Integrando nuevas estrategias terapeticas en CNMP estadios precoces






- Slides: 41

Integrando nuevas estrategias terapeúticas en CNMP estadios precoces Enriqueta Felip Hospital Vall d’Hebron, Barcelona Madrid, 25 de Octubre 2017 #SEOM 2017

#SEOM 2017

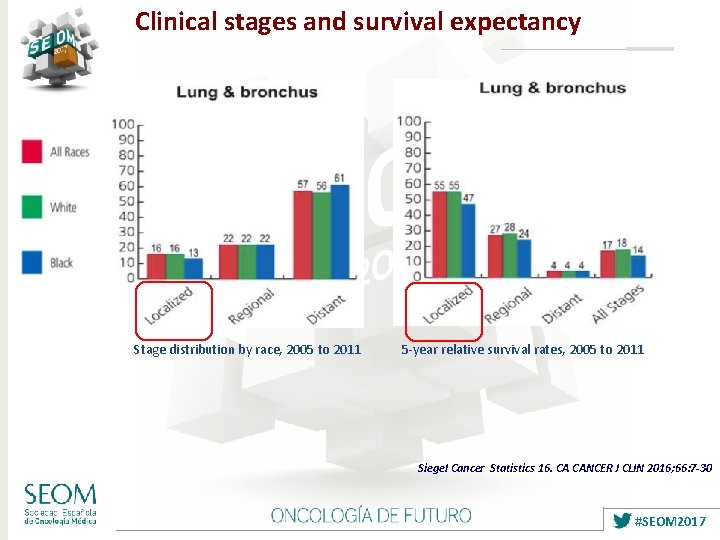
Clinical stages and survival expectancy Stage distribution by race, 2005 to 2011 5 -year relative survival rates, 2005 to 2011 Siegel Cancer Statistics 16. CA CANCER J CLIN 2016; 66: 7 -30 #SEOM 2017

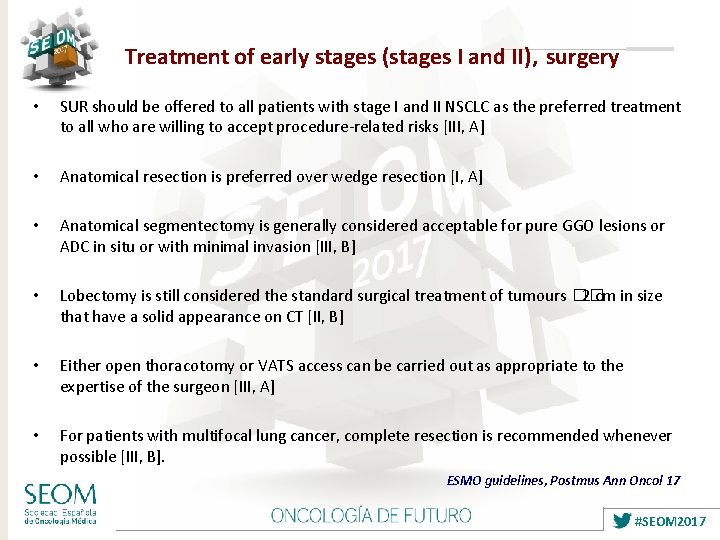
Treatment of early stages (stages I and II), surgery • SUR should be offered to all patients with stage I and II NSCLC as the preferred treatment to all who are willing to accept procedure-related risks [III, A] • Anatomical resection is preferred over wedge resection [I, A] • Anatomical segmentectomy is generally considered acceptable for pure GGO lesions or ADC in situ or with minimal invasion [III, B] • Lobectomy is still considered the standard surgical treatment of tumours �� 2 cm in size that have a solid appearance on CT [II, B] • Either open thoracotomy or VATS access can be carried out as appropriate to the expertise of the surgeon [III, A] • For patients with multifocal lung cancer, complete resection is recommended whenever possible [III, B]. ESMO guidelines, Postmus Ann Oncol 17 #SEOM 2017

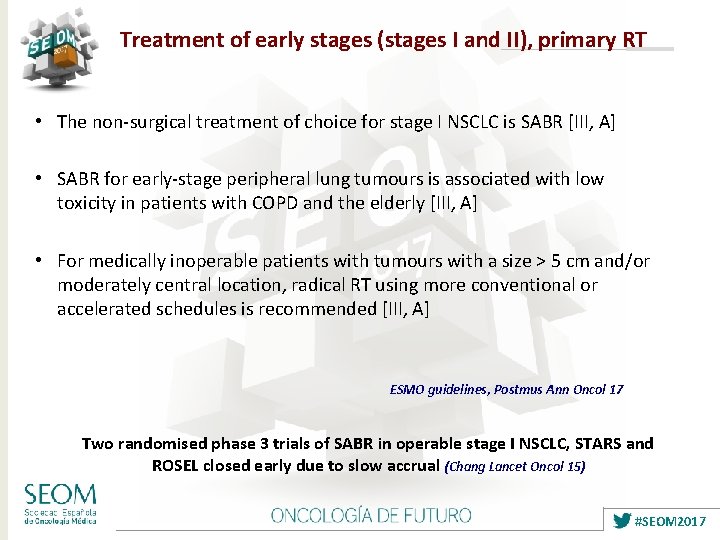
Treatment of early stages (stages I and II), primary RT • The non-surgical treatment of choice for stage I NSCLC is SABR [III, A] • SABR for early-stage peripheral lung tumours is associated with low toxicity in patients with COPD and the elderly [III, A] • For medically inoperable patients with tumours with a size > 5 cm and/or moderately central location, radical RT using more conventional or accelerated schedules is recommended [III, A] ESMO guidelines, Postmus Ann Oncol 17 Two randomised phase 3 trials of SABR in operable stage I NSCLC, STARS and ROSEL closed early due to slow accrual (Chang Lancet Oncol 15) #SEOM 2017

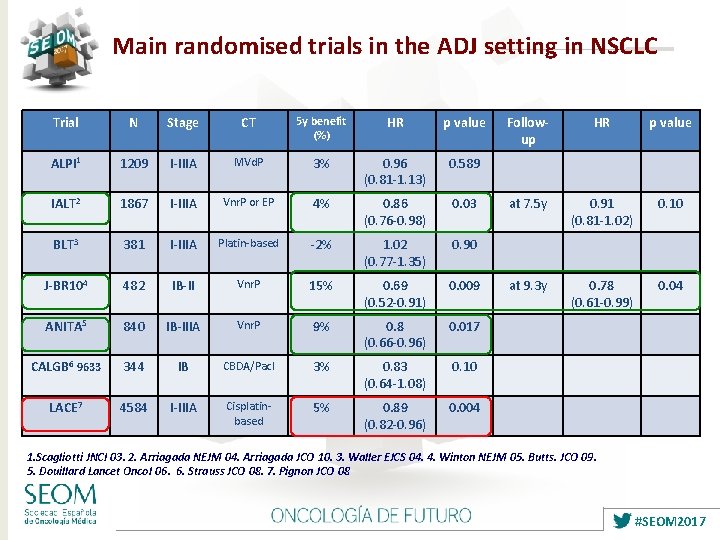
Main randomised trials in the ADJ setting in NSCLC Trial N Stage CT 5 y benefit (%) HR p value ALPI 1 1209 I-IIIA MVd. P 3% 0. 96 (0. 81 -1. 13) 0. 589 IALT 2 1867 I-IIIA Vnr. P or EP 4% 0. 86 (0. 76 -0. 98) 0. 03 BLT 3 381 I-IIIA Platin-based -2% 1. 02 (0. 77 -1. 35) 0. 90 J-BR 104 482 IB-II Vnr. P 15% 0. 69 (0. 52 -0. 91) 0. 009 ANITA 5 840 IB-IIIA Vnr. P 9% 0. 8 (0. 66 -0. 96) 0. 017 CALGB 6 9633 344 IB CBDA/Pacl 3% 0. 83 (0. 64 -1. 08) 0. 10 LACE 7 4584 I-IIIA Cisplatinbased 5% 0. 89 (0. 82 -0. 96) 0. 004 Followup HR p value at 7. 5 y 0. 91 (0. 81 -1. 02) 0. 10 at 9. 3 y 0. 78 (0. 61 -0. 99) 0. 04 1. Scagliotti JNCI 03. 2. Arriagada NEJM 04. Arriagada JCO 10. 3. Waller EJCS 04. 4. Winton NEJM 05. Butts. JCO 09. 5. Douillard Lancet Oncol 06. 6. Strauss JCO 08. 7. Pignon JCO 08 #SEOM 2017

