In Vitro Gliadin Challenge Diagnostic Accuracy and Utility


















- Slides: 28

In Vitro Gliadin Challenge: Diagnostic Accuracy and Utility for the Difficult Diagnosis of Celiac Disease Am J Gastroenterol 2012; 107: 111– 117; Raffaella Tortora MD et al.

Editor: Dr Mohammad Sadrkabir

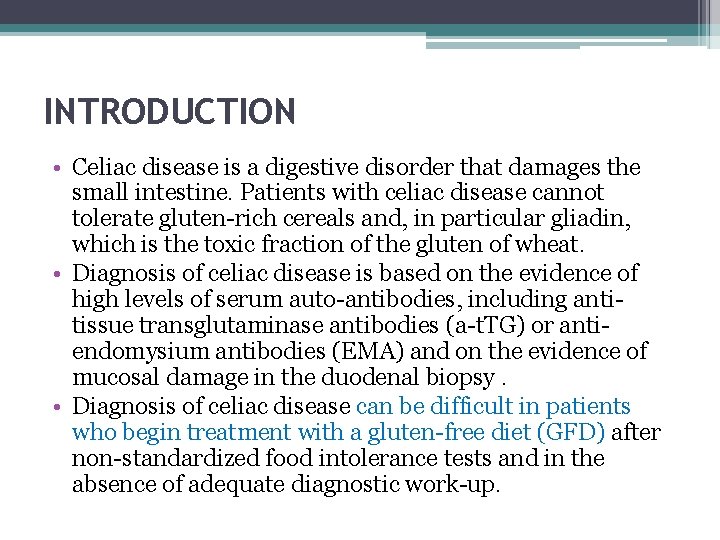
INTRODUCTION • Celiac disease is a digestive disorder that damages the small intestine. Patients with celiac disease cannot tolerate gluten-rich cereals and, in particular gliadin, which is the toxic fraction of the gluten of wheat. • Diagnosis of celiac disease is based on the evidence of high levels of serum auto-antibodies, including antitissue transglutaminase antibodies (a-t. TG) or antiendomysium antibodies (EMA) and on the evidence of mucosal damage in the duodenal biopsy. • Diagnosis of celiac disease can be difficult in patients who begin treatment with a gluten-free diet (GFD) after non-standardized food intolerance tests and in the absence of adequate diagnostic work-up.

• Once this treatment is initiated, diagnosis of celiac disease requires the reintroduction of gluten into the patient's diet for an appropriate period. • Diagnosis of celiac disease can be difficult when the search of serum antibodies and the duodenal biopsy results are not concordant. • Genetic markers as the HLA DQ 2–DQ 8 genes are of help only in excluding celiac disease as their presence is common also in non-celiac subjects. • In the presence of gliadin, the duodenal mucosa of celiac patients modifications that, in part, can be reproduced in vitro.

• The short-term in vitro exposure of the duodenal mucosa of celiac patients to gliadin induces crypt hyperplasia, villus atrophy, and T lymphocyte recruitment in the lamina propria, increasing the number of intraepithelial T lymphocytes. • The in vitro response of the duodenal mucosa to gliadin is generally defined as the gliadin challenge, a test which could represent a tool for objective assessment of celiac disease when diagnosis is difficult. • The present study aimed to evaluate the diagnostic accuracy of the in vitro gliadin challenge in patients without celiac disease, with celiac disease, and in patients with difficult diagnosis.

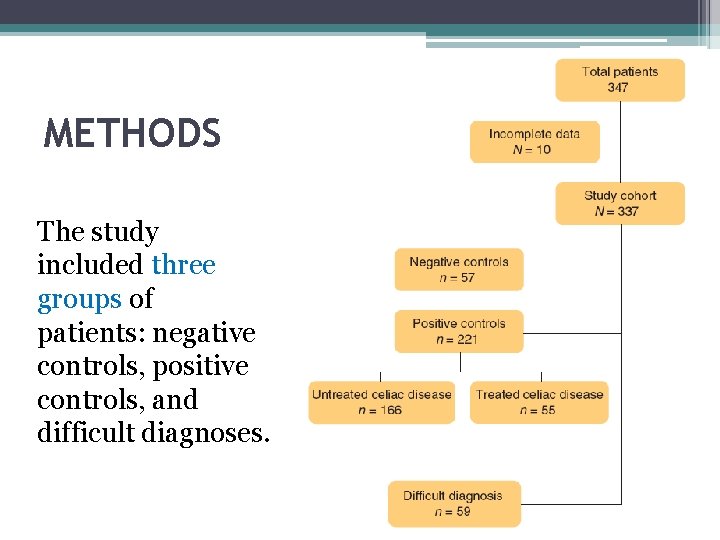
METHODS The study included three groups of patients: negative controls, positive controls, and difficult diagnoses.

