Isotopes Average Atomic Mass Quiz Today Know your






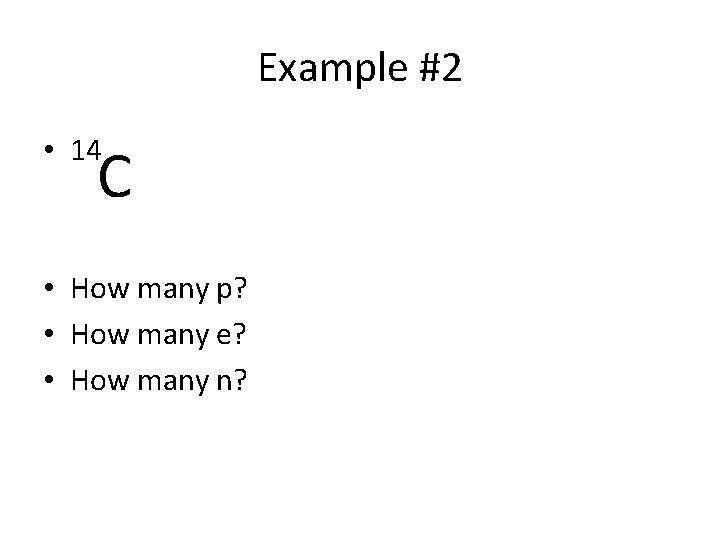

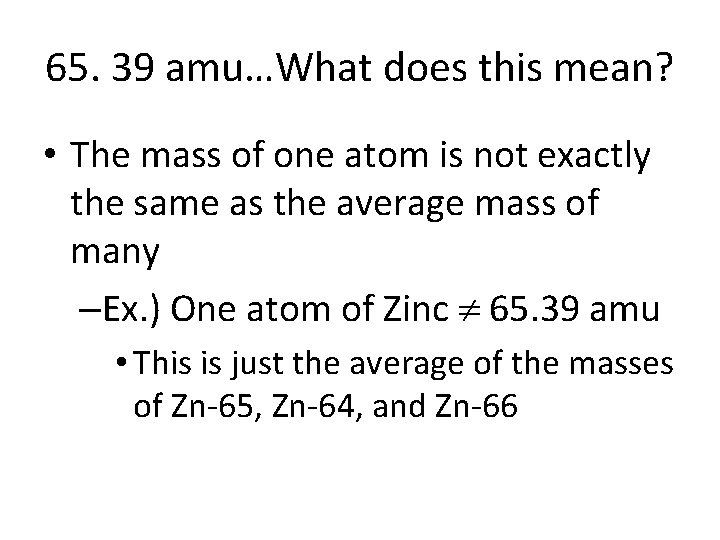
- Slides: 18

Isotopes & Average Atomic Mass • Quiz Today!!! Know your scientists! • Notebook Add-ons: – P. 29: LEAVE BLANK for Quiz 2. 1 – P. 30: Glue in “Isotopes Activity” – P. 31: Title “Notes: Isotopes & Average Atomic Mass” – P. 32: Glue in “Average Calculations Worksheet” • Homework: – Finish “Average Calculations Worksheet” – Study for Quiz!!!

Review!!! • An neutral atom has an Atomic Number of 82 and a Mass Number of 207. • • What is this element? How many protons? How many electrons? How many neutrons?

Review! • Discuss with your tablemates the experiments and conclusions of each scientist. • • • Who did the gold foil experiment? Who discovered the electron? Who discovered the nucleus? Who used the cathode ray tube? What are Dalton’s Postulates? What is the Bohr Model of the atom?

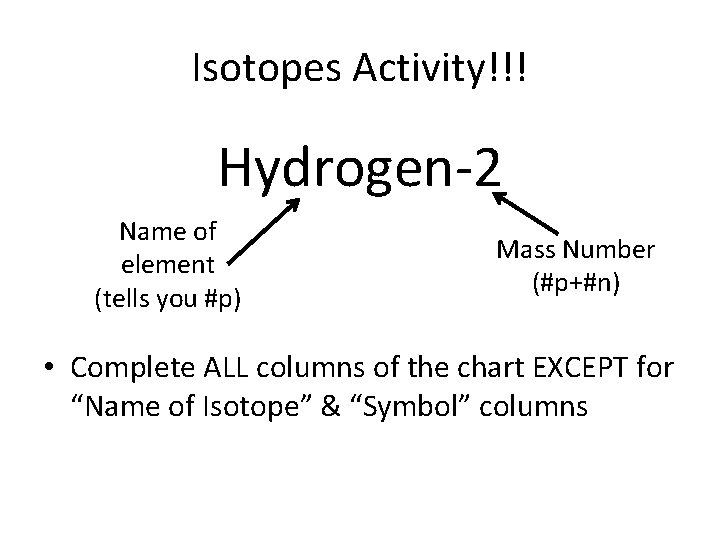
Isotopes Activity!!! Hydrogen-2 Name of element (tells you #p) Mass Number (#p+#n) • Complete ALL columns of the chart EXCEPT for “Name of Isotope” & “Symbol” columns

