species from the vapor phase to the condensed phase as HNO₃. • The are involved in catalytic cycles with chlorine and bromine compounds that speed the reaction of ozone with itself to form oxygen. • They move chlorine from the reservoir species HCl and Cl. ONO₂ to Cl. Ox.

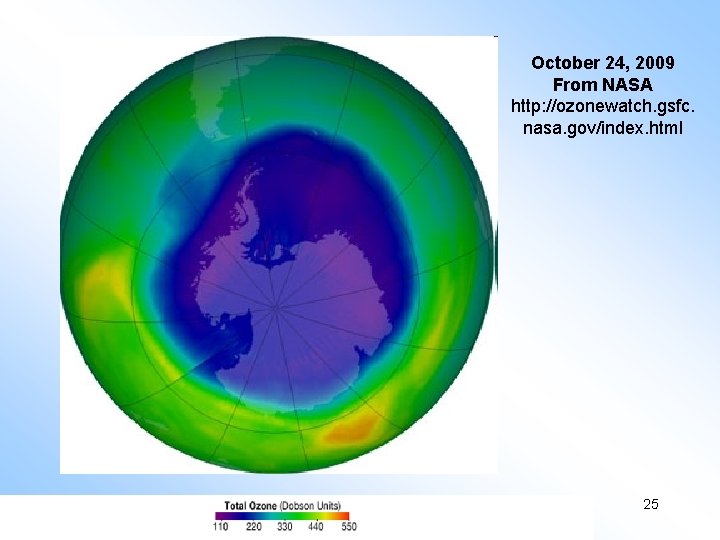
October 24, 2009 From NASA http: //ozonewatch. gsfc. nasa. gov/index. html Copyright © 2010 R. R. Dickerson & Z. Q. Li 25

Airborne Antarctic Ozone Expedition: Punta Arenas, Chile, 1987 Anderson et al. , Science, 1991 Copyright © 2010 R. R. Dickerson & Z. Q. Li 26

Copyright © 2010 R. R. Polar Stratospheric Clouds (PSCs) Dickerson & Z. Q. Li 27

Copyright © 2010 R. R. Dickerson & Z. Q. Li 28

Copyright © 2010 R. R. Dickerson & Z. Q. Li 29

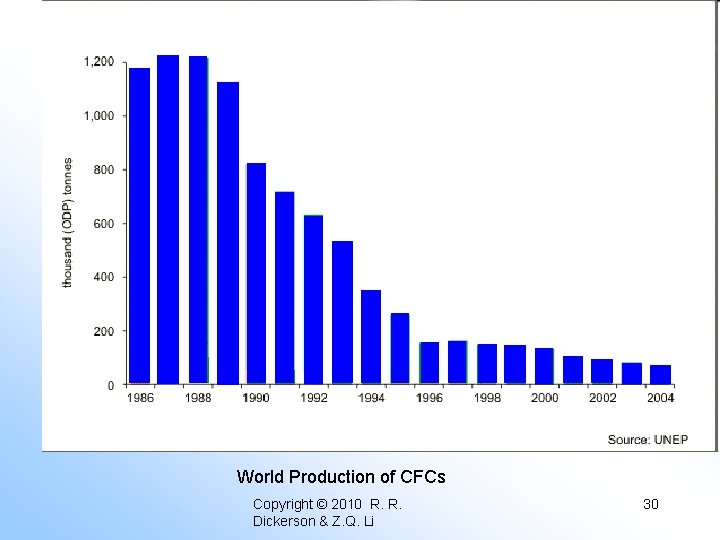
World Production of CFCs Copyright © 2010 R. R. Dickerson & Z. Q. Li 30

Copyright © 2010 R. R. Dickerson & Z. Q. Li 31

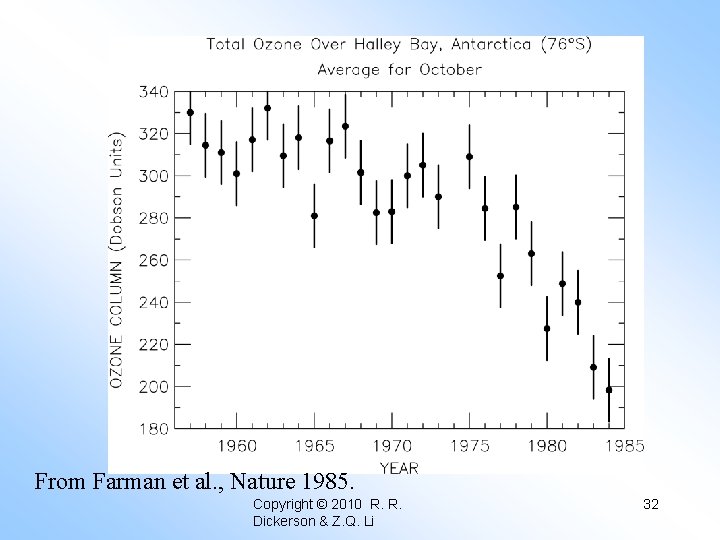
From Farman et al. , Nature 1985. Copyright © 2010 R. R. Dickerson & Z. Q. Li 32

