Average Atomic Mass Measuring Atomic Mass Unit is

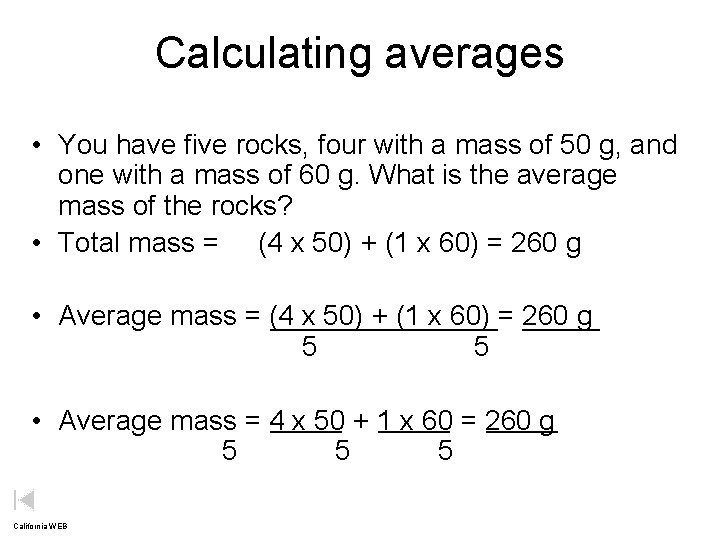
- Slides: 12

Average Atomic Mass

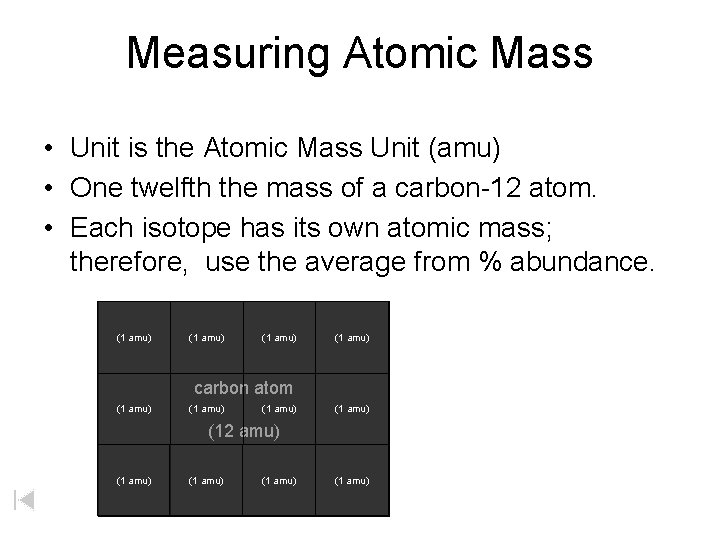
Measuring Atomic Mass • Unit is the Atomic Mass Unit (amu) • One twelfth the mass of a carbon-12 atom. • Each isotope has its own atomic mass; therefore, use the average from % abundance. (1 amu) carbon atom (1 amu) (12 amu) (1 amu)
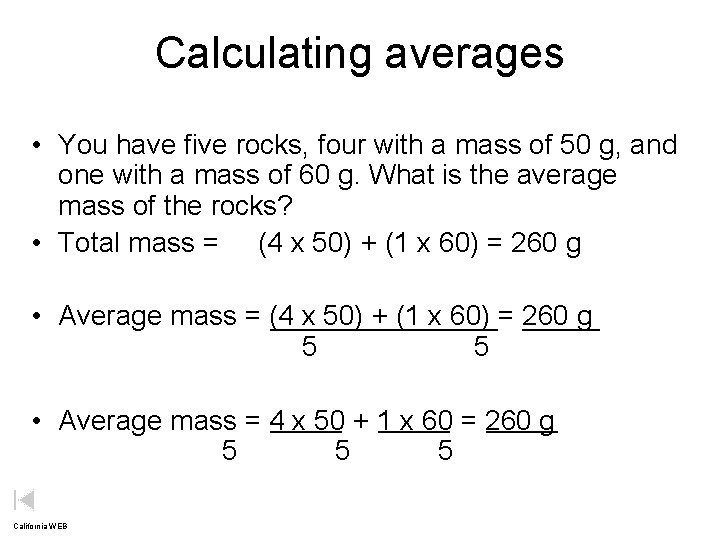
Calculating averages • You have five rocks, four with a mass of 50 g, and one with a mass of 60 g. What is the average mass of the rocks? • Total mass = (4 x 50) + (1 x 60) = 260 g • Average mass = (4 x 50) + (1 x 60) = 260 g 5 5 • Average mass = 4 x 50 + 1 x 60 = 260 g 5 5 5 California WEB

