4 5 Isotopes and Atomic Mass The atomic
- Slides: 21

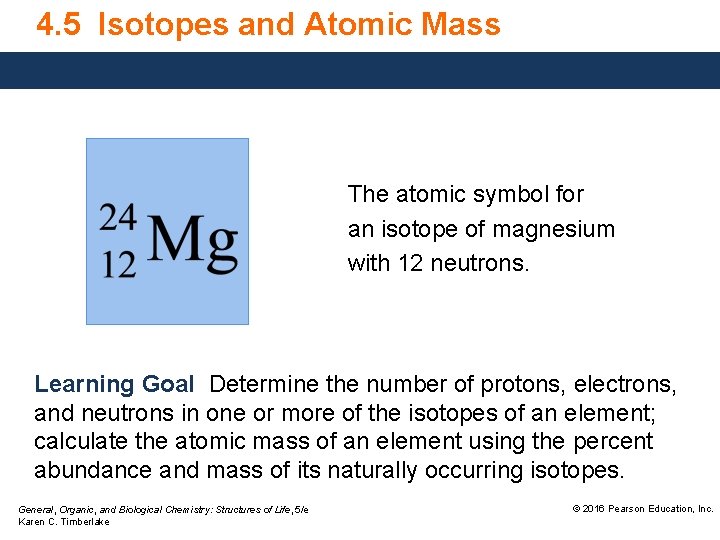
4. 5 Isotopes and Atomic Mass The atomic symbol for an isotope of magnesium with 12 neutrons. Learning Goal Determine the number of protons, electrons, and neutrons in one or more of the isotopes of an element; calculate the atomic mass of an element using the percent abundance and mass of its naturally occurring isotopes. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

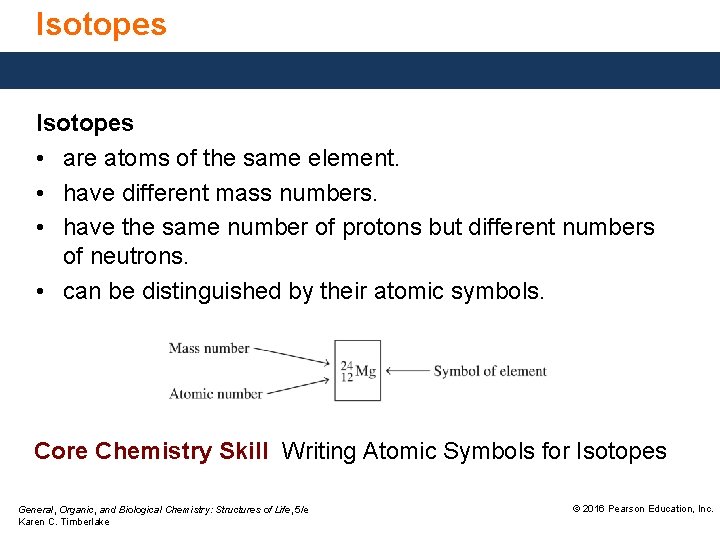
Isotopes • are atoms of the same element. • have different mass numbers. • have the same number of protons but different numbers of neutrons. • can be distinguished by their atomic symbols. Core Chemistry Skill Writing Atomic Symbols for Isotopes General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

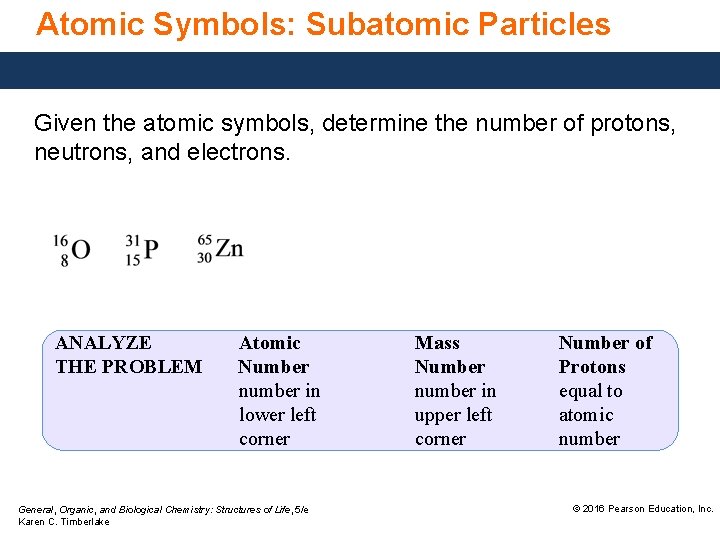
Atomic Symbols: Subatomic Particles Given the atomic symbols, determine the number of protons, neutrons, and electrons. ANALYZE THE PROBLEM Atomic Number number in lower left corner General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Mass Number number in upper left corner Number of Protons equal to atomic number © 2016 Pearson Education, Inc.