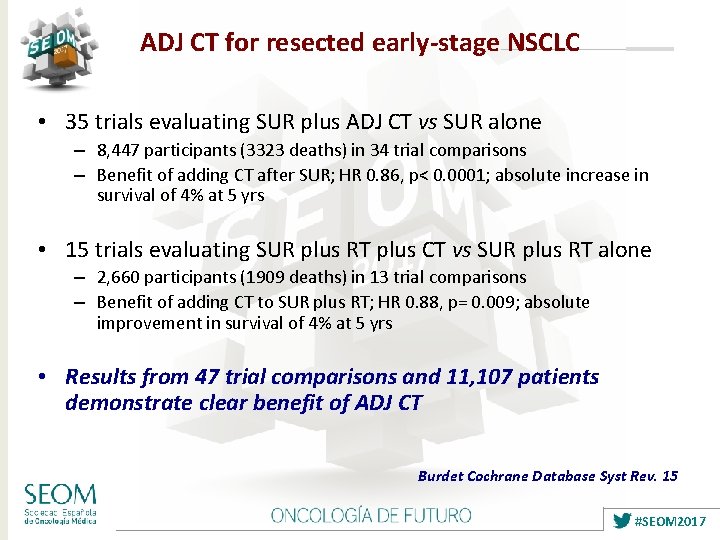
ADJ CT for resected early-stage NSCLC • 35 trials evaluating SUR plus ADJ CT vs SUR alone – 8, 447 participants (3323 deaths) in 34 trial comparisons – Benefit of adding CT after SUR; HR 0. 86, p< 0. 0001; absolute increase in survival of 4% at 5 yrs • 15 trials evaluating SUR plus RT plus CT vs SUR plus RT alone – 2, 660 participants (1909 deaths) in 13 trial comparisons – Benefit of adding CT to SUR plus RT; HR 0. 88, p= 0. 009; absolute improvement in survival of 4% at 5 yrs • Results from 47 trial comparisons and 11, 107 patients demonstrate clear benefit of ADJ CT Burdet Cochrane Database Syst Rev. 15 #SEOM 2017

ADJ CT in stage IB • A pooled exploratory analysis of the effect of tumor size on survival benefit from ADJ platinum-based CT in node-negative NSCLC (Cuffe JTO 12) – 461 node-negative p (CALGB 9633 and JBR. 10) reclassified as T 2 a, T 2 b or T 3 – A non-significant trend for increased CT effect on OS with advancing T-size (HR T 2 a 0. 90, T 2 b 0. 69, T 3 0. 57) Tumor size is a relevant factor to consider to decide ADJ CT in stage I, although not prospectively validated #SEOM 2017

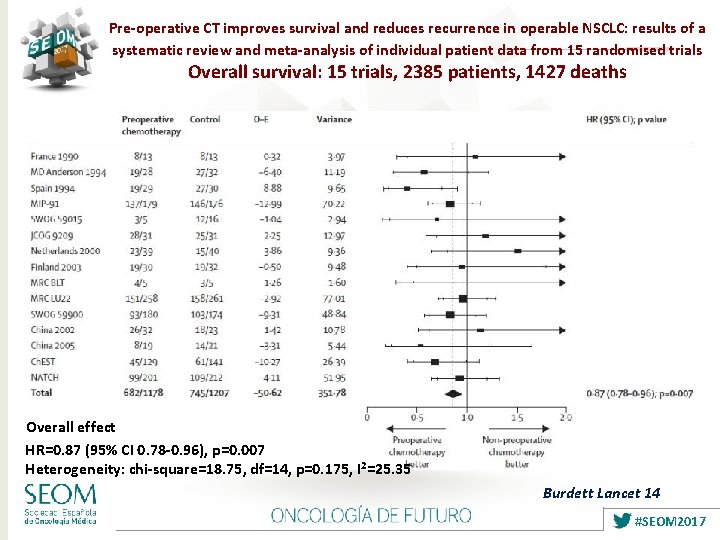
Pre-operative CT improves survival and reduces recurrence in operable NSCLC: results of a systematic review and meta-analysis of individual patient data from 15 randomised trials Overall survival: 15 trials, 2385 patients, 1427 deaths Overall effect HR=0. 87 (95% CI 0. 78 -0. 96), p=0. 007 Heterogeneity: chi-square=18. 75, df=14, p=0. 175, I 2=25. 35 Burdett Lancet 14 #SEOM 2017

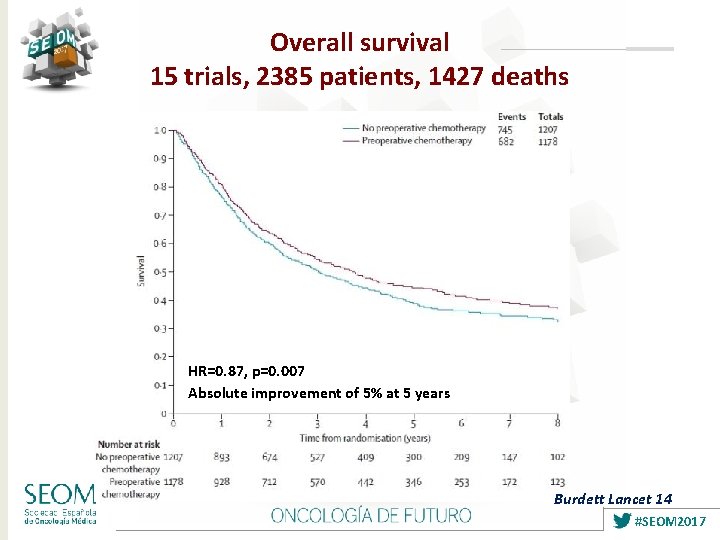
Overall survival 15 trials, 2385 patients, 1427 deaths HR=0. 87, p=0. 007 Absolute improvement of 5% at 5 years Burdett Lancet 14 #SEOM 2017