Isotopes & Average Atomic Mass p. 31

Isotopes

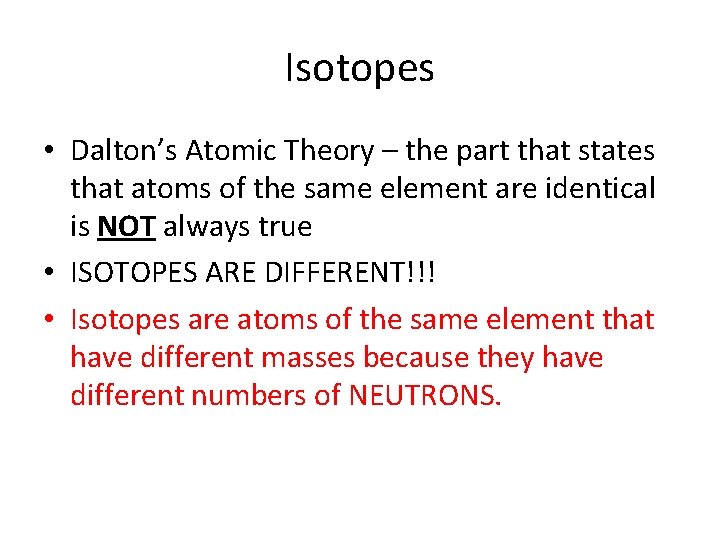
Isotopes • Dalton’s Atomic Theory – the part that states that atoms of the same element are identical is NOT always true • ISOTOPES ARE DIFFERENT!!! • Isotopes are atoms of the same element that have different masses because they have different numbers of NEUTRONS.

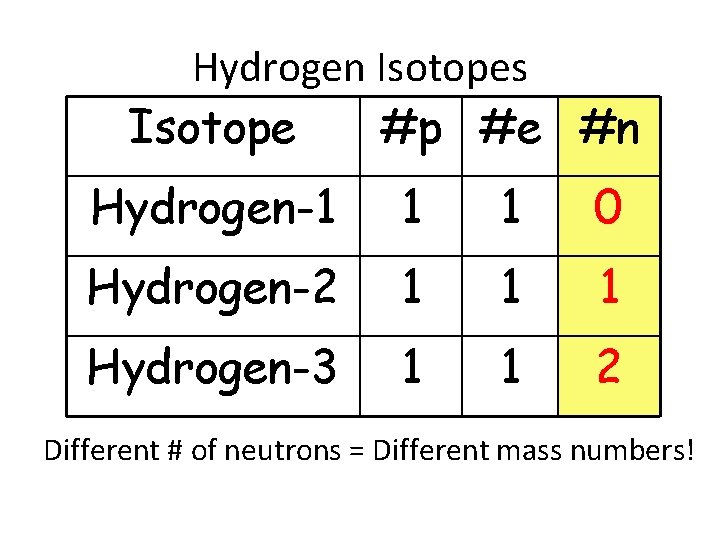
Hydrogen Isotopes Isotope #p #e #n Hydrogen-1 1 1 0 Hydrogen-2 1 1 1 Hydrogen-3 1 1 2 Different # of neutrons = Different mass numbers!