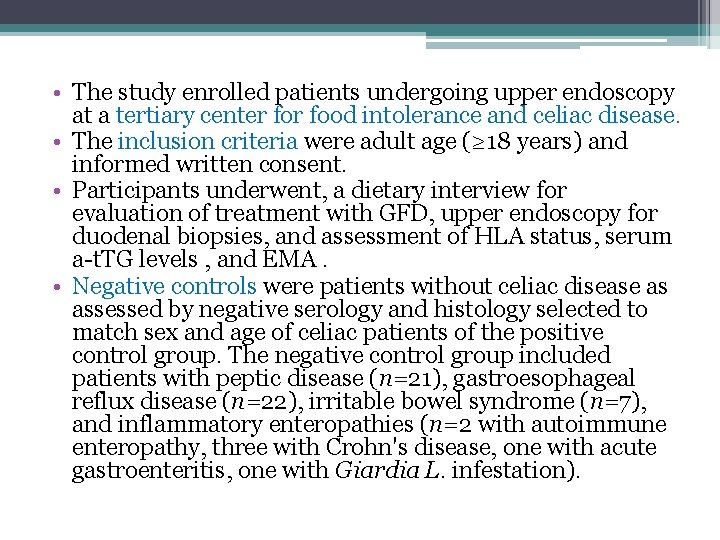
• The study enrolled patients undergoing upper endoscopy at a tertiary center food intolerance and celiac disease. • The inclusion criteria were adult age (≥ 18 years) and informed written consent. • Participants underwent, a dietary interview for evaluation of treatment with GFD, upper endoscopy for duodenal biopsies, and assessment of HLA status, serum a-t. TG levels , and EMA. • Negative controls were patients without celiac disease as assessed by negative serology and histology selected to match sex and age of celiac patients of the positive control group. The negative control group included patients with peptic disease (n=21), gastroesophageal reflux disease (n=22), irritable bowel syndrome (n=7), and inflammatory enteropathies (n=2 with autoimmune enteropathy, three with Crohn's disease, one with acute gastroenteritis, one with Giardia L. infestation).

• Positive controls included celiac disease patients under treated and untreated conditions. Treated celiac patients were on GFD since at least 1 year after complete diagnostic work-up including serology and histology. Untreated celiac patients were cases with positive serology who underwent upper endoscopy for completion of diagnostic work-up. • Difficult diagnosis group was made of patients with suspected celiac disease in whom diagnosis could not be made because the diagnostic work-up was initiated while on treatment with GFD and/or of non-concordant diagnostic tests. The list of combination of nonconcordant tests included the presence of EMA antibodies with the absence of a-t. TG or vice versa and the presence of antibodies with the absence of histological abnormalities in the duodenal mucosa.

Follow-up studies • After completion of the gliadin challenge, participants of the difficult diagnosis group were prescribed a gluten-containing diet and were followed up for up 1 year with assessments of serum a-t. TG and EMA every 3 months. Celiac disease diagnosis was made during follow-up if celiac disease-specific serology was consistently positive for both a-t. TG and EMA. If the serology was constantly negative during the follow-up, celiac disease was excluded.

Gliadin challenge • The in vitro gliadin challenge was performed by expert biologists who were blind to the characteristics of the patients. • Eight duodenal fragments were collected from each patient. • Two fragments were obtained for routine histology. • The remaining six fragments were used for gliadin challenge and placed in ice-cold tissue-culture medium within 20 min and cultured as follows. • The gliadin challenge was performed adding a gliadin digest (1 mg/ml) to four samples.