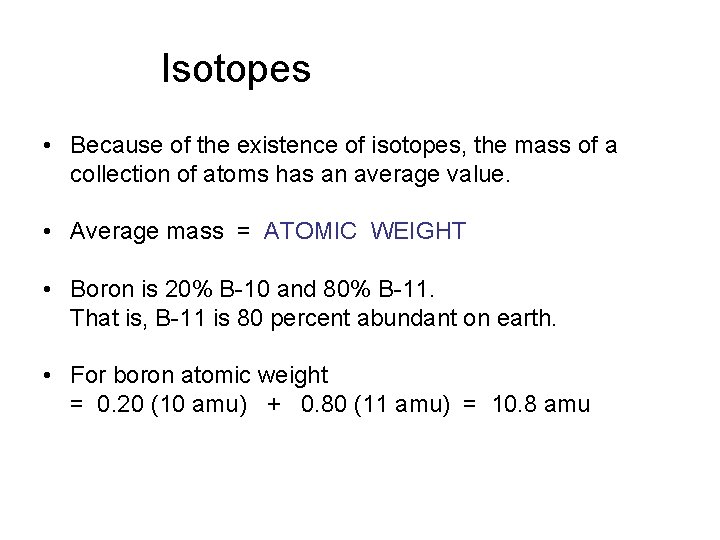
Isotopes • Because of the existence of isotopes, the mass of a collection of atoms has an average value. • Average mass = ATOMIC WEIGHT • Boron is 20% B-10 and 80% B-11. That is, B-11 is 80 percent abundant on earth. • For boron atomic weight = 0. 20 (10 amu) + 0. 80 (11 amu) = 10. 8 amu

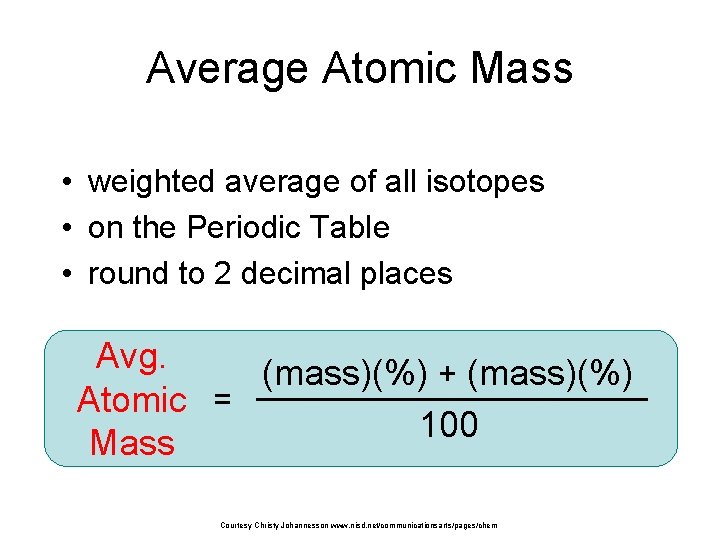
Average Atomic Mass • weighted average of all isotopes • on the Periodic Table • round to 2 decimal places Avg. (mass)(%) + (mass)(%) Atomic = 100 Mass Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