Treatment of early stages (stages I and II), systemic therapy • ADJ CT should be offered to patients with resected stage II-III [I, A] and can be considered in patients with resected stage IB and primary tumour > 4 cm [II, B] • For ADJ CT, a two-drug combination with cis is preferable [I, A]. The most frequently studied regimen is cis–vinorelbine • At the present time, the choice of ADJ therapy should not be guided by molecular analyses, e. g. ERCC 1 mutation testing [IV, B] • In the current state of knowledge, targeted agents should not be used in the ADJ setting [II, A] • In view of the equivalence of NEO and ADJ CT for OS, the consistent results and broad evidence base support ADJ CT as the timing of choice [II, C] ESMO guidelines, Postmus Ann Oncol 17 #SEOM 2017

12 #SEOM 2017

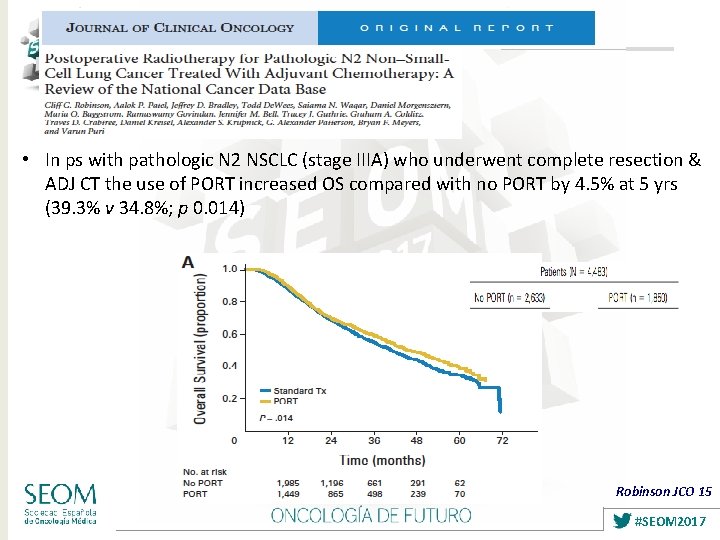
• In ps with pathologic N 2 NSCLC (stage IIIA) who underwent complete resection & ADJ CT the use of PORT increased OS compared with no PORT by 4. 5% at 5 yrs (39. 3% v 34. 8%; p 0. 014) Robinson JCO 15 #SEOM 2017

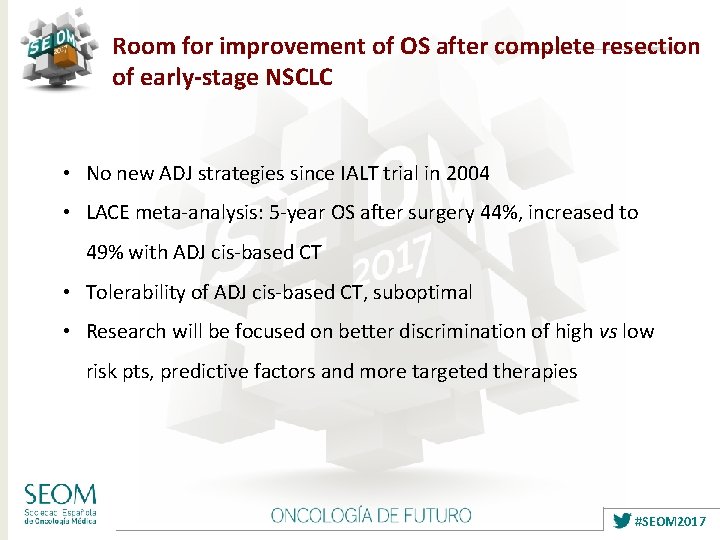
Room for improvement of OS after complete resection of early-stage NSCLC • No new ADJ strategies since IALT trial in 2004 • LACE meta-analysis: 5 -year OS after surgery 44%, increased to 49% with ADJ cis-based CT • Tolerability of ADJ cis-based CT, suboptimal • Research will be focused on better discrimination of high vs low risk pts, predictive factors and more targeted therapies #SEOM 2017

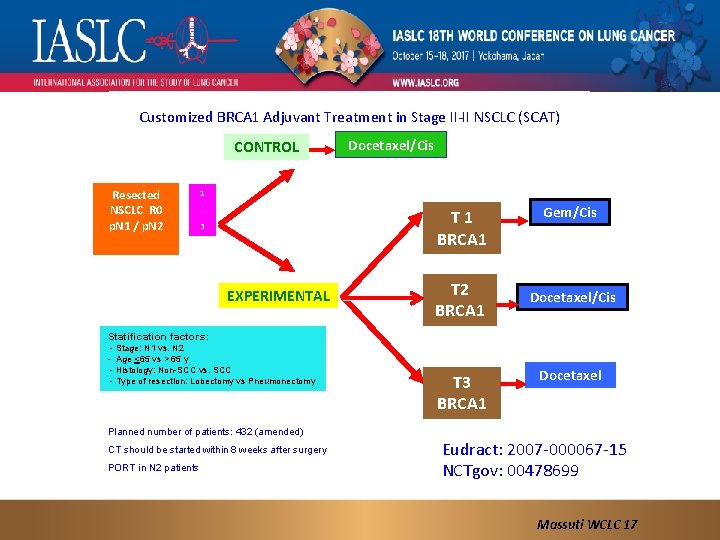
Customized BRCA 1 Adjuvant Treatment in Stage II-II NSCLC (SCAT) CONTROL Resected NSCLC R 0 p. N 1 / p. N 2 Docetaxel/Cis 1 : T 1 BRCA 1 3 EXPERIMENTAL T 2 BRCA 1 Gem/Cis Docetaxel/Cis Statification factors: - Stage: N 1 vs. N 2 - Age <65 vs > 65 y - Histology: Non-SCC vs. SCC - Type of resection: Lobectomy vs Pneumonectomy T 3 BRCA 1 Docetaxel Planned number of patients: 432 (amended) CT should be started within 8 weeks after surgery PORT in N 2 patients Eudract: 2007 -000067 -15 NCTgov: 00478699 Massuti WCLC 17

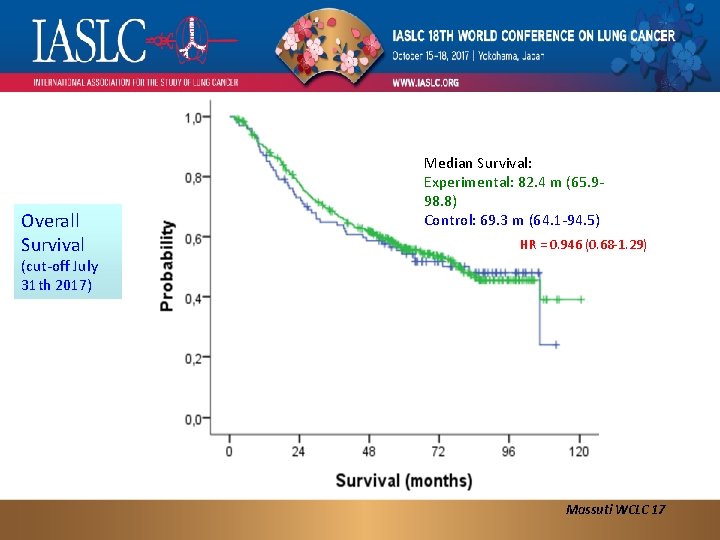
Overall Survival Median Survival: Experimental: 82. 4 m (65. 998. 8) Control: 69. 3 m (64. 1 -94. 5) HR = 0. 946 (0. 68 -1. 29) (cut-off July 31 th 2017) Massuti WCLC 17

