LECTURE 12 AOSC 434 AIR POLLUTION RUSSELL R






- Slides: 19

LECTURE 12 AOSC 434 AIR POLLUTION RUSSELL R. DICKERSON

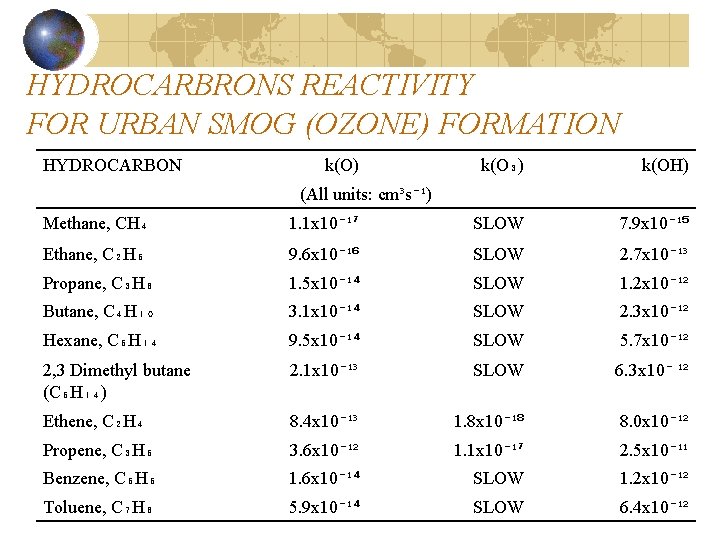
HYDROCARBRONS REACTIVITY FOR URBAN SMOG (OZONE) FORMATION HYDROCARBON k(O) k(O₃) k(OH) (All units: cm³s⁻¹) Methane, CH₄ 1. 1 x 10⁻¹⁷ SLOW 7. 9 x 10⁻¹⁵ Ethane, C₂H₆ 9. 6 x 10⁻¹⁶ SLOW 2. 7 x 10⁻¹³ Propane, C₃H₈ 1. 5 x 10⁻¹⁴ SLOW 1. 2 x 10⁻¹² Butane, C₄H₁₀ 3. 1 x 10⁻¹⁴ SLOW 2. 3 x 10⁻¹² Hexane, C₆H₁₄ 9. 5 x 10⁻¹⁴ SLOW 5. 7 x 10⁻¹² 2, 3 Dimethyl butane (C₆H₁₄) 2. 1 x 10⁻¹³ SLOW 6. 3 x 10⁻ ¹² Ethene, C₂H₄ 8. 4 x 10⁻¹³ 1. 8 x 10⁻¹⁸ 8. 0 x 10⁻¹² Propene, C₃H₆ 3. 6 x 10⁻¹² 1. 1 x 10⁻¹⁷ 2. 5 x 10⁻¹¹ Benzene, C₆H₆ 1. 6 x 10⁻¹⁴ SLOW 1. 2 x 10⁻¹² Toluene, C₇H₈ 5. 9 x 10⁻¹⁴ SLOW 6. 4 x 10⁻¹²