• Two fragments were cultured for 3 h for evaluation of early markers of inflammation namely: PY 99 (antiphospho-tyrosine-m. Ab), HLA-DR , and ICAM-1 (intercellular cell adhesion molecule). • Two fragments were cultured for up to 24 h for evaluation of the delayed markers of inflammation, namely CD 3 (a marker of mature T lymphocyte), CD 25 (interleukin-2 receptor in both lymphoid and myeloid cells), and CD 69 (a marker of T-cell activation). • The remaining two fragments served as controls and were cultured for 3 or 24 h in the same way without the addition of gliadin (blank samples). • All samples underwent also the search for the transglutaminase 2 Ig. A (TG 2–Ig. A) deposits before and after 3 and 24 h.

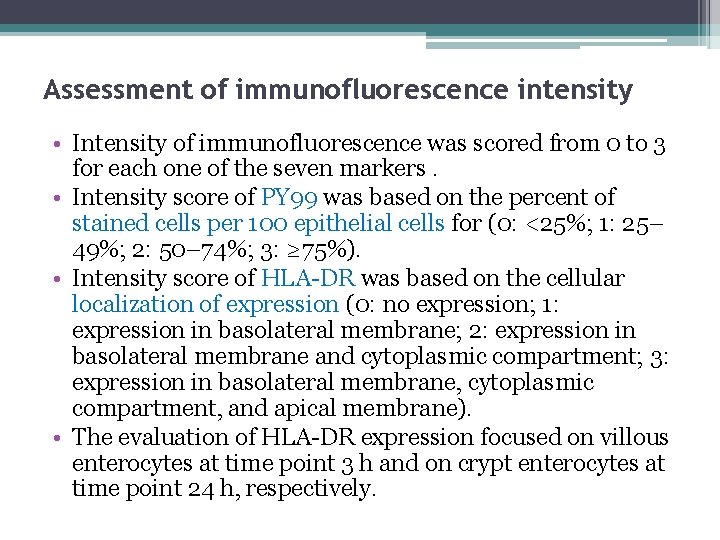
Assessment of immunofluorescence intensity • Intensity of immunofluorescence was scored from 0 to 3 for each one of the seven markers. • Intensity score of PY 99 was based on the percent of stained cells per 100 epithelial cells for (0: <25%; 1: 25– 49%; 2: 50– 74%; 3: ≥ 75%). • Intensity score of HLA-DR was based on the cellular localization of expression (0: no expression; 1: expression in basolateral membrane; 2: expression in basolateral membrane and cytoplasmic compartment; 3: expression in basolateral membrane, cytoplasmic compartment, and apical membrane). • The evaluation of HLA-DR expression focused on villous enterocytes at time point 3 h and on crypt enterocytes at time point 24 h, respectively.

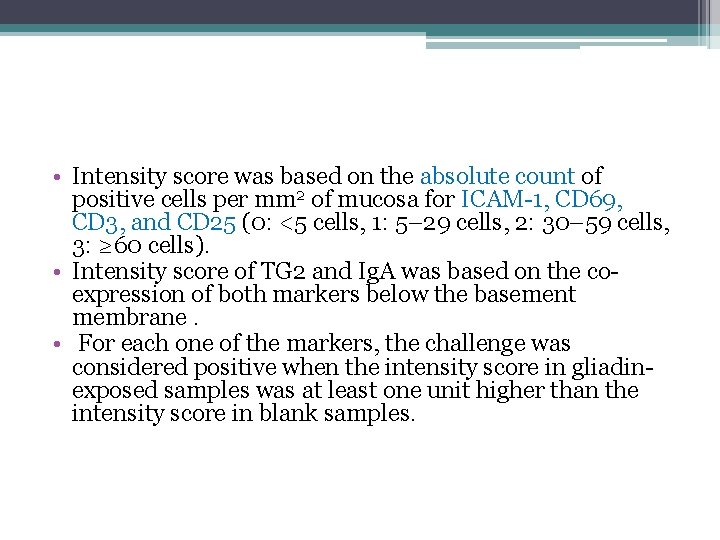
• Intensity score was based on the absolute count of positive cells per mm 2 of mucosa for ICAM-1, CD 69, CD 3, and CD 25 (0: <5 cells, 1: 5– 29 cells, 2: 30– 59 cells, 3: ≥ 60 cells). • Intensity score of TG 2 and Ig. A was based on the coexpression of both markers below the basement membrane. • For each one of the markers, the challenge was considered positive when the intensity score in gliadinexposed samples was at least one unit higher than the intensity score in blank samples.