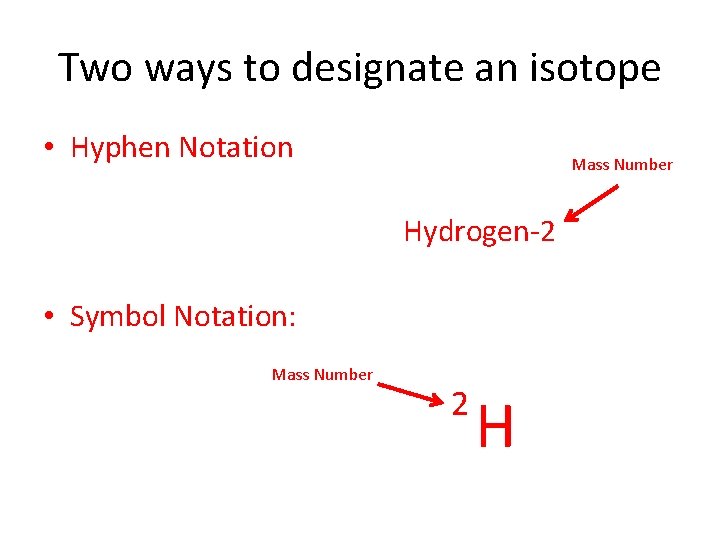
Two ways to designate an isotope • Hyphen Notation Mass Number Hydrogen-2 • Symbol Notation: Mass Number 2 H

Example #1 • Nitrogen-15 • How many p? • How many e? • How many n?
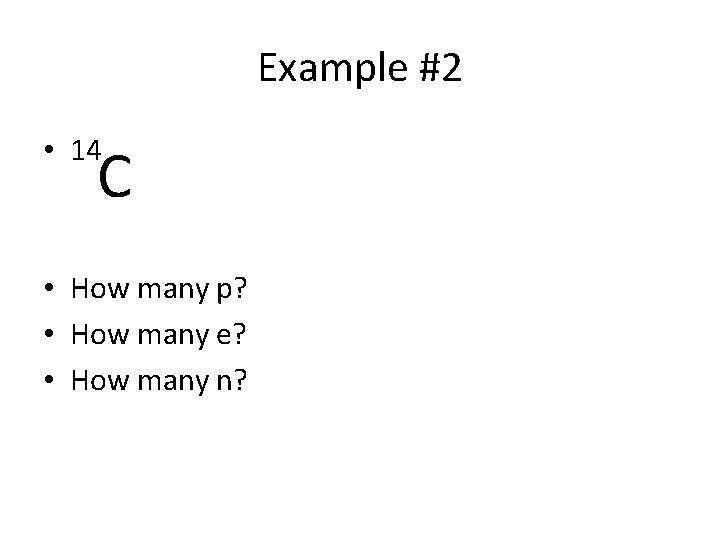
Example #2 • 14 C • How many p? • How many e? • How many n?

Average Atomic Mass (Relative Abundance)

Quick Question! • How do teachers (and gradebooks) calculate your grade in your class? • Remember that you have Daily Grades, Quiz Grades, and Test grades (each worth different amounts)? • Your total average in your class is a WEIGHTED AVERAGE of all of your grade categories!

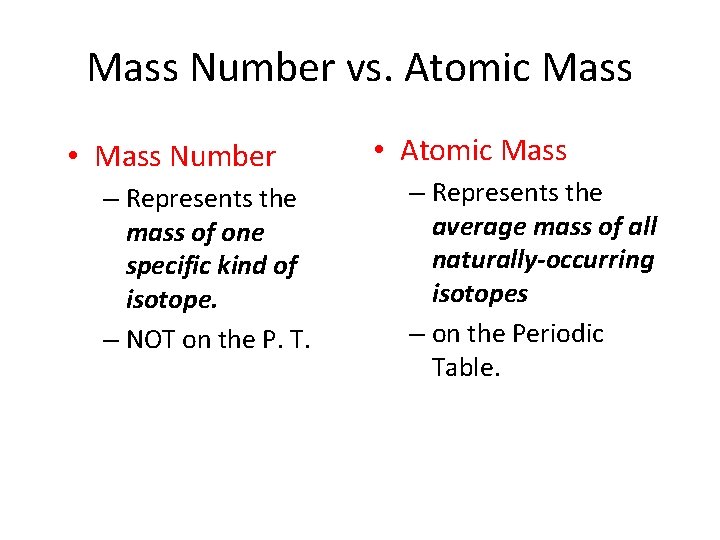
Mass Number vs. Atomic Mass • Mass Number – Represents the mass of one specific kind of isotope. – NOT on the P. T. • Atomic Mass – Represents the average mass of all naturally-occurring isotopes – on the Periodic Table.