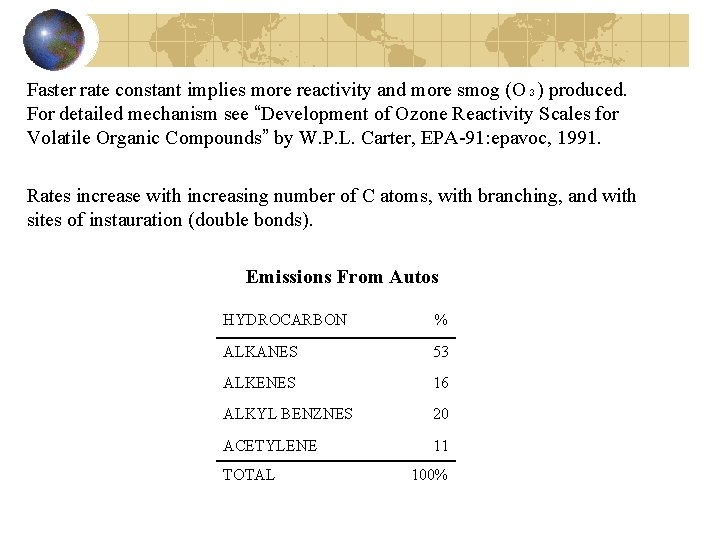
Faster rate constant implies more reactivity and more smog (O₃) produced. For detailed mechanism see “Development of Ozone Reactivity Scales for Volatile Organic Compounds” by W. P. L. Carter, EPA-91: epavoc, 1991. Rates increase with increasing number of C atoms, with branching, and with sites of instauration (double bonds). Emissions From Autos HYDROCARBON % ALKANES 53 ALKENES 16 ALKYL BENZNES 20 ACETYLENE 11 TOTAL 100%

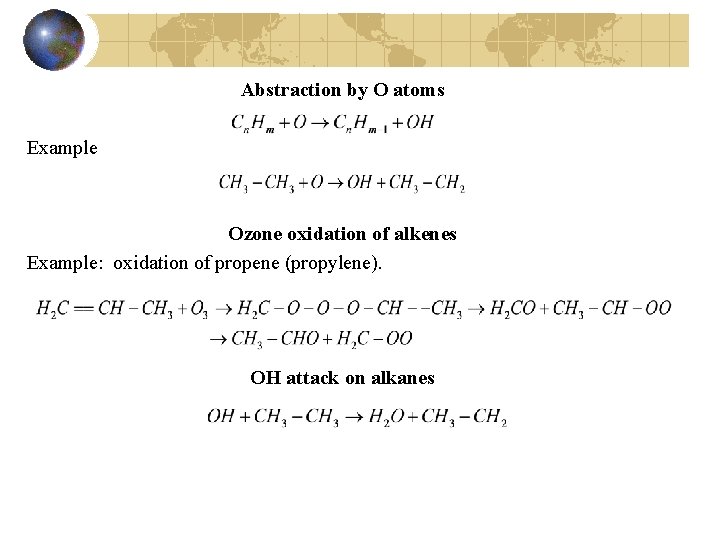
Abstraction by O atoms Example Ozone oxidation of alkenes Example: oxidation of propene (propylene). OH attack on alkanes

SINKS OF AIR POLLUTANTS I. RAINOUT/WASHOUT Only for soluble gases and particles Lifetime the same as that for water ~10 days Lifetime increases with altitude II. DRY DEPOSITION Only for “sticky” or reactive gases and particles Rate determined by atmospheric turbulence, chemical and physical properties of both the atmospheric species and the surface, i. e. , bare soil, vegetation etc. III. REACTIONS Transformation to other species, usually by oxidation.