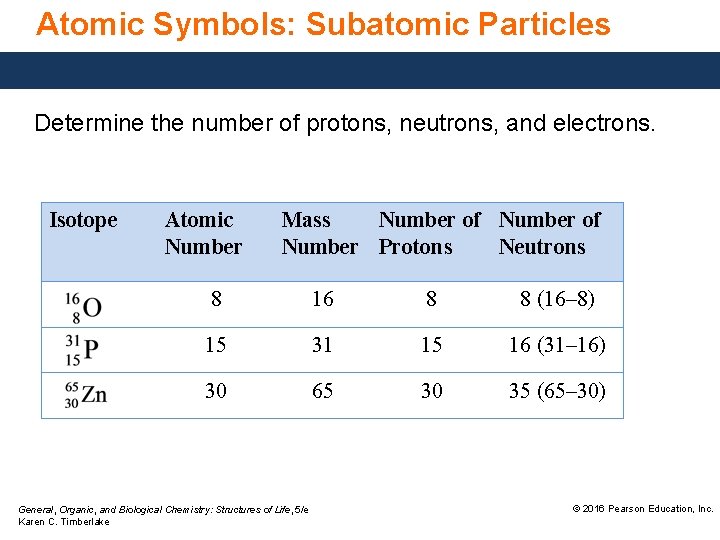
Atomic Symbols: Subatomic Particles Determine the number of protons, neutrons, and electrons. Isotope Atomic Number Mass Number of Number Protons Neutrons 8 16 8 8 (16– 8) 15 31 15 16 (31– 16) 30 65 30 35 (65– 30) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

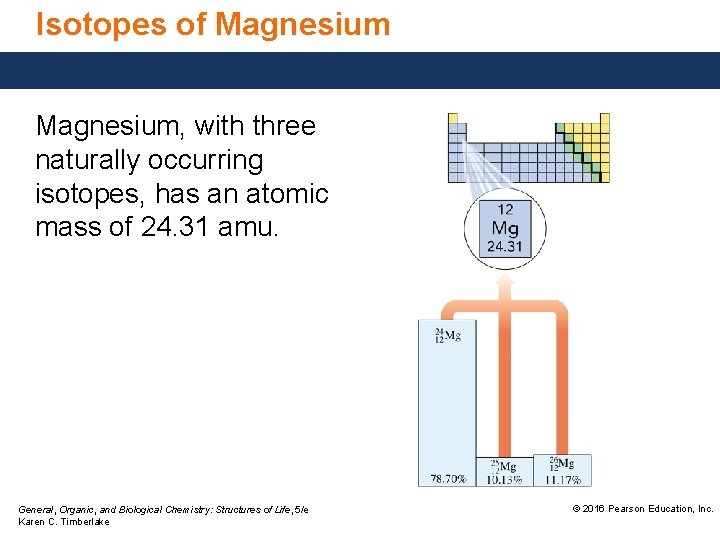
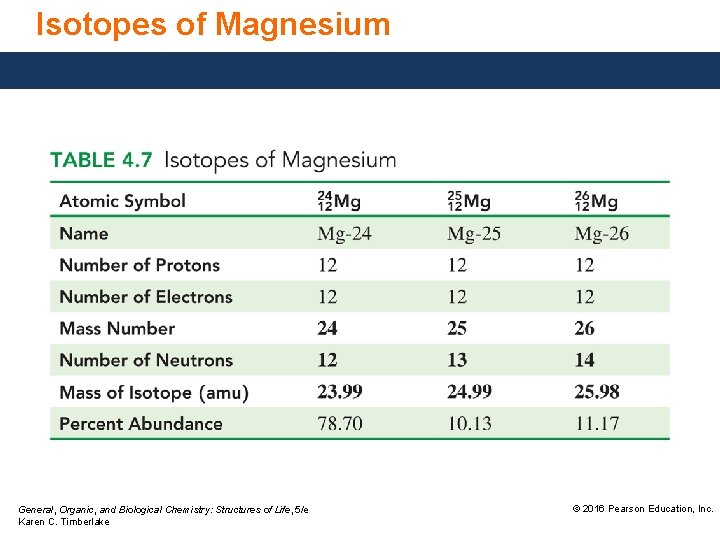
Isotopes of Magnesium, with three naturally occurring isotopes, has an atomic mass of 24. 31 amu. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Isotopes of Magnesium General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Study Check Naturally occurring carbon consists of three isotopes: 12 C, 13 C, and 14 C. State the number of protons, neutrons, and electrons in each of the three isotopes. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