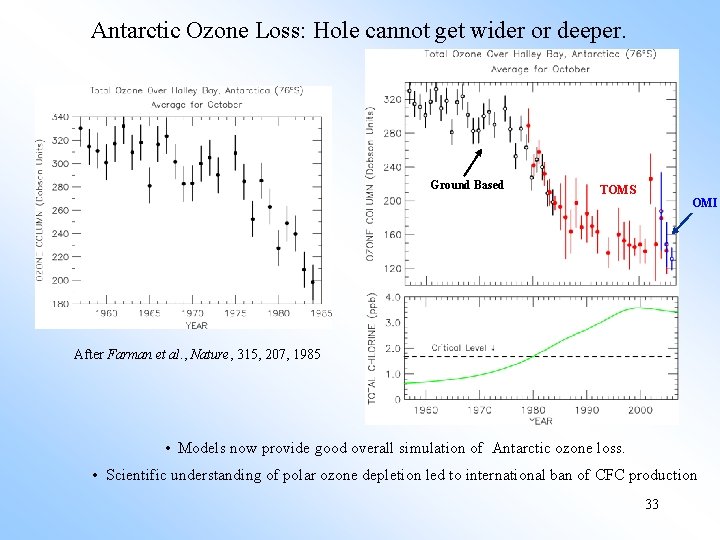
Antarctic Ozone Loss: Hole cannot get wider or deeper. Ground Based TOMS OMI After Farman et al. , Nature, 315, 207, 1985 • Models now provide good overall simulation of Antarctic ozone loss. • Scientific understanding of polar ozone depletion led to international ban of CFC production 33

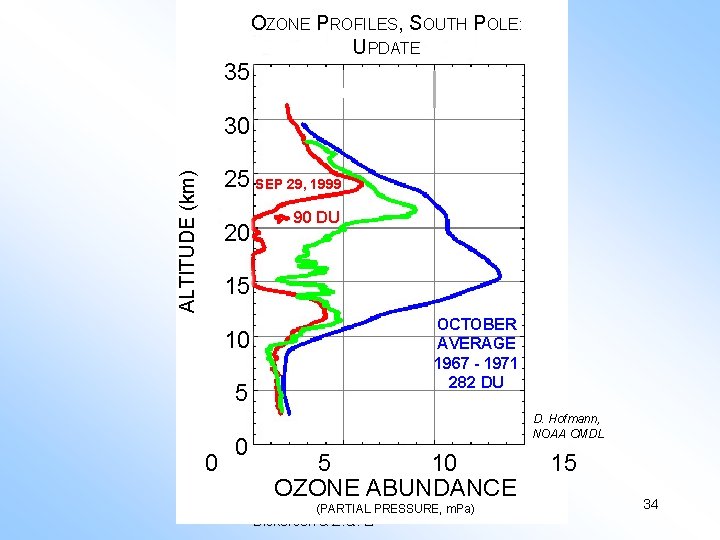
OZONE PROFILES, SOUTH POLE: UPDATE 35 Ozone Hole Update, II 30 ALTITUDE (km) 25 SEP 29, 1999 20 90 DU 15 10 5 0 0 OCTOBER AVERAGE 1967 - 1971 282 DU D. Hofmann, NOAA CMDL 5 10 OZONE ABUNDANCE Copyright (PARTIAL © 2010 R. R. PRESSURE, m. Pa) Dickerson & Z. Q. Li 15 34

Accommodation Coefficients • Condensed phase has lower entropy than gas phase. • Accommodation coefficients (reaction probabilities) should be greater at lower temperatures. Copyright © 2010 R. R. Dickerson & Z. Q. Li 35

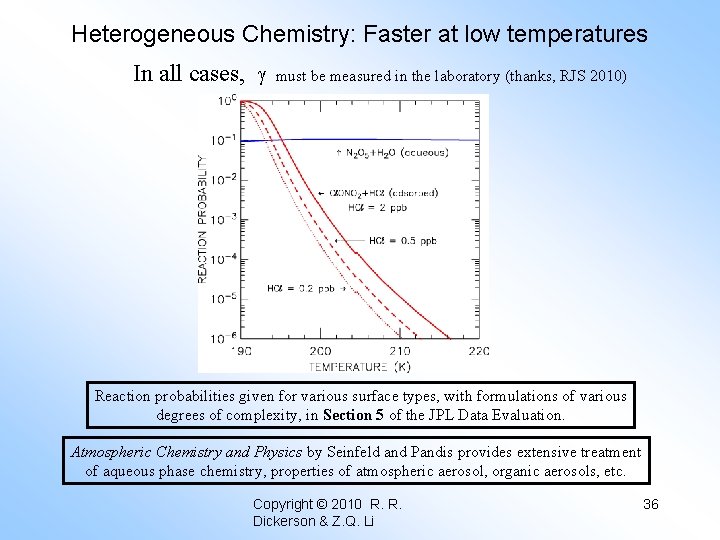
Heterogeneous Chemistry: Faster at low temperatures In all cases, must be measured in the laboratory (thanks, RJS 2010) Reaction probabilities given for various surface types, with formulations of various degrees of complexity, in Section 5 of the JPL Data Evaluation. Atmospheric Chemistry and Physics by Seinfeld and Pandis provides extensive treatment of aqueous phase chemistry, properties of atmospheric aerosol, organic aerosols, etc. Copyright © 2010 R. R. Dickerson & Z. Q. Li 36