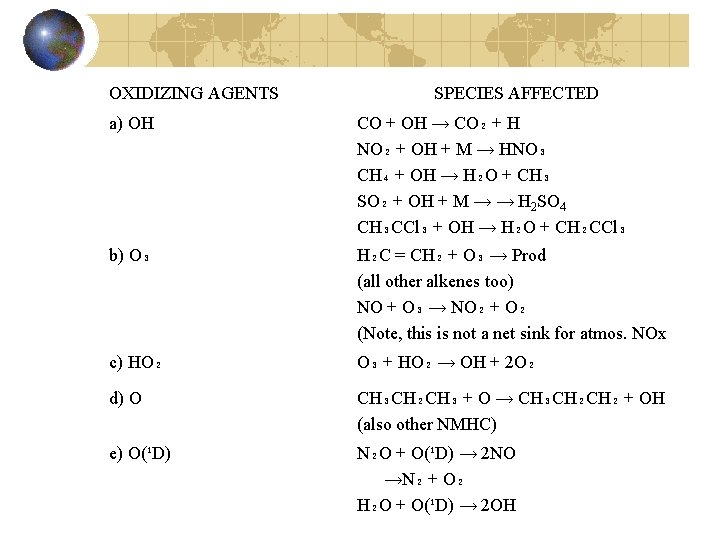
OXIDIZING AGENTS SPECIES AFFECTED a) OH CO + OH → CO₂ + H NO₂ + OH + M → HNO₃ CH₄ + OH → H₂O + CH₃ SO₂ + OH + M → → H 2 SO 4 CH₃CCl₃ + OH → H₂O + CH₂CCl₃ b) O₃ H₂C = CH₂ + O₃ → Prod (all other alkenes too) NO + O₃ → NO₂ + O₂ (Note, this is not a net sink for atmos. NOx c) HO₂ O₃ + HO₂ → OH + 2 O₂ d) O CH₃CH₂CH₃ + O → CH₃CH₂CH₂ + OH (also other NMHC) e) O(¹D) N₂O + O(¹D) → 2 NO →N₂ + O₂ H₂O + O(¹D) → 2 OH

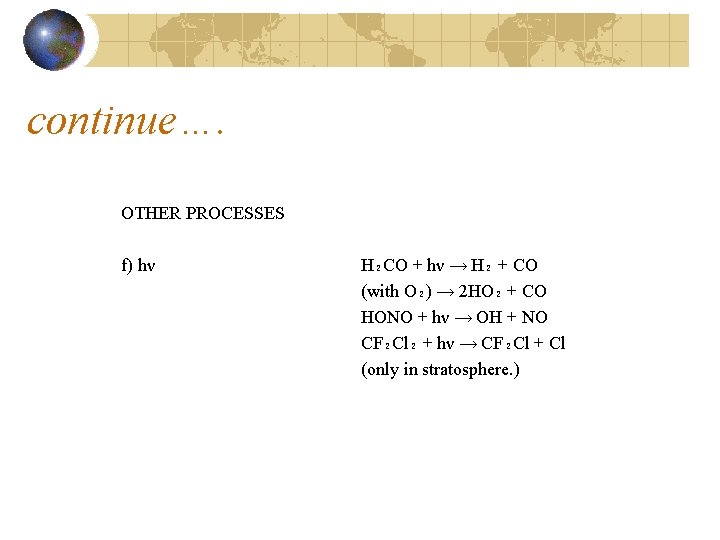
continue…. OTHER PROCESSES f) hν H₂CO + hν → H₂ + CO (with O₂) → 2 HO₂ + CO HONO + hν → OH + NO CF₂Cl₂ + hν → CF₂Cl + Cl (only in stratosphere. )