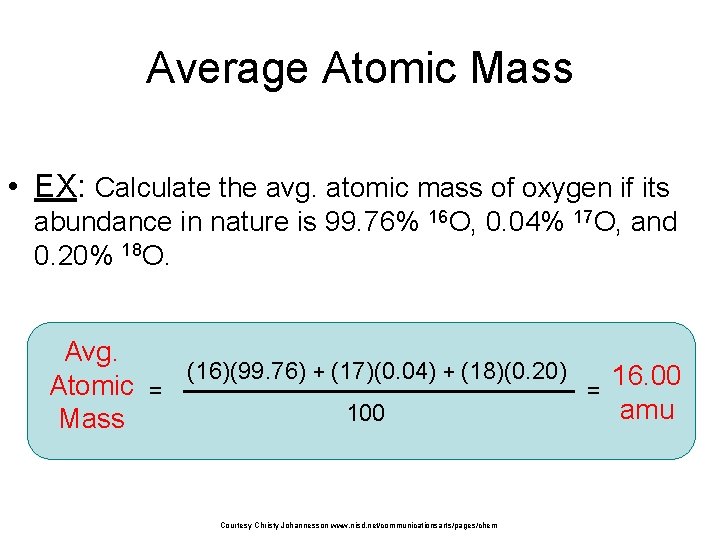
Average Atomic Mass • EX: Calculate the avg. atomic mass of oxygen if its abundance in nature is 99. 76% 16 O, 0. 04% 17 O, and 0. 20% 18 O. Avg. (16)(99. 76) + (17)(0. 04) + (18)(0. 20) 16. 00 Atomic = = amu 100 Mass Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

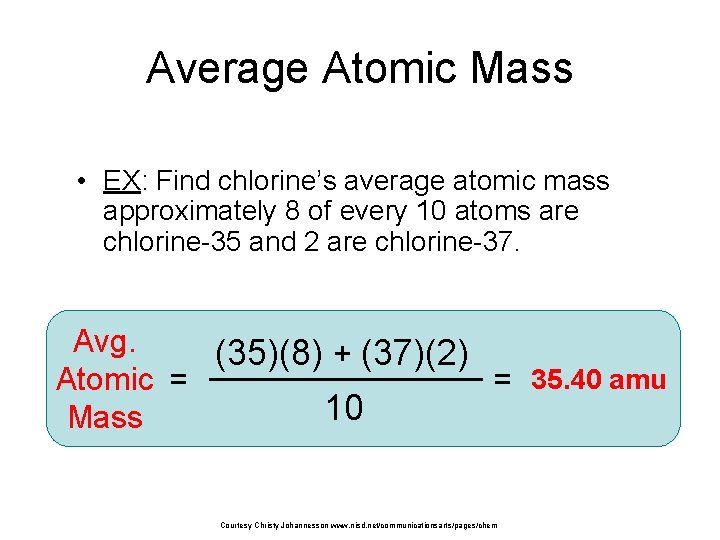
Average Atomic Mass • EX: Find chlorine’s average atomic mass approximately 8 of every 10 atoms are chlorine-35 and 2 are chlorine-37. Avg. (35)(8) + (37)(2) Atomic = = 35. 40 amu 10 Mass Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem i

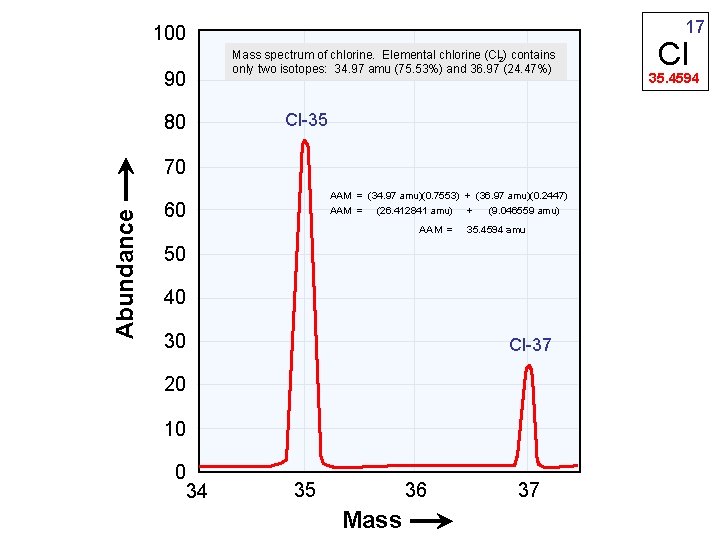
17 100 Mass spectrum of chlorine. Elemental chlorine (Cl 2) contains only two isotopes: 34. 97 amu (75. 53%) and 36. 97 (24. 47%) 90 Cl-35 80 Abundance 70 AAM = (34. 97 amu)(0. 7553) + (36. 97 amu)(0. 2447) AAM = (26. 412841 amu) + (9. 046559 amu) 60 AAM = 35. 4594 amu 50 40 30 Cl-37 20 10 0 34 36 35 Mass 37 Cl 35. 4594