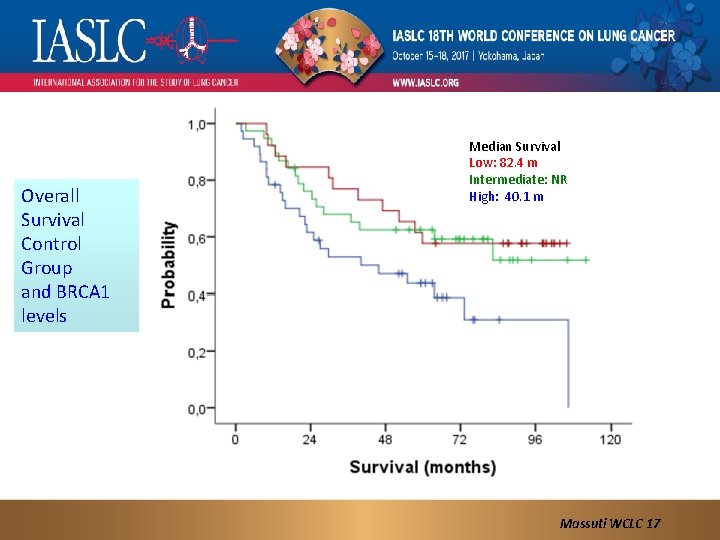
Overall Survival Control Group and BRCA 1 levels Median Survival Low: 82. 4 m Intermediate: NR High: 40. 1 m Massuti WCLC 17

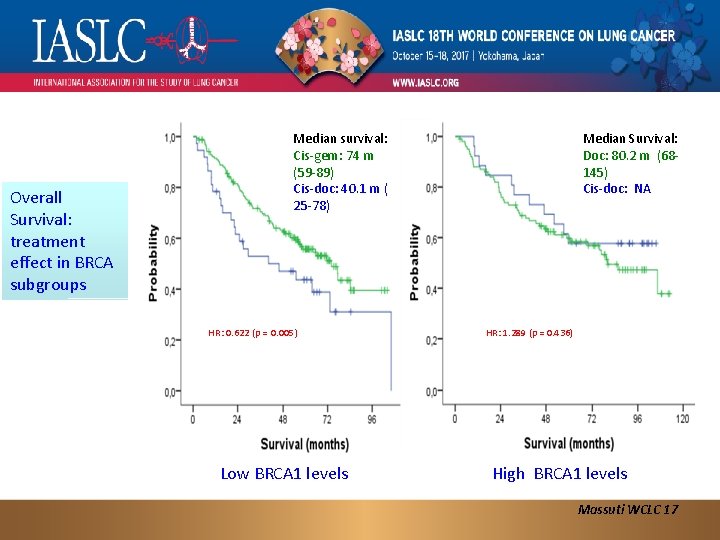
Overall Survival: treatment effect in BRCA subgroups Median survival: Cis-gem: 74 m (59 -89) Cis-doc: 40. 1 m ( 25 -78) HR: 0. 622 (p = 0. 005) Low BRCA 1 levels Median Survival: Doc: 80. 2 m (68145) Cis-doc: NA HR: 1. 289 (p = 0. 436) High BRCA 1 levels Massuti WCLC 17

#SEOM 2017

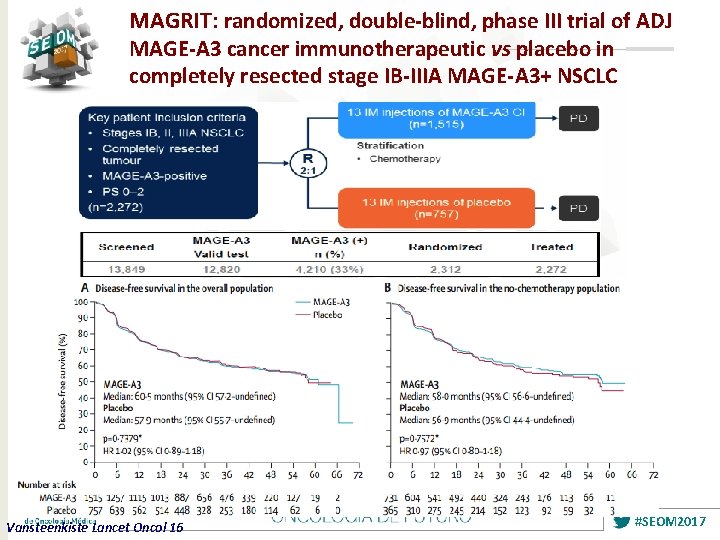
MAGRIT: randomized, double-blind, phase III trial of ADJ MAGE-A 3 cancer immunotherapeutic vs placebo in completely resected stage IB-IIIA MAGE-A 3+ NSCLC Vansteenkiste Lancet Oncol 16 #SEOM 2017

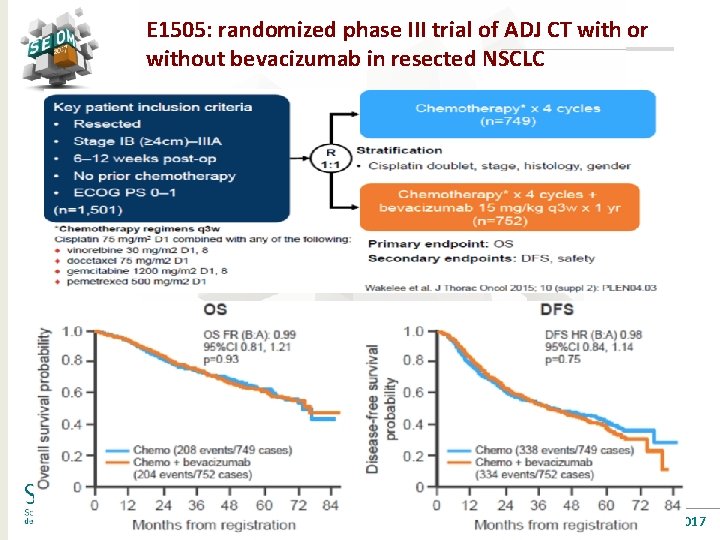
E 1505: randomized phase III trial of ADJ CT with or without bevacizumab in resected NSCLC #SEOM 2017

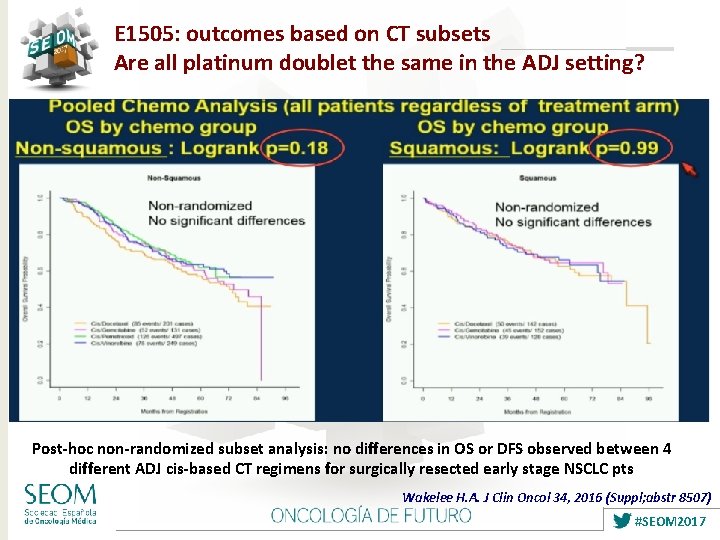
E 1505: outcomes based on CT subsets Are all platinum doublet the same in the ADJ setting? Post-hoc non-randomized subset analysis: no differences in OS or DFS observed between 4 different ADJ cis-based CT regimens for surgically resected early stage NSCLC pts Wakelee H. A. J Clin Oncol 34, 2016 (Suppl; abstr 8507) #SEOM 2017