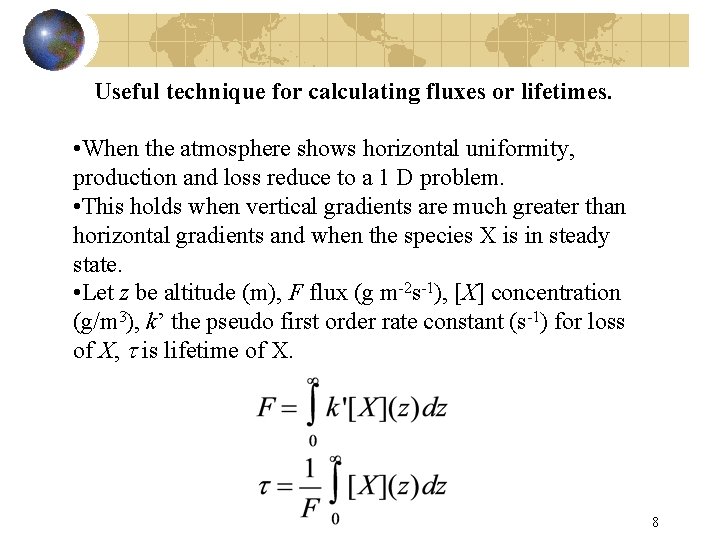
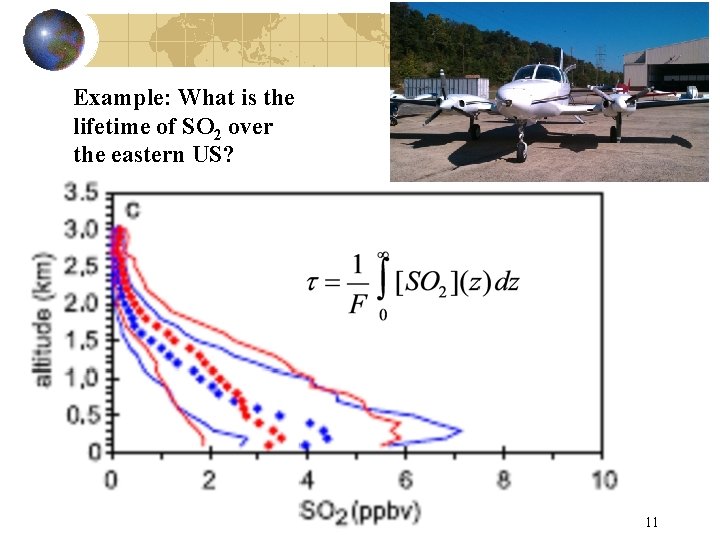
Useful technique for calculating fluxes or lifetimes. • When the atmosphere shows horizontal uniformity, production and loss reduce to a 1 D problem. • This holds when vertical gradients are much greater than horizontal gradients and when the species X is in steady state. • Let z be altitude (m), F flux (g m-2 s-1), [X] concentration (g/m 3), k’ the pseudo first order rate constant (s-1) for loss of X, t is lifetime of X. 8

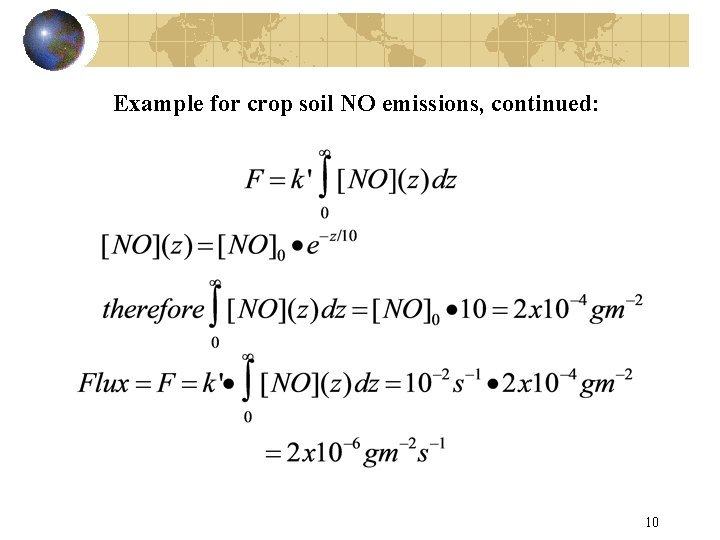
Example for fertilized soil NO emissions: • We want to know the emission rate. • We have the NO profile at night; this only works at night. • NO goes from 20 mg/m 3 at the surface to essentially zero at 100 m with a scale height of 10 m. • The column content is therefore 10 m*20 x 10 -6 g m-3 = 2 x 10 -4 g m-2 • We know ozone is roughly constant at 50 ppb, therefore at RTP the lifetime is ~100 s. More generally, you can integrate with [O 3](z) and k(z). • If t is a constant then k’ is a constant: 9