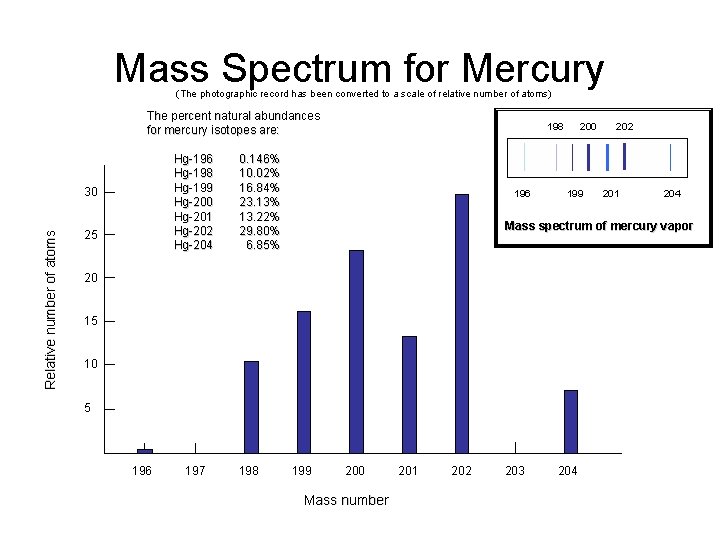
Mass Spectrum for Mercury (The photographic record has been converted to a scale of relative number of atoms) The percent natural abundances for mercury isotopes are: Hg-196 Hg-198 Hg-199 Hg-200 Hg-201 Hg-202 Hg-204 Relative number of atoms 30 25 198 0. 146% 10. 02% 16. 84% 23. 13% 13. 22% 29. 80% 6. 85% 196 200 199 15 10 5 197 198 201 204 Mass spectrum of mercury vapor 20 196 202 199 200 Mass number 201 202 203 204

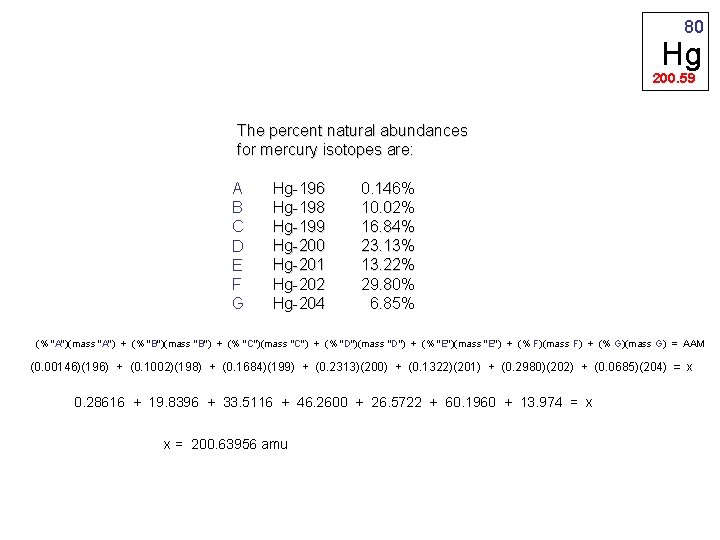
80 Hg 200. 59 The percent natural abundances for mercury isotopes are: A B C D E F G Hg-196 Hg-198 Hg-199 Hg-200 Hg-201 Hg-202 Hg-204 0. 146% 10. 02% 16. 84% 23. 13% 13. 22% 29. 80% 6. 85% (% "A")(mass "A") + (% "B")(mass "B") + (% "C")(mass "C") + (% "D")(mass "D") + (% "E")(mass "E") + (% F)(mass F) + (% G)(mass G) = AAM (0. 00146)(196) + (0. 1002)(198) + (0. 1684)(199) + (0. 2313)(200) + (0. 1322)(201) + (0. 2980)(202) + (0. 0685)(204) = x 0. 28616 + 19. 8396 + 33. 5116 + 46. 2600 + 26. 5722 + 60. 1960 + 13. 974 = x x = 200. 63956 amu