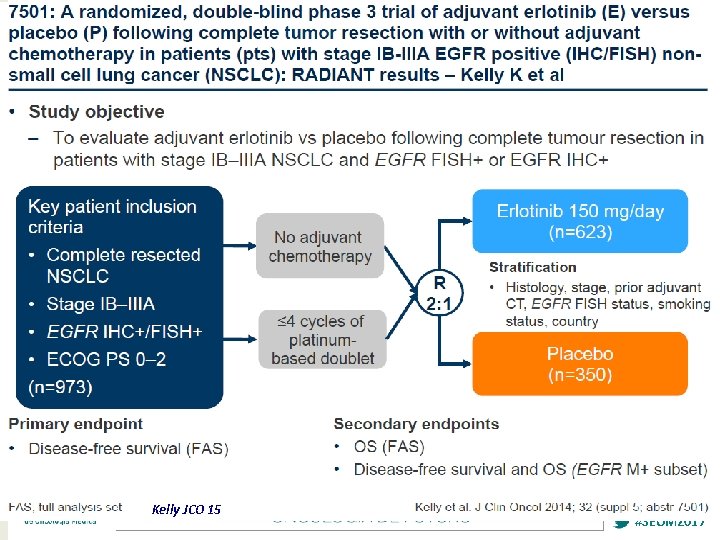
Kelly JCO 15 #SEOM 2017

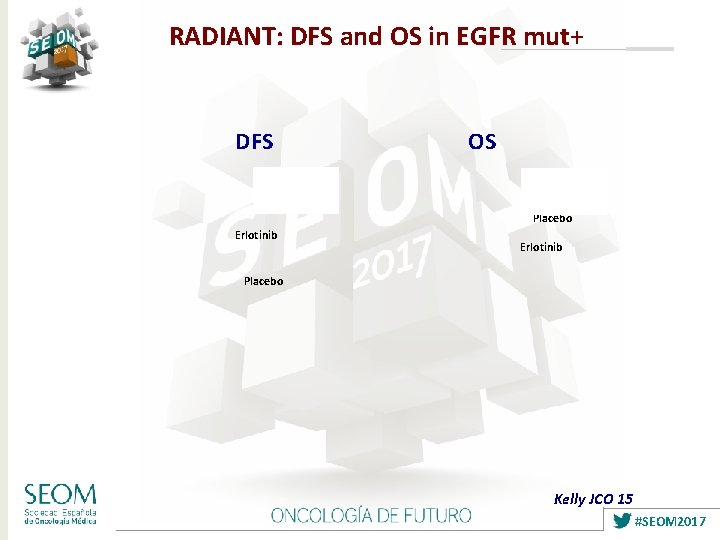
RADIANT: DFS and OS in EGFR mut+ DFS OS Placebo Erlotinib Placebo Kelly JCO 15 #SEOM 2017

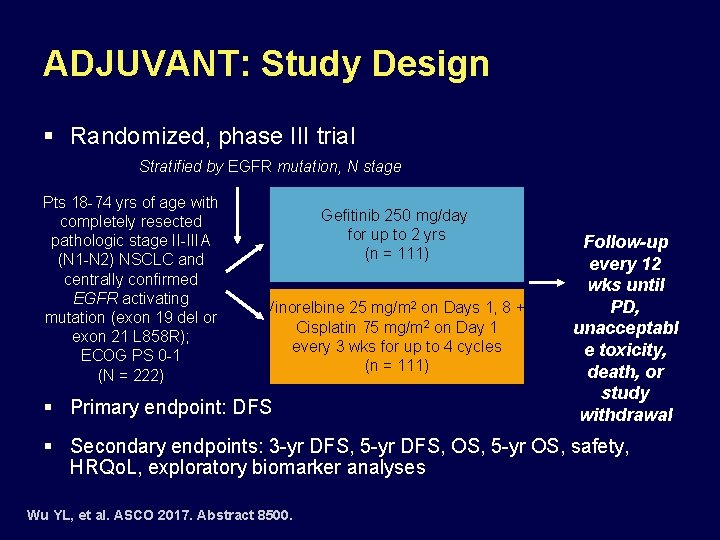
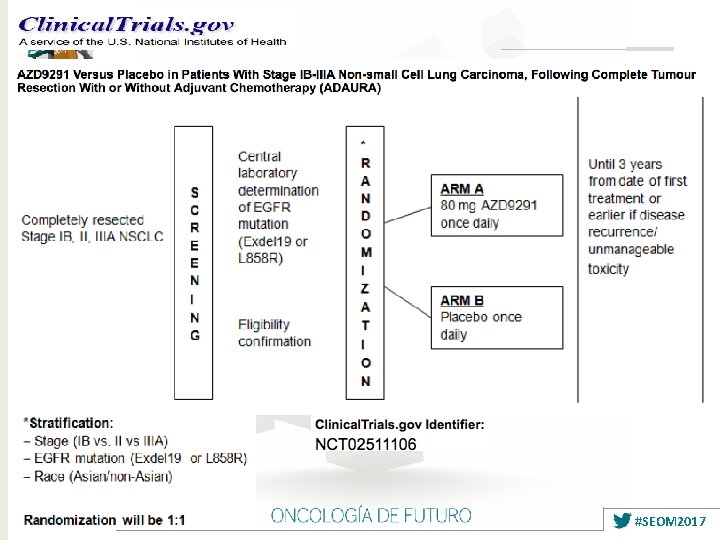
ADJUVANT: Study Design § Randomized, phase III trial Stratified by EGFR mutation, N stage Pts 18 -74 yrs of age with completely resected pathologic stage II-IIIA (N 1 -N 2) NSCLC and centrally confirmed EGFR activating mutation (exon 19 del or exon 21 L 858 R); ECOG PS 0 -1 (N = 222) Gefitinib 250 mg/day for up to 2 yrs (n = 111) Vinorelbine 25 mg/m 2 on Days 1, 8 + Cisplatin 75 mg/m 2 on Day 1 every 3 wks for up to 4 cycles (n = 111) § Primary endpoint: DFS Follow-up every 12 wks until PD, unacceptabl e toxicity, death, or study withdrawal § Secondary endpoints: 3 -yr DFS, 5 -yr DFS, OS, 5 -yr OS, safety, HRQo. L, exploratory biomarker analyses Wu YL, et al. ASCO 2017. Abstract 8500.