Example for crop soil NO emissions, continued: 10

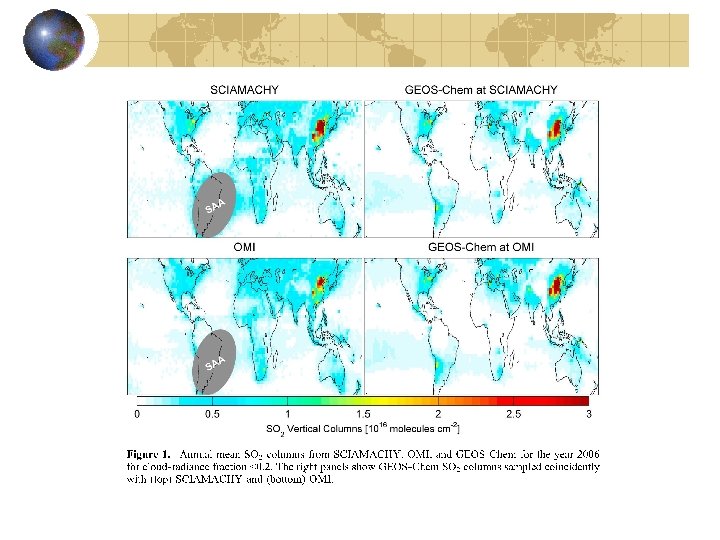
Example: What is the lifetime of SO 2 over the eastern US? 11

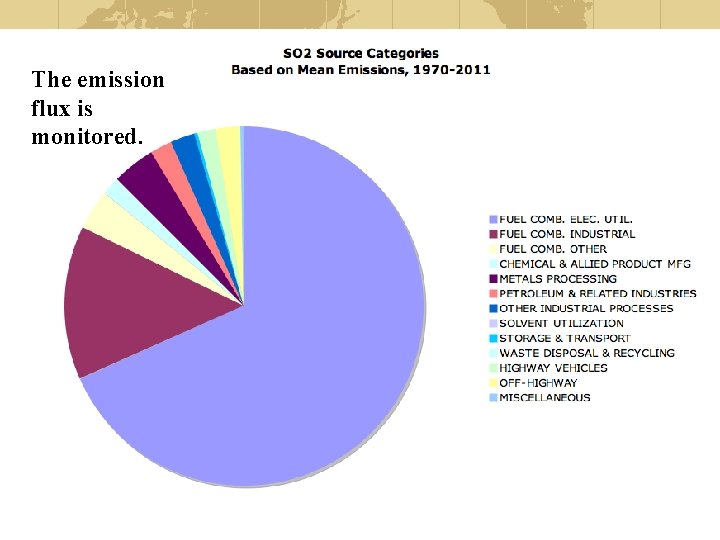
The emission flux is monitored.


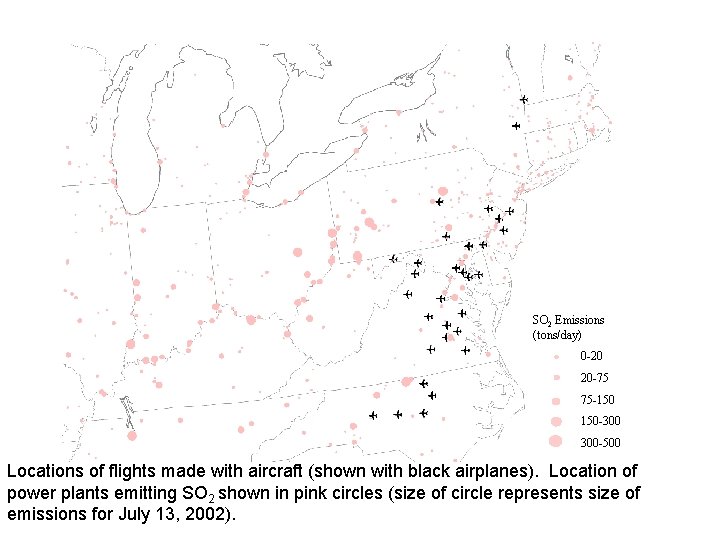
Figure IIa SO 2 Emissions (tons/day) 0 -20 20 -75 75 -150 150 -300 300 -500 Locations of flights made with aircraft (shown with black airplanes). Location of power plants emitting SO 2 shown in pink circles (size of circle represents size of emissions for July 13, 2002).

