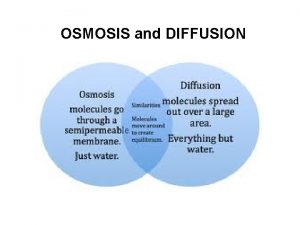
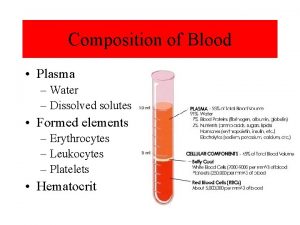
Interpreting profiles of pore water solutes First solute








![A mineral, undersaturated in seawater apparently simple dissolution kinetics… What do we expect [Si(OH)4] A mineral, undersaturated in seawater apparently simple dissolution kinetics… What do we expect [Si(OH)4]](https://slidetodoc.com/presentation_image_h2/58b442888fad9d7cb7464cb6311133f9/image-20.jpg)




- Slides: 25

Interpreting profiles of pore water solutes

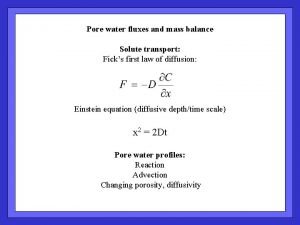
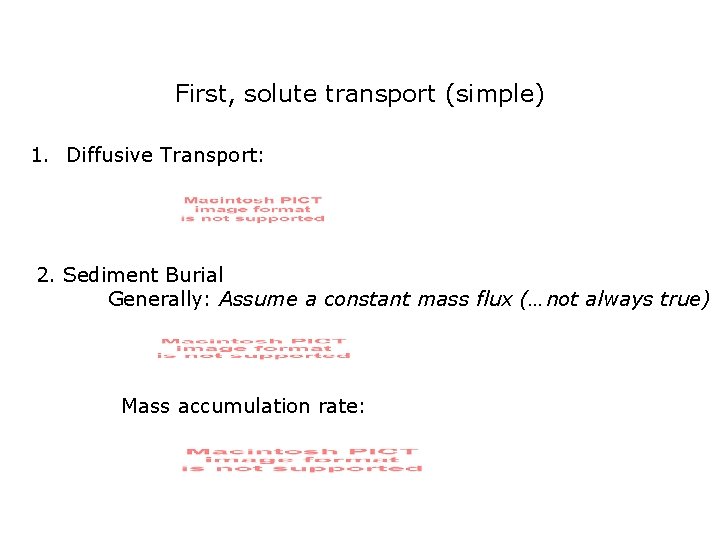
First, solute transport (simple) 1. Diffusive Transport: 2. Sediment Burial Generally: Assume a constant mass flux (…not always true) Mass accumulation rate:

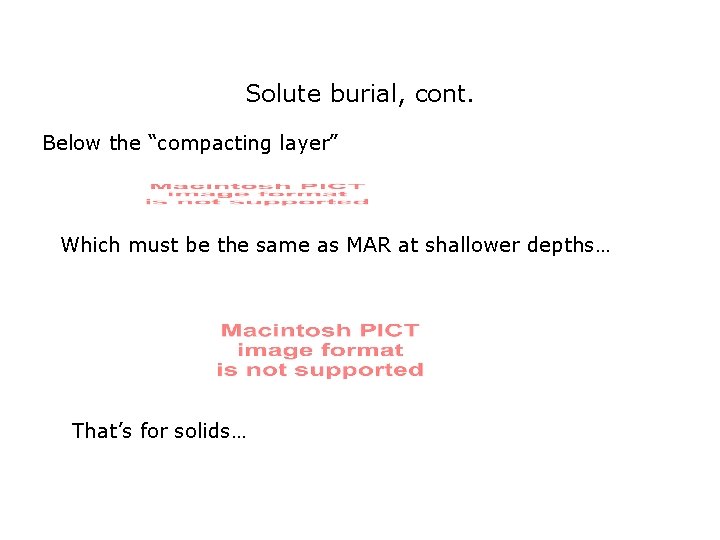
Solute burial, cont. Below the “compacting layer” Which must be the same as MAR at shallower depths… That’s for solids…