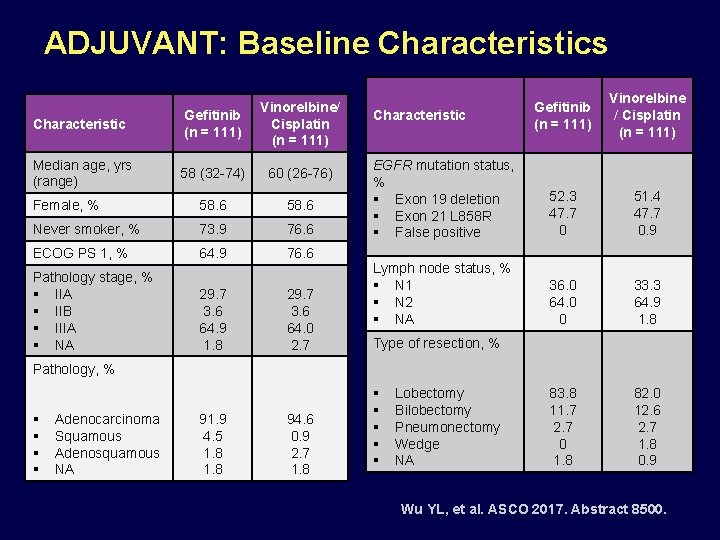
ADJUVANT: Baseline Characteristics Characteristic Gefitinib (n = 111) Vinorelbine/ Cisplatin (n = 111) Median age, yrs (range) 58 (32 -74) 60 (26 -76) Female, % 58. 6 Never smoker, % 73. 9 76. 6 ECOG PS 1, % 64. 9 76. 6 Pathology stage, % § IIA § IIB § IIIA § NA 29. 7 3. 6 64. 9 1. 8 29. 7 3. 6 64. 0 2. 7 Gefitinib (n = 111) Vinorelbine / Cisplatin (n = 111) EGFR mutation status, % § Exon 19 deletion § Exon 21 L 858 R § False positive 52. 3 47. 7 0 51. 4 47. 7 0. 9 Lymph node status, % § N 1 § N 2 § NA 36. 0 64. 0 0 33. 3 64. 9 1. 8 83. 8 11. 7 2. 7 0 1. 8 82. 0 12. 6 2. 7 1. 8 0. 9 Characteristic Type of resection, % Pathology, % § § Adenocarcinoma Squamous Adenosquamous NA 91. 9 4. 5 1. 8 94. 6 0. 9 2. 7 1. 8 § § § Lobectomy Bilobectomy Pneumonectomy Wedge NA Wu YL, et al. ASCO 2017. Abstract 8500.

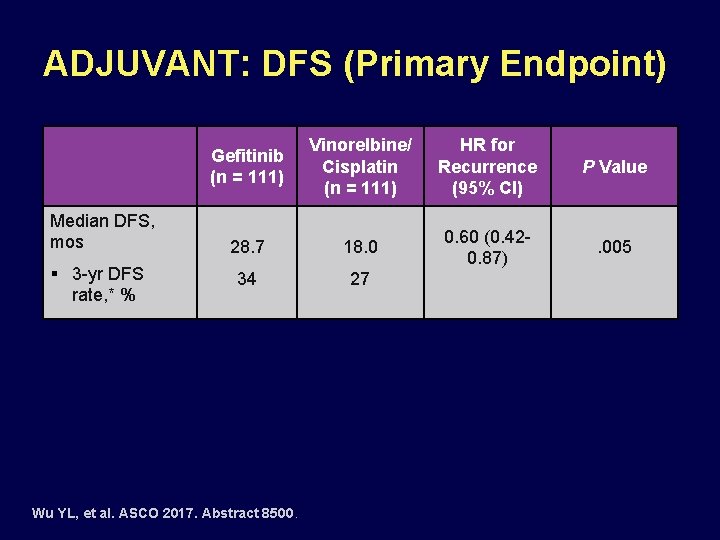
ADJUVANT: DFS (Primary Endpoint) Median DFS, mos § 3 -yr DFS rate, * % Gefitinib (n = 111) Vinorelbine/ Cisplatin (n = 111) HR for Recurrence (95% CI) P Value 28. 7 18. 0 0. 60 (0. 420. 87) . 005 34 27 Wu YL, et al. ASCO 2017. Abstract 8500.

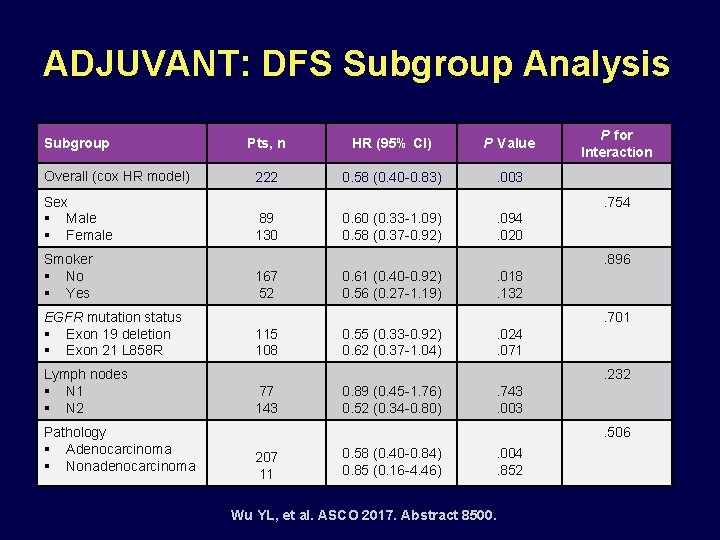
ADJUVANT: DFS Subgroup Analysis Subgroup Overall (cox HR model) Sex § Male § Female Smoker § No § Yes EGFR mutation status § Exon 19 deletion § Exon 21 L 858 R Lymph nodes § N 1 § N 2 Pathology § Adenocarcinoma § Nonadenocarcinoma Pts, n HR (95% CI) P Value 222 0. 58 (0. 40 -0. 83) . 003 P for Interaction. 754 89 130 0. 60 (0. 33 -1. 09) 0. 58 (0. 37 -0. 92) . 094. 020. 896 167 52 0. 61 (0. 40 -0. 92) 0. 56 (0. 27 -1. 19) . 018. 132. 701 115 108 0. 55 (0. 33 -0. 92) 0. 62 (0. 37 -1. 04) . 024. 071. 232 77 143 0. 89 (0. 45 -1. 76) 0. 52 (0. 34 -0. 80) . 743. 003. 506 207 11 0. 58 (0. 40 -0. 84) 0. 85 (0. 16 -4. 46) . 004. 852 Wu YL, et al. ASCO 2017. Abstract 8500.

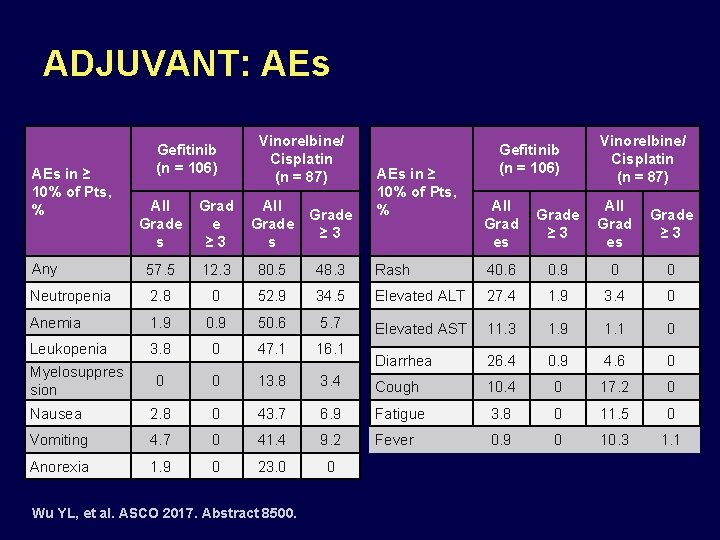
ADJUVANT: AEs in ≥ 10% of Pts, % Gefitinib (n = 106) Vinorelbine/ Cisplatin (n = 87) All Grad es Grade ≥ 3 Rash 40. 6 0. 9 0 0 34. 5 Elevated ALT 27. 4 1. 9 3. 4 0 50. 6 5. 7 Elevated AST 11. 3 1. 9 1. 1 0 0 47. 1 16. 1 Diarrhea 26. 4 0. 9 4. 6 0 0 0 13. 8 3. 4 Cough 10. 4 0 17. 2 0 Nausea 2. 8 0 43. 7 6. 9 Fatigue 3. 8 0 11. 5 0 Vomiting 4. 7 0 41. 4 9. 2 Fever 0. 9 0 10. 3 1. 1 Anorexia 1. 9 0 23. 0 0 All Grade s Grad e ≥ 3 All Grade s Grade ≥ 3 Any 57. 5 12. 3 80. 5 48. 3 Neutropenia 2. 8 0 52. 9 Anemia 1. 9 0. 9 Leukopenia 3. 8 Myelosuppres sion Wu YL, et al. ASCO 2017. Abstract 8500.