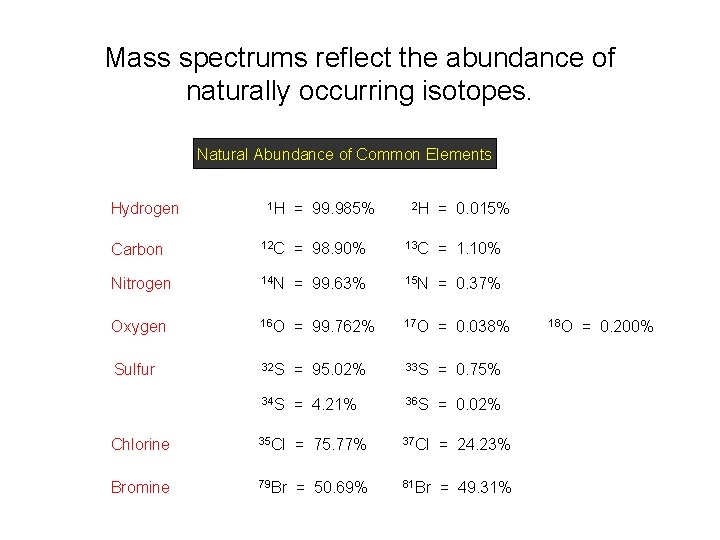
Mass spectrums reflect the abundance of naturally occurring isotopes. Natural Abundance of Common Elements Hydrogen 1 H = 99. 985% 2 H = 0. 015% Carbon 12 C = 98. 90% 13 C = 1. 10% Nitrogen 14 N = 99. 63% 15 N = 0. 37% Oxygen 16 O = 99. 762% 17 O = 0. 038% Sulfur 32 S = 95. 02% 33 S = 0. 75% 34 S = 4. 21% 36 S = 0. 02% Chlorine 35 Cl = 75. 77% 37 Cl = 24. 23% Bromine 79 Br = 50. 69% 81 Br = 49. 31% 18 O = 0. 200%

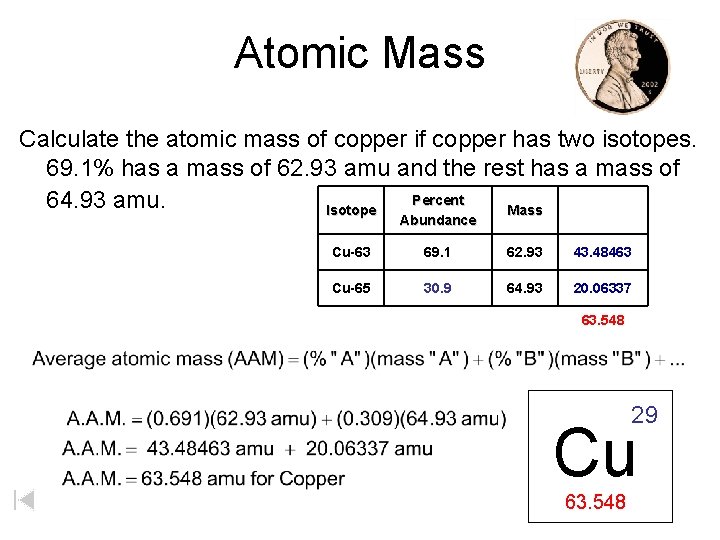
Atomic Mass Calculate the atomic mass of copper if copper has two isotopes. 69. 1% has a mass of 62. 93 amu and the rest has a mass of Percent 64. 93 amu. Isotope Mass Abundance Cu-63 69. 1 62. 93 43. 48463 Cu-65 30. 9 64. 93 20. 06337 63. 548 29 Cu 63. 548