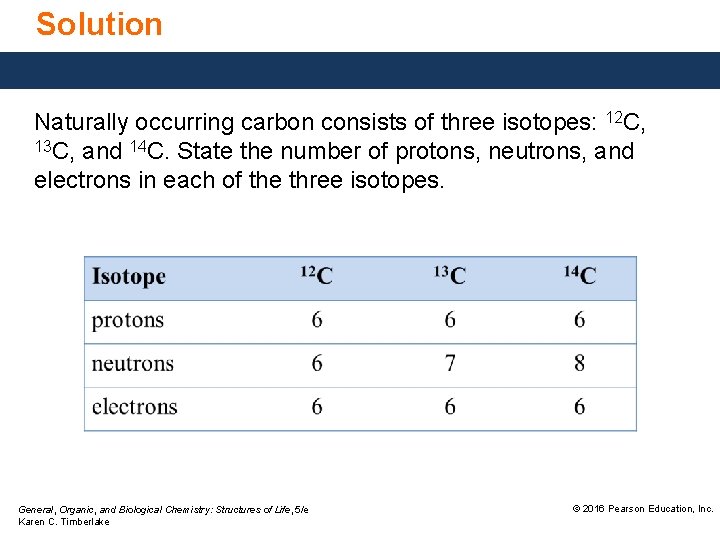
Solution Naturally occurring carbon consists of three isotopes: 12 C, 13 C, and 14 C. State the number of protons, neutrons, and electrons in each of the three isotopes. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

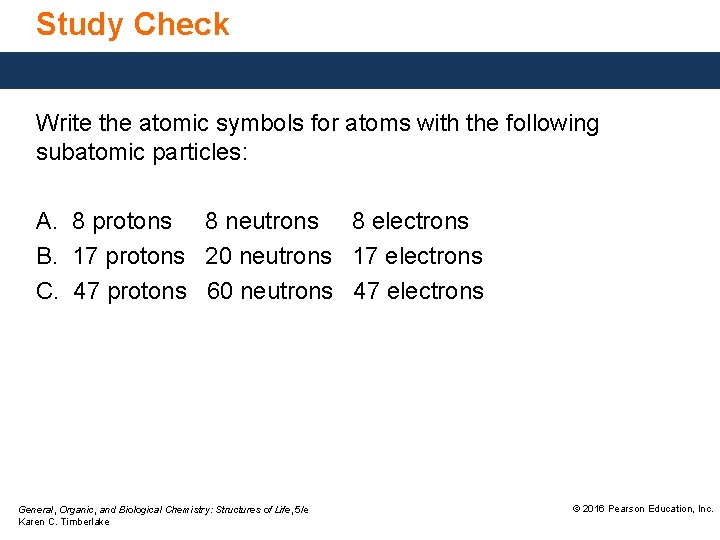
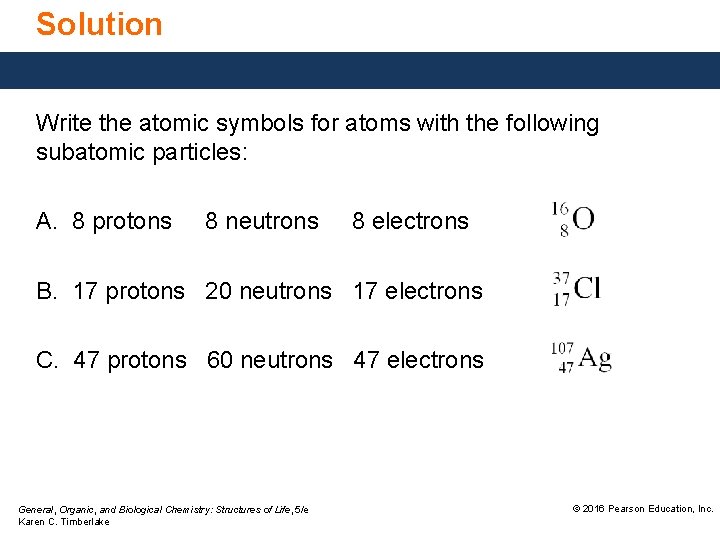
Study Check Write the atomic symbols for atoms with the following subatomic particles: A. 8 protons 8 neutrons 8 electrons B. 17 protons 20 neutrons 17 electrons C. 47 protons 60 neutrons 47 electrons General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution Write the atomic symbols for atoms with the following subatomic particles: A. 8 protons 8 neutrons 8 electrons B. 17 protons 20 neutrons 17 electrons C. 47 protons 60 neutrons 47 electrons General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