#SEOM 2017

#SEOM 2017

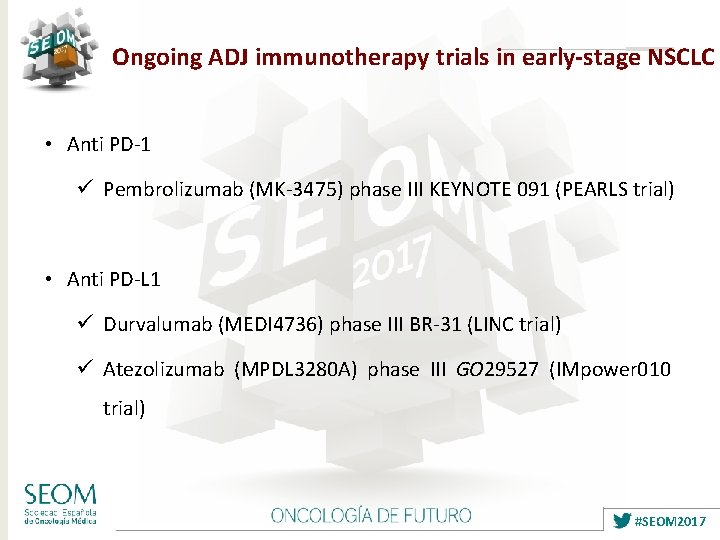
Ongoing ADJ immunotherapy trials in early-stage NSCLC • Anti PD-1 ü Pembrolizumab (MK-3475) phase III KEYNOTE 091 (PEARLS trial) • Anti PD-L 1 ü Durvalumab (MEDI 4736) phase III BR-31 (LINC trial) ü Atezolizumab (MPDL 3280 A) phase III GO 29527 (IMpower 010 trial) #SEOM 2017

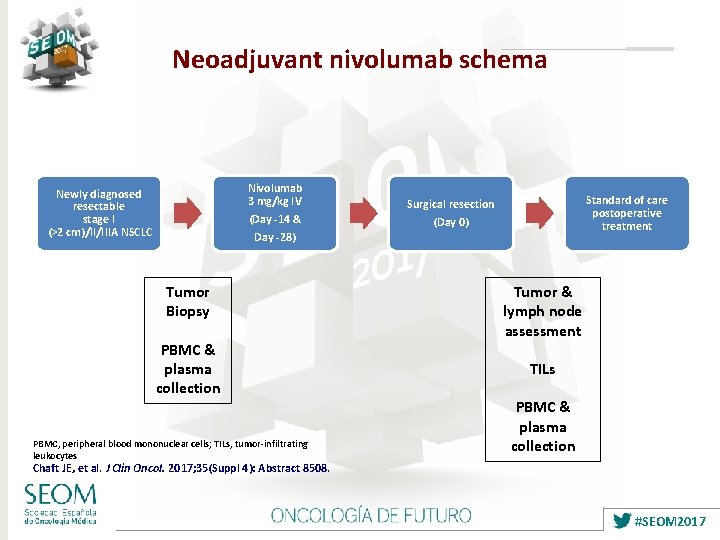
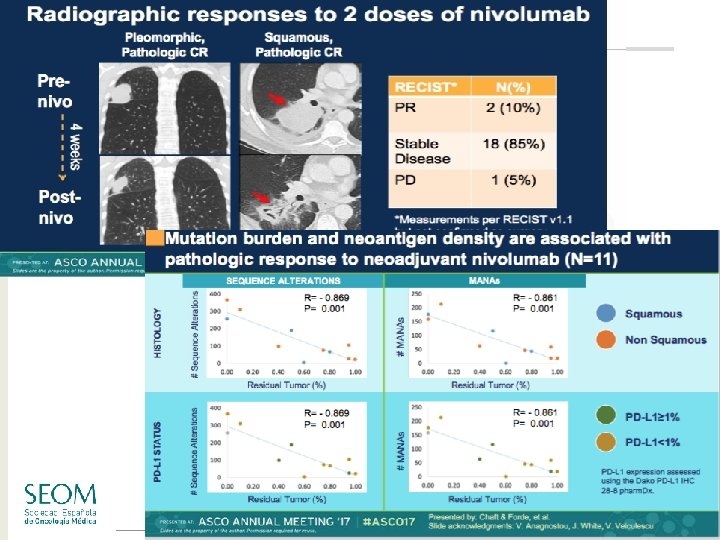
Neoadjuvant nivolumab schema Nivolumab 3 mg/kg IV Newly diagnosed resectable stage I (>2 cm)/II/IIIA NSCLC (Day -14 & Day -28) Tumor Biopsy PBMC & plasma collection PBMC, peripheral blood mononuclear cells; TILs, tumor-infiltrating leukocytes Standard of care postoperative treatment Surgical resection (Day 0) Tumor & lymph node assessment TILs PBMC & plasma collection Chaft JE, et al. J Clin Oncol. 2017; 35(Suppl 4): Abstract 8508. #SEOM 2017

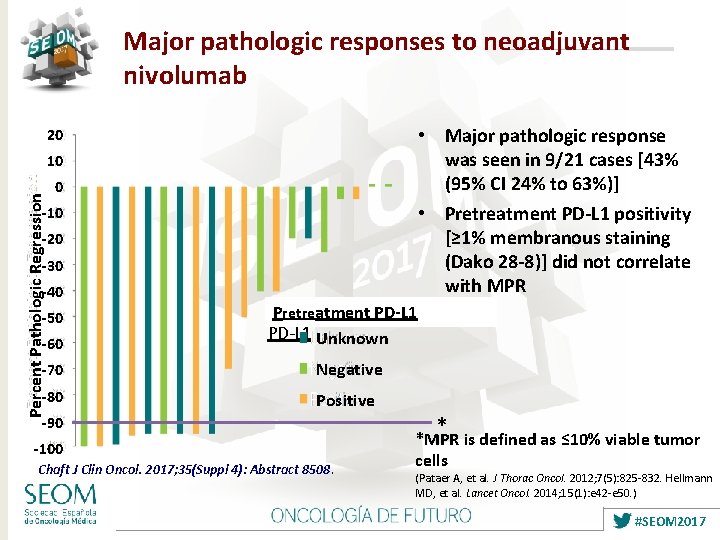
Major pathologic responses to neoadjuvant nivolumab • Major pathologic response was seen in 9/21 cases [43% (95% CI 24% to 63%)] • Pretreatment PD-L 1 positivity [≥ 1% membranous staining (Dako 28 -8)] did not correlate with MPR 20 Percent Pathologic Regression 10 0 -10 -20 -30 -40 -50 -60 Pre-treatment Pretreatment PD-L 1 Unknown -70 Negative -80 Positive -90 -100 Chaft J Clin Oncol. 2017; 35(Suppl 4): Abstract 8508. * *MPR is defined as ≤ 10% viable tumor cells (Pataer A, et al. J Thorac Oncol. 2012; 7(5): 825 -832. Hellmann MD, et al. Lancet Oncol. 2014; 15(1): e 42 -e 50. ) #SEOM 2017