Statistical analysis • Analysis of variance was used for comparisons of continuous variables among the groups. • For each marker, the rate of positive cases in negative controls and of negative cases in positive controls was used to calculate the area under the receptor-operated curve (ROC).

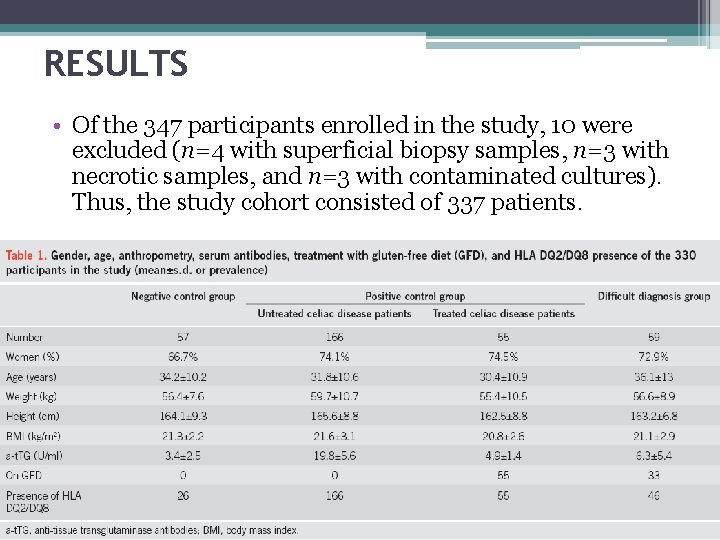
RESULTS • Of the 347 participants enrolled in the study, 10 were excluded (n=4 with superficial biopsy samples, n=3 with necrotic samples, and n=3 with contaminated cultures). Thus, the study cohort consisted of 337 patients.

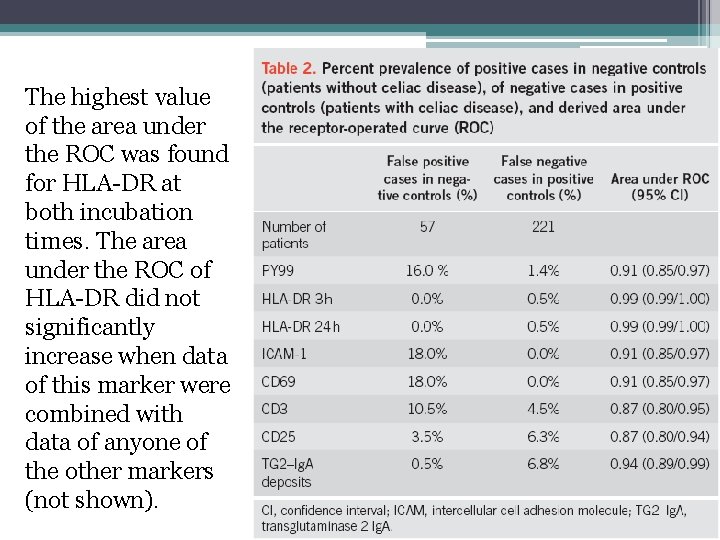
Challenge in negative controls and positive controls • Table 1 reports for each marker the prevalence of positive cases (i. e. , false-positive cases) in negative controls and of negative cases positive controls (i. e. , false-negative cases). • PY 99 had the second highest rate of false-positive cases. • HLA-DR had no false-positive case at both times of incubation and only one false-negative case. • ICAM-1 and CD 69 had the highest rate of false-positive cases and no false-negative case. • CD 3 and CD 25 had high rates of false-positive cases and of false-negative cases. • TG 2–Ig. A deposits had the highest rate of false-negative cases.