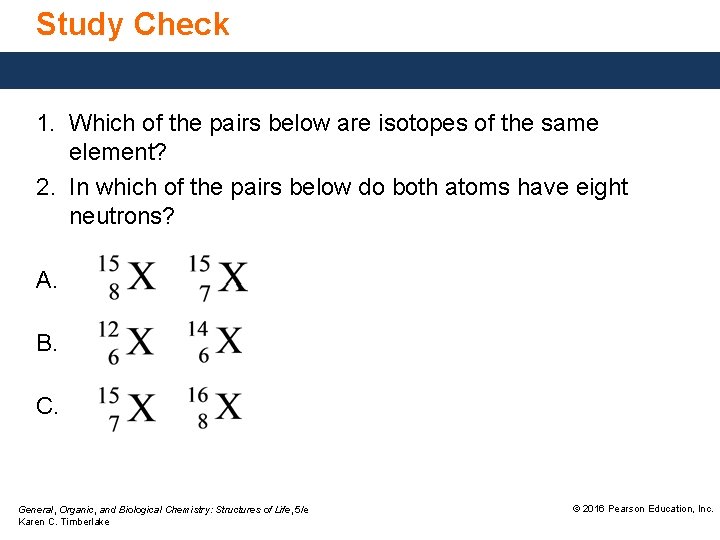
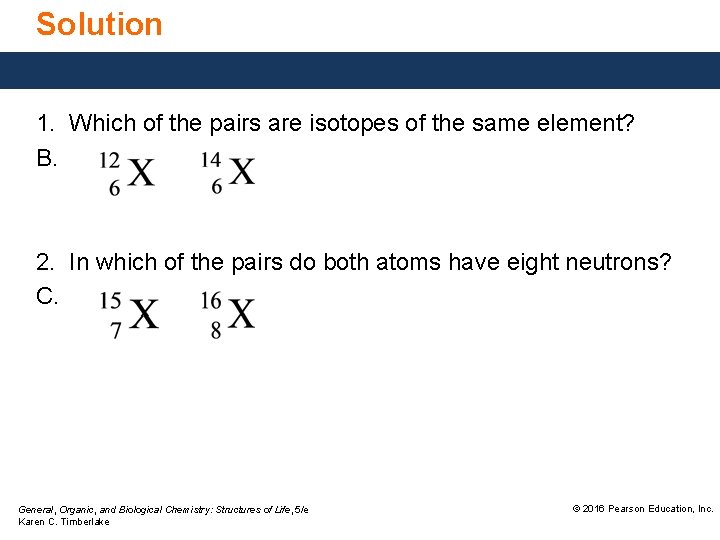
Study Check 1. Which of the pairs below are isotopes of the same element? 2. In which of the pairs below do both atoms have eight neutrons? A. B. C. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution 1. Which of the pairs are isotopes of the same element? B. 2. In which of the pairs do both atoms have eight neutrons? C. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