#SEOM 2017

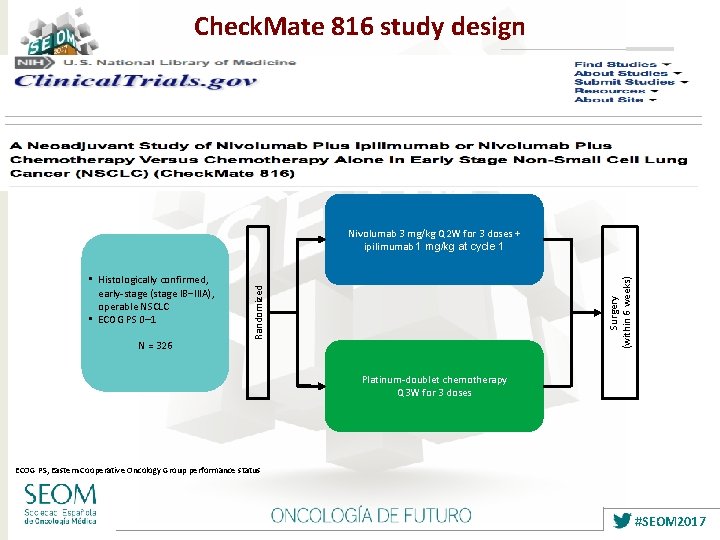
Check. Mate 816 study design N = 326 Randomized • Histologically confirmed, early-stage (stage IB‒IIIA), operable NSCLC • ECOG PS 0– 1 Surgery (within 6 weeks) Nivolumab 3 mg/kg Q 2 W for 3 doses + ipilimumab 1 mg/kg at cycle 1 Platinum-doublet chemotherapy Q 3 W for 3 doses ECOG PS, Eastern Cooperative Oncology Group performance status #SEOM 2017

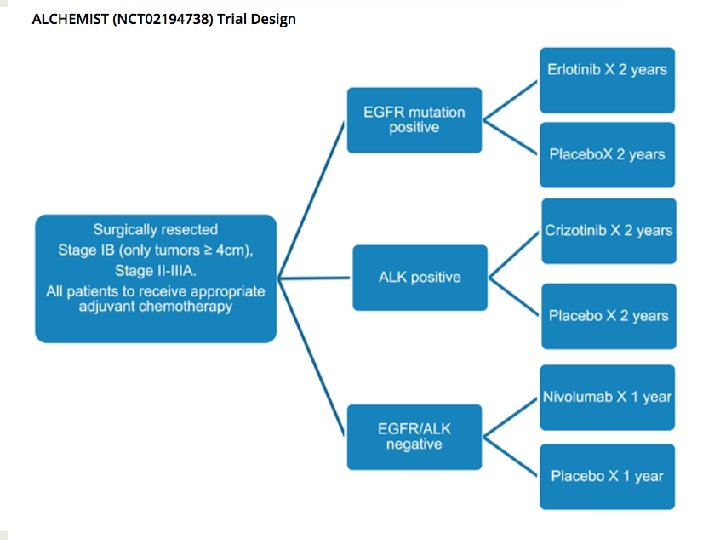
Early-stage NSCLC what will happen in the next 10 years? • Large percentage of lung cancer patients diagnosed with early-stage disease expected (screening strategies) • Ongoing studies of ADJ TKI in resected EGFR-mut / ALK + patients ü Prospectively designed in marker+ population • Ongoing ADJ studies with anti-PD 1/anti-PDL 1 strategies ü Different compounds, large sample size • Ongoing NEO trials with immunotherapy ü Promising results in small studies #SEOM 2017

Early-stage NSCLC what will happen in the next 10 years? • Optimism for positive results in early-stage NSCLC ü Heterogeneity in patients included (staging, different surgery standards) ü Long follow-up needed ü How will treatments at recurrence affect study outcomes • Molecular testing also in early-stage disease #SEOM 2017

Integrando nuevas estrategias terapéuticas en CNMP estadios precoces • • • Cribado Biopsias liquidas (ct. DNA, CTCs. . ) Biología del CPNM en estadio precoz Agentes dirigidos Inmunoterapia #SEOM 2017

Gracias!!! efelip@vhebron. net #SEOM 2017
 Conduccion anisotropica
Conduccion anisotropica Integrando
Integrando Integrando
Integrando Manejo rciu
Manejo rciu Um consumidor desconfia que a balança
Um consumidor desconfia que a balança Cifras tension arterial
Cifras tension arterial Estadios de nolla
Estadios de nolla Drc estagios
Drc estagios Clasificacion de crisis asmatica
Clasificacion de crisis asmatica Dilemas morales
Dilemas morales Estadio de la adolescencia wallon
Estadio de la adolescencia wallon Que es ser responsable
Que es ser responsable Son estadios del esqueleto
Son estadios del esqueleto Estadios de desarrollo moral
Estadios de desarrollo moral Estadios insuficiencia cardiaca
Estadios insuficiencia cardiaca Taenia saginata huevo
Taenia saginata huevo Teorias del desarrollo moral
Teorias del desarrollo moral Pseudopuberta
Pseudopuberta Estadios irc
Estadios irc Tfg estadios
Tfg estadios Estadios gasometricos asma
Estadios gasometricos asma Estadios de tanner edades
Estadios de tanner edades Esquema de jean piaget
Esquema de jean piaget Círculos bíblicos
Círculos bíblicos Insulina nph esquema
Insulina nph esquema In the previous lesson you have learned
In the previous lesson you have learned Inclusion de nuevas tareas
Inclusion de nuevas tareas Contigo aprendi que existen nuevas y mejores emociones
Contigo aprendi que existen nuevas y mejores emociones Neuropsicosis de defensa
Neuropsicosis de defensa Yo hago nuevas todas las cosas evangelio
Yo hago nuevas todas las cosas evangelio Nuevas perspectivas 1
Nuevas perspectivas 1 Les gusta probar ideas teorías y técnicas nuevas
Les gusta probar ideas teorías y técnicas nuevas Nuevas tecnologias
Nuevas tecnologias 7 nuevas maravillas
7 nuevas maravillas Nuevas validaciones sii
Nuevas validaciones sii Sobrerrecorrido
Sobrerrecorrido Frases bonitas para la vida
Frases bonitas para la vida Nuevos retos nuevas oportunidades
Nuevos retos nuevas oportunidades Preposicin
Preposicin Las nuevas palabras fontanarrosa
Las nuevas palabras fontanarrosa Dios hara cosas nuevas
Dios hara cosas nuevas Estrategias de expresión
Estrategias de expresión