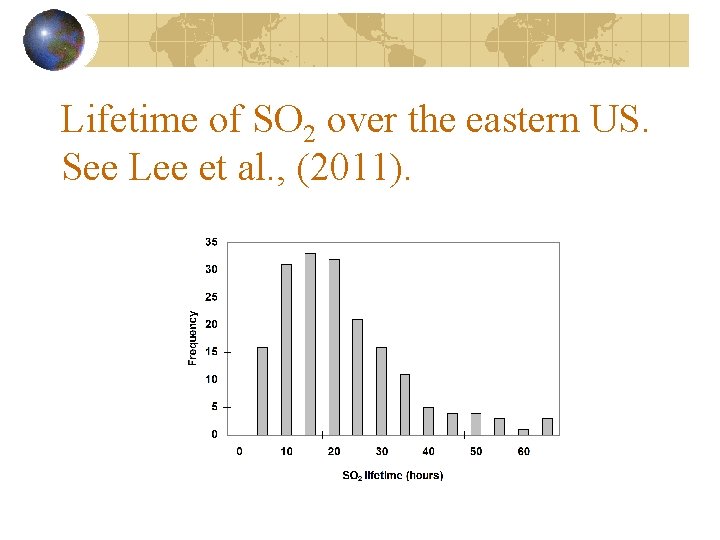
Lifetime of SO 2 over the eastern US. See Lee et al. , (2011).




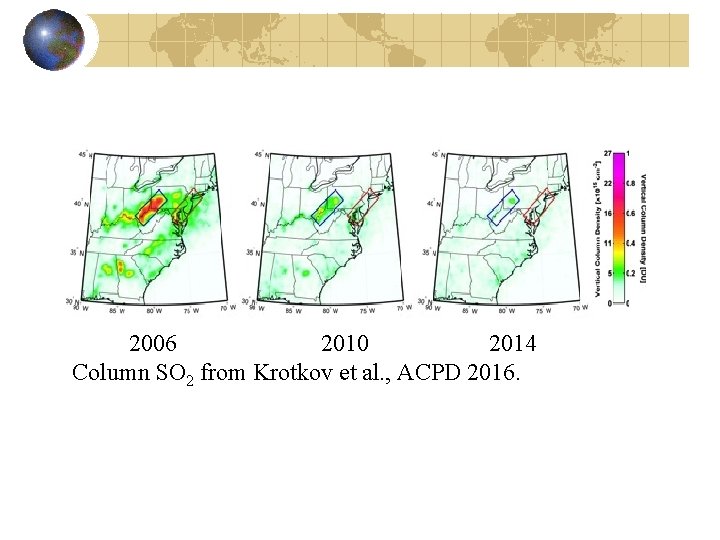
2006 2010 2014 Column SO 2 from Krotkov et al. , ACPD 2016.
 Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Air higroskopis air kapiler dan air gravitasi
Air higroskopis air kapiler dan air gravitasi Ece 434
Ece 434 Pile retention test
Pile retention test Ece 434
Ece 434 Anatomy team 434
Anatomy team 434 434 x 2
434 x 2 Kj 434 allah adalah kasih dan sumber kasih
Kj 434 allah adalah kasih dan sumber kasih Ece 434
Ece 434 Ece 434
Ece 434 Anatomy team 434
Anatomy team 434 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Air pollution
Air pollution Air pollution wildfires
Air pollution wildfires Indoor air pollution examples
Indoor air pollution examples Air pollution
Air pollution Air pollution
Air pollution Baghouse filter diagram
Baghouse filter diagram Main cause of air pollution
Main cause of air pollution