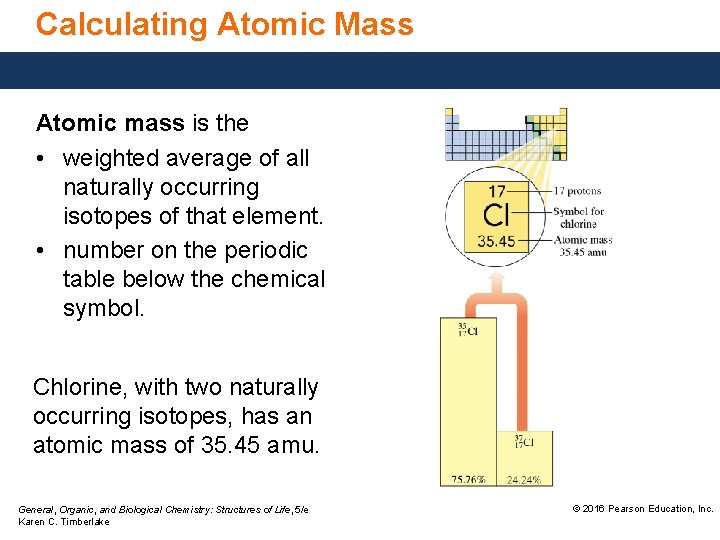
Calculating Atomic Mass Atomic mass is the • weighted average of all naturally occurring isotopes of that element. • number on the periodic table below the chemical symbol. Chlorine, with two naturally occurring isotopes, has an atomic mass of 35. 45 amu. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

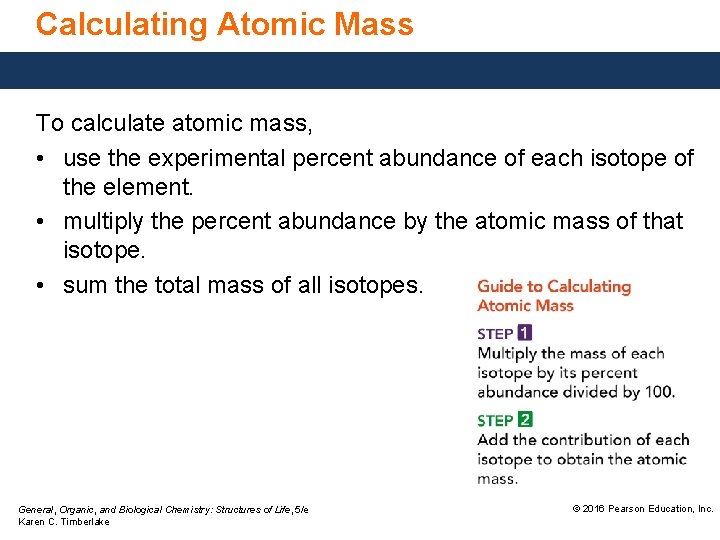
Calculating Atomic Mass To calculate atomic mass, • use the experimental percent abundance of each isotope of the element. • multiply the percent abundance by the atomic mass of that isotope. • sum the total mass of all isotopes. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

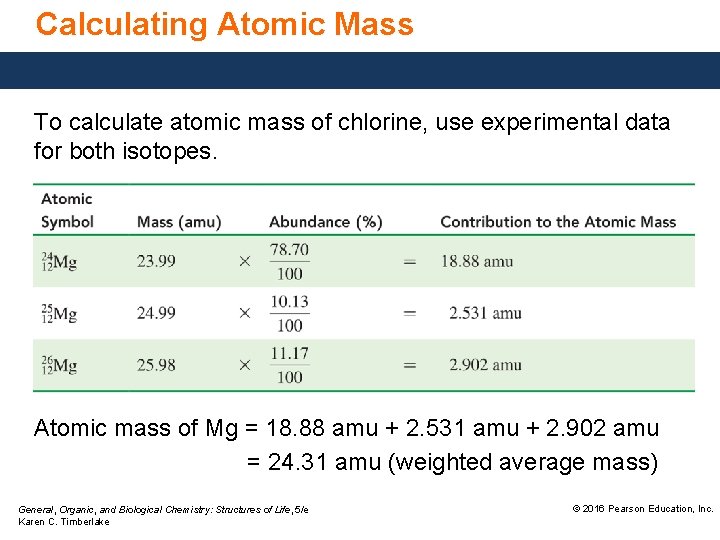
Calculating Atomic Mass To calculate atomic mass of chlorine, use experimental data for both isotopes. Atomic mass of Mg = 18. 88 amu + 2. 531 amu + 2. 902 amu = 24. 31 amu (weighted average mass) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