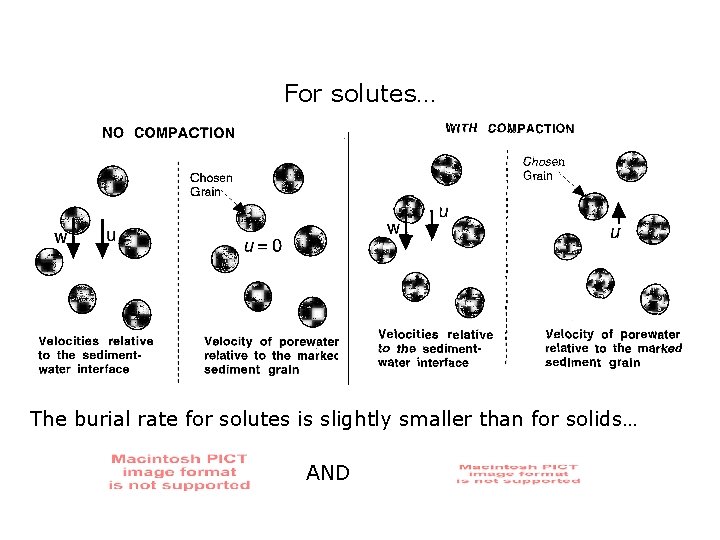
For solutes… The burial rate for solutes is slightly smaller than for solids… AND

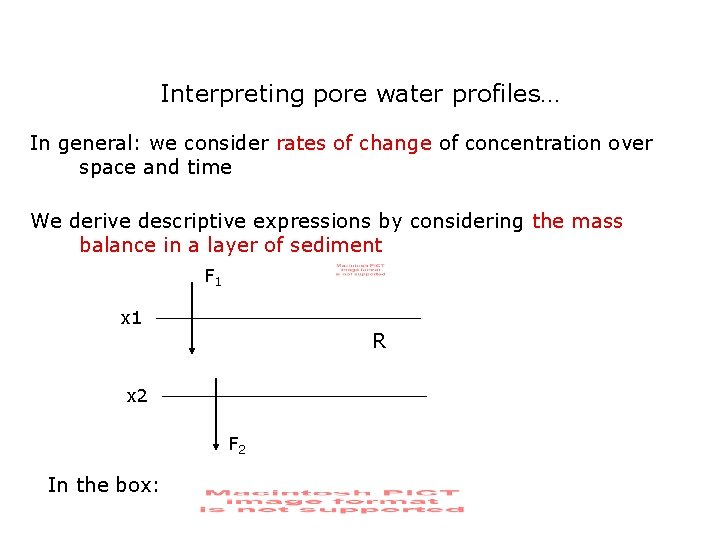
Interpreting pore water profiles… In general: we consider rates of change of concentration over space and time We derive descriptive expressions by considering the mass balance in a layer of sediment F 1 x 1 R x 2 F 2 In the box:

Interpreting, cont. (x 2 - x 1) --> small… Since…

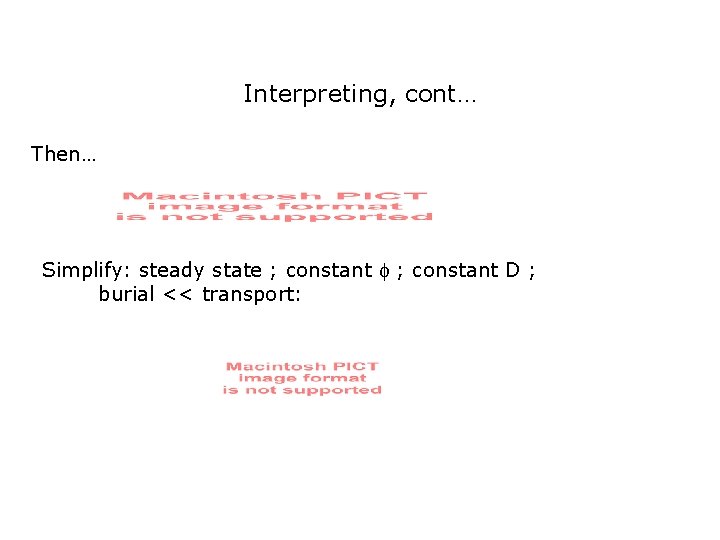
Interpreting, cont… Then… Simplify: steady state ; constant D ; burial << transport:

==> A simple interpretation of pw profiles



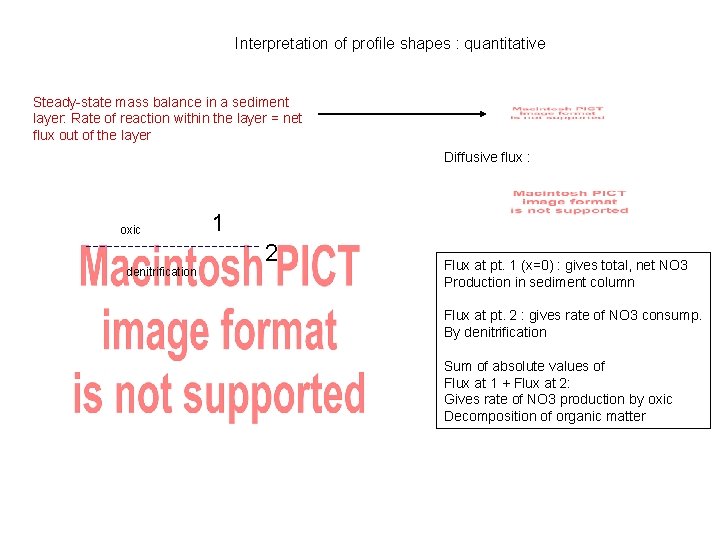
Interpretation of profile shapes : quantitative Steady-state mass balance in a sediment layer: Rate of reaction within the layer = net flux out of the layer Diffusive flux : oxic denitrification 1 2 Flux at pt. 1 (x=0) : gives total, net NO 3 Production in sediment column Flux at pt. 2 : gives rate of NO 3 consump. By denitrification Sum of absolute values of Flux at 1 + Flux at 2: Gives rate of NO 3 production by oxic Decomposition of organic matter

But we can get more information… What else do we need to solve this equation?

But we can get more information… Boundary conditions! At sediment-water interface (x=0) At depth in the sediments:

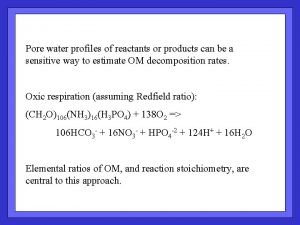
What about R ? Example : organic matter oxidation by O 2 What solutes could you measure to define reaction rate?

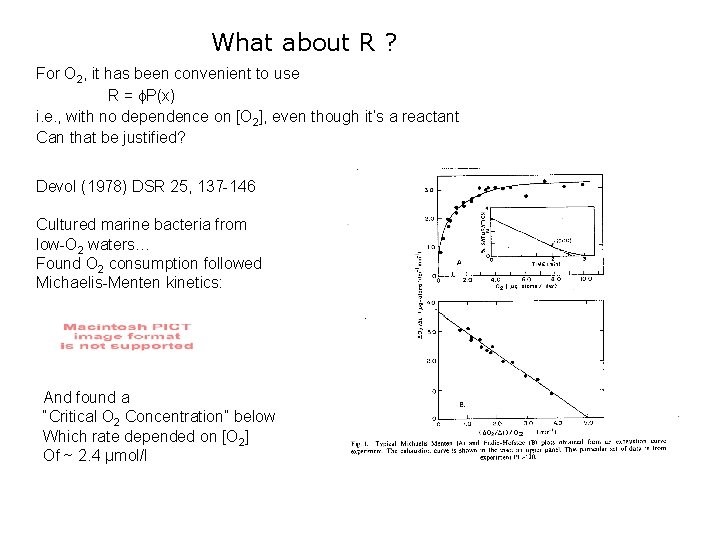
What about R ? For O 2, it has been convenient to use R = P(x) i. e. , with no dependence on [O 2], even though it’s a reactant Can that be justified? Devol (1978) DSR 25, 137 -146 Cultured marine bacteria from low-O 2 waters… Found O 2 consumption followed Michaelis-Menten kinetics: And found a “Critical O 2 Concentration” below Which rate depended on [O 2] Of ~ 2. 4 µmol/l

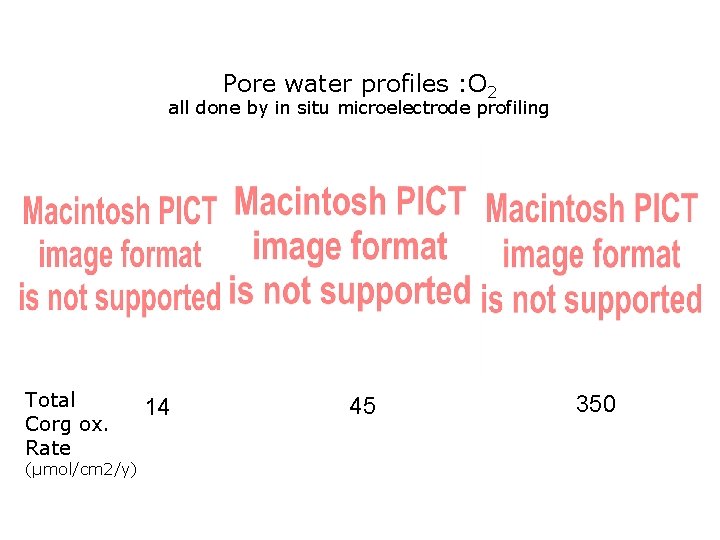
Pore water profiles : O 2 all done by in situ microelectrode profiling Total Corg ox. Rate (µmol/cm 2/y) 14 45 350