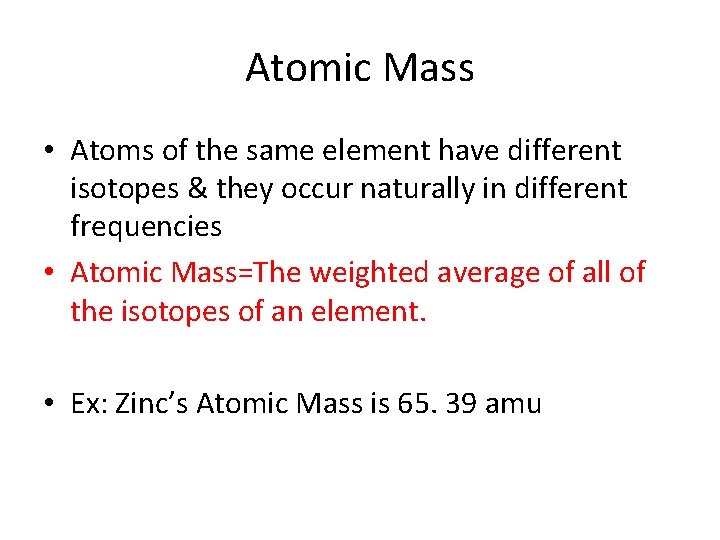
Atomic Mass • Atoms of the same element have different isotopes & they occur naturally in different frequencies • Atomic Mass=The weighted average of all of the isotopes of an element. • Ex: Zinc’s Atomic Mass is 65. 39 amu
65. 39 amu…What does this mean? • The mass of one atom is not exactly the same as the average mass of many –Ex. ) One atom of Zinc 65. 39 amu • This is just the average of the masses of Zn-65, Zn-64, and Zn-66

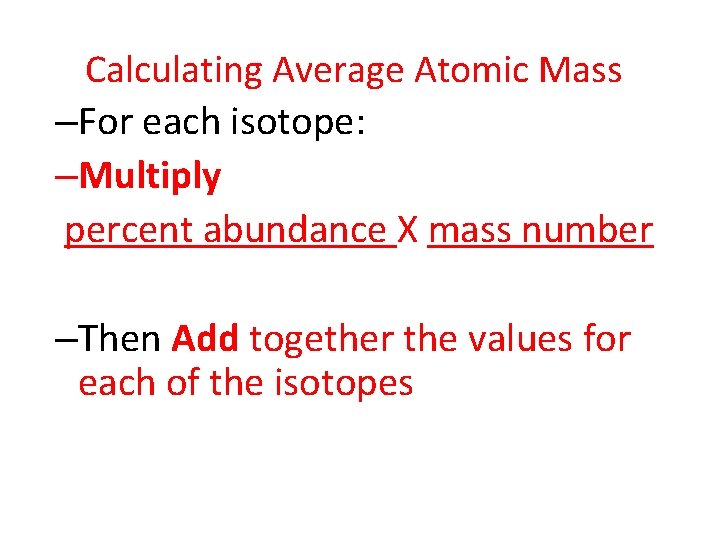
Calculating Average Atomic Mass –For each isotope: –Multiply percent abundance X mass number –Then Add together the values for each of the isotopes

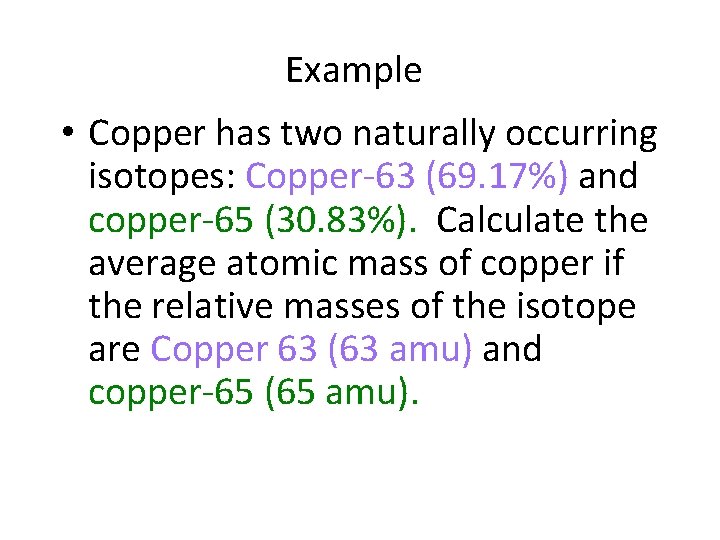
Example • Copper has two naturally occurring isotopes: Copper-63 (69. 17%) and copper-65 (30. 83%). Calculate the average atomic mass of copper if the relative masses of the isotope are Copper 63 (63 amu) and copper-65 (65 amu).