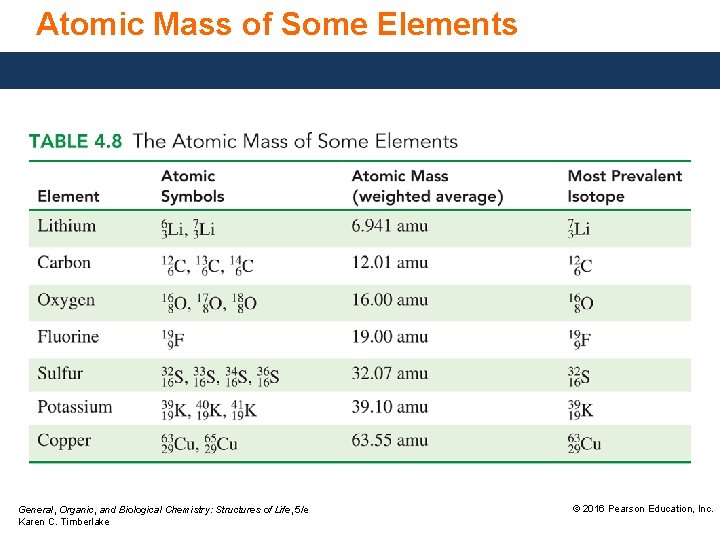
Atomic Mass of Some Elements General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

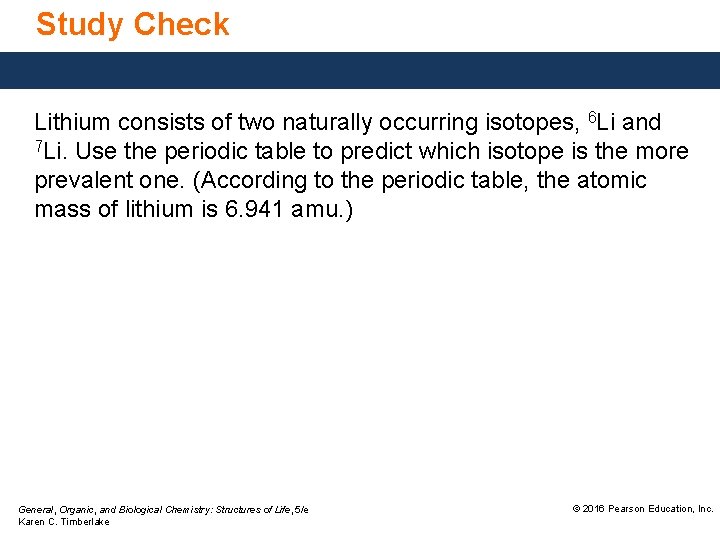
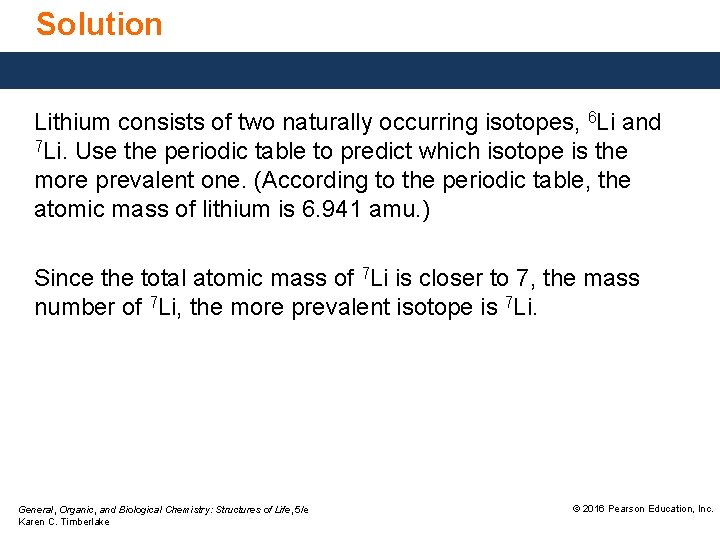
Study Check Lithium consists of two naturally occurring isotopes, 6 Li and 7 Li. Use the periodic table to predict which isotope is the more prevalent one. (According to the periodic table, the atomic mass of lithium is 6. 941 amu. ) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution Lithium consists of two naturally occurring isotopes, 6 Li and 7 Li. Use the periodic table to predict which isotope is the more prevalent one. (According to the periodic table, the atomic mass of lithium is 6. 941 amu. ) Since the total atomic mass of 7 Li is closer to 7, the mass number of 7 Li, the more prevalent isotope is 7 Li. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