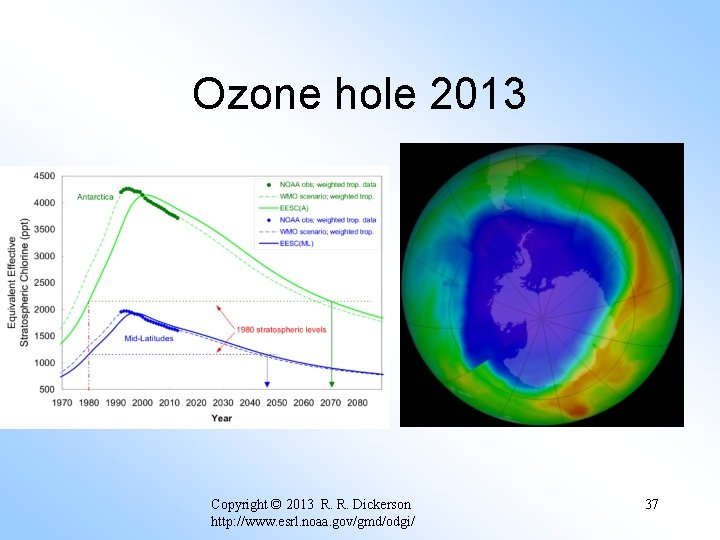
Ozone hole 2013 Copyright © 2013 R. R. Dickerson http: //www. esrl. noaa. gov/gmd/odgi/ 37

Summary of Ozone Hole Formation • • Threat to DNA-based life forms. Not predicted by any models First observed by Farman et al. , (Nature 1985). Ozone destruction nearly complete. Halogen (Cl & Br) reactions are responsible. Polar stratospheric Clouds play a central role. Multiphase (heterogeneous) reactions denitrify stratosphere. • Reaction rates depend on accommodation coefficients, f(T). • Replacement of CFC’s should heal ozone hole. Copyright © 2010 R. R. Dickerson & Z. Q. Li 38

Summary of Ozone Hole Formation • • Threat to DNA-based life forms. Not predicted by any models First observed by Farman et al. , (Nature 1985). Ozone destruction nearly complete. Halogen (Cl & Br) reactions are responsible. Polar stratospheric Clouds play a central role. Multiphase (heterogeneous) reactions denitrify stratosphere. • Reaction rates depend on accommodation coefficients, f(T). • Replacement of CFC’s should heal ozone hole. Copyright © 2010 R. R. Dickerson & Z. Q. Li 39
 Chapter 12 air section 1
Chapter 12 air section 1 Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Air higroskopis air kapiler dan air gravitasi
Air higroskopis air kapiler dan air gravitasi Ece 434
Ece 434 Anatomy team 434
Anatomy team 434 Ece 434
Ece 434 Astm d4685
Astm d4685 Ece 434
Ece 434 Anatomy team 434
Anatomy team 434 434 x 2
434 x 2 Kj 434
Kj 434 Ece 434
Ece 434 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Objective of pollution
Objective of pollution Conclusion of soil pollution
Conclusion of soil pollution Indoor air pollution sources
Indoor air pollution sources Air pollution wildfires
Air pollution wildfires Brainpop canada
Brainpop canada Two sources of air pollution
Two sources of air pollution Example of environmental sustainability
Example of environmental sustainability Air pollution box model example
Air pollution box model example Introduction about air pollution
Introduction about air pollution Air pollution specialist
Air pollution specialist Air pollution wildfires
Air pollution wildfires Air pollution
Air pollution Indoor air pollution examples
Indoor air pollution examples Air pollution
Air pollution Air pollution
Air pollution Main cause of air pollution
Main cause of air pollution What is mobile source
What is mobile source Baghouse filter diagram
Baghouse filter diagram Air pollution
Air pollution Air pollution control methods
Air pollution control methods 5 effects of air pollution
5 effects of air pollution Air pollution mexico
Air pollution mexico General effects of air pollution
General effects of air pollution Air pollution
Air pollution Air pollution effects on plant
Air pollution effects on plant Prevention of indoor air pollution
Prevention of indoor air pollution Air pollution simulator
Air pollution simulator