Continental margin sediments: * large organic matter flux * electron acceptors other than O 2 Let’s consider a sediment dominated by sulfate reduction: Defining P as the production rate of a solute, What would we predict pore water profiles of these 3 solutes to look like? Solution:

Solve the equation for each solute: Assume P 0 = 100, p 1 = 0. 2 Assume porosity = 0. 8 and Dsed = Dsw x (porosity)2 … then DHCO 3 - = 323 cm 2/y , DNH 4+ = 543 cm 2/y, DHPO 42 - = 208 cm 2/y

Plotting the concentration of one solute vs. another… Interpreting the slopes: At any depth, Therefore, the slopes imply
![A mineral undersaturated in seawater apparently simple dissolution kinetics What do we expect SiOH4 A mineral, undersaturated in seawater apparently simple dissolution kinetics… What do we expect [Si(OH)4]](https://slidetodoc.com/presentation_image_h2/58b442888fad9d7cb7464cb6311133f9/image-20.jpg)
A mineral, undersaturated in seawater apparently simple dissolution kinetics… What do we expect [Si(OH)4] in pore water to look like? CBW Concentration Diagenesis of a solid, undersaturated in bw CSAT Asymptotic approach to Saturation in pore water

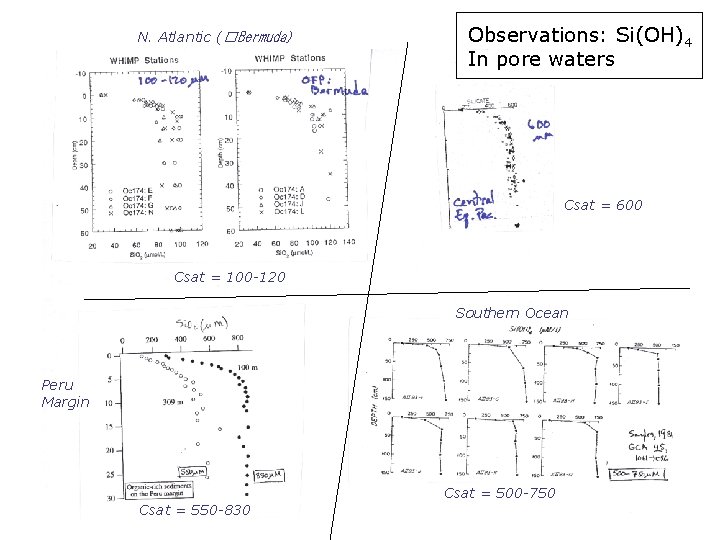
N. Atlantic (�Bermuda) Observations: Si(OH)4 In pore waters Csat = 600 Csat = 100 -120 Southern Ocean Peru Margin Csat = 500 -750 Csat = 550 -830




 Solute and solvent worksheet for grade 7
Solute and solvent worksheet for grade 7 Unit 4 lesson 2 solutes and solvents
Unit 4 lesson 2 solutes and solvents Water and water and water water
Water and water and water water Taste pore
Taste pore Pore size distribution calculation
Pore size distribution calculation Artistry pore cleansing masque
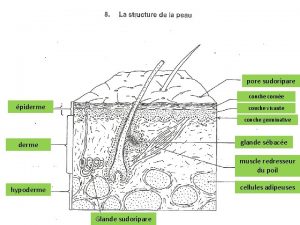
Artistry pore cleansing masque Pore sudoripare
Pore sudoripare Anal pore
Anal pore Anal pore
Anal pore Portuguese man of war characteristics
Portuguese man of war characteristics Surfapore rd
Surfapore rd Pore bearer
Pore bearer Porifera cellular organization
Porifera cellular organization Anglometre
Anglometre Pore pressure parameters
Pore pressure parameters Pore pressure parameters
Pore pressure parameters Taste pore
Taste pore Excessive concavity
Excessive concavity Tectonic hazard profiles
Tectonic hazard profiles Jean louise to kill a mockingbird
Jean louise to kill a mockingbird Profiles of the gifted and talented
Profiles of the gifted and talented Process discriminants in software project management
Process discriminants in software project management Alma publishing profiles
Alma publishing profiles Onf profile
Onf profile Net wlan show profiles
Net wlan show profiles H.264 profiles and levels
H.264 profiles and levels