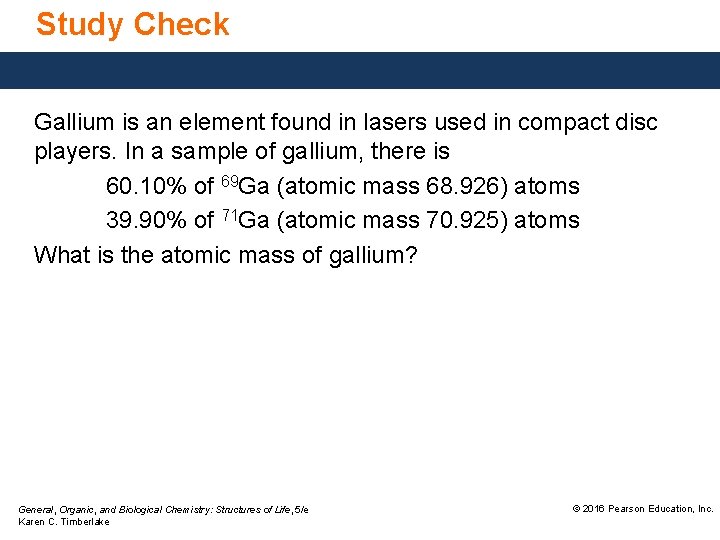
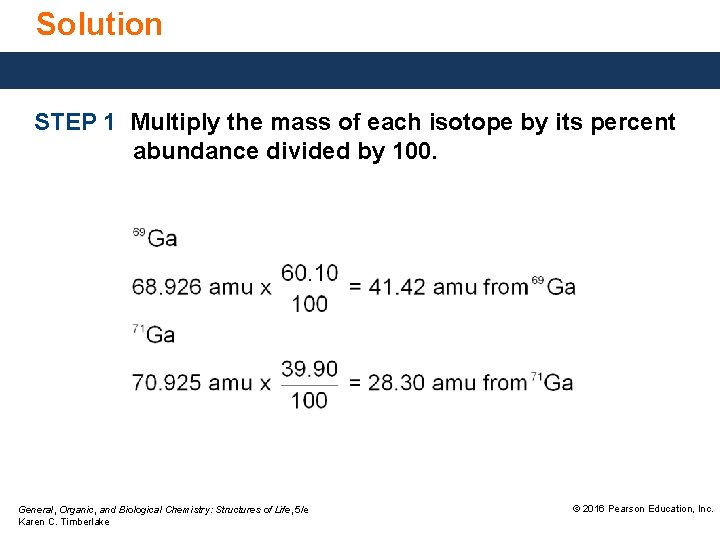
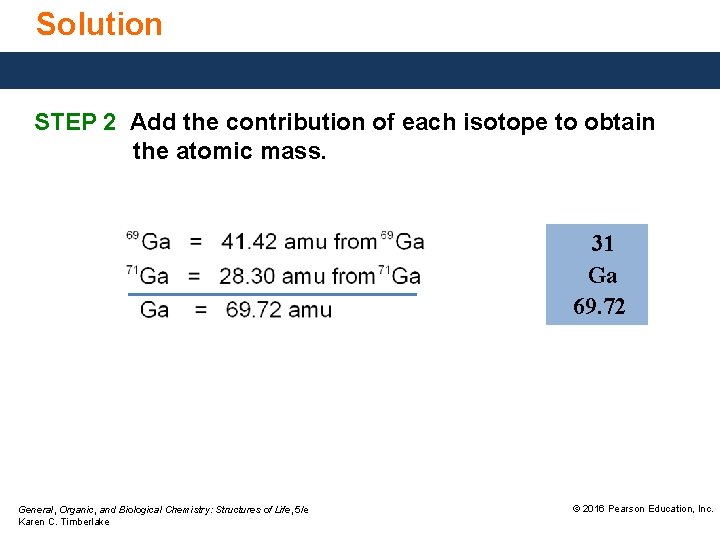
Study Check Gallium is an element found in lasers used in compact disc players. In a sample of gallium, there is 60. 10% of 69 Ga (atomic mass 68. 926) atoms 39. 90% of 71 Ga (atomic mass 70. 925) atoms What is the atomic mass of gallium? General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution STEP 1 Multiply the mass of each isotope by its percent abundance divided by 100. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution STEP 2 Add the contribution of each isotope to obtain the atomic mass. 31 Ga 69. 72 General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.