The highest value of the area under the ROC was found for HLA-DR at both incubation times. The area under the ROC of HLA-DR did not significantly increase when data of this marker were combined with data of anyone of the other markers (not shown).

Examples of immunostaining in samples of negative and positive controls after incubation with gliadin-free medium (blank) and with gliadinrich medium (PT-gliadin). ICAM, intercellular cell adhesion molecule; TG 2/Ig. A, transglutaminase 2/Ig. A.

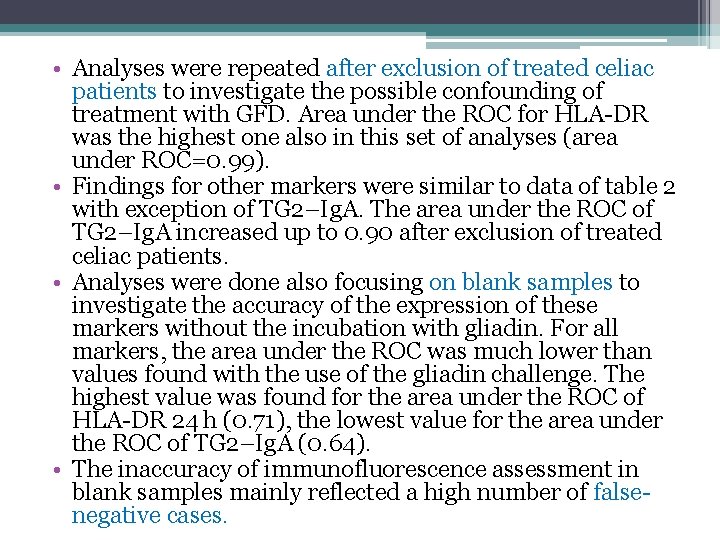
• Analyses were repeated after exclusion of treated celiac patients to investigate the possible confounding of treatment with GFD. Area under the ROC for HLA-DR was the highest one also in this set of analyses (area under ROC=0. 99). • Findings for other markers were similar to data of table 2 with exception of TG 2–Ig. A. The area under the ROC of TG 2–Ig. A increased up to 0. 90 after exclusion of treated celiac patients. • Analyses were done also focusing on blank samples to investigate the accuracy of the expression of these markers without the incubation with gliadin. For all markers, the area under the ROC was much lower than values found with the use of the gliadin challenge. The highest value was found for the area under the ROC of HLA-DR 24 h (0. 71), the lowest value for the area under the ROC of TG 2–Ig. A (0. 64). • The inaccuracy of immunofluorescence assessment in blank samples mainly reflected a high number of falsenegative cases.

Gliadin challenge results in difficult diagnosis group • Thirty-nine patients of the difficult diagnosis group accepted to stop GFD after the gliadin challenge to undergo a re-assessment of the celiac disease-specific serology. • Table 3 reports data about accuracy of markers of the gliadin challenge in these 39 patients divided by presence/absence of celiac disease-specific antibodies in the analyses repeated under untreated conditions. • The comparison between these two subgroups tended to give results, which were similar to the results of the comparison between negative controls and positive controls. HLA-DR had the highest area under the ROC also in this set of analyses.



DISCUSSION • The present study shows that in the gliadin challenge test, HLA-DR has high accuracy for celiac disease diagnosis because of high specificity and high sensitivity. • Findings for this marker were consistent at both incubation times, in the absence and in the presence of treatment with GFD, and in patients with difficult diagnosis because of non-concordant tests and/or the confounding of GFD initiated before an appropriate diagnosis of celiac disease. • Findings for the other markers of in vitro mucosal response to gliadin were less accurate and did not increase the accuracy of information derived by the evaluation of HLA-DR alone.

• The data from this study indicate the diagnostic accuracy of the in vitro gliadin challenge in the identification of celiac disease in the event of a difficult diagnosis. • In the present study, we observed a clear modification in the expression of markers of the Tcell immune response following in vitro gliadin challenge almost exclusively in celiac disease. • We experienced a 3% failure rate in the ability to cultivate biopsies for the in vitro challenge with gliadin; however, standardized procedures may lower the percentage of these technical problems (such as obtaining superficial tissue samples).

• Several patients in the difficult diagnosis group were on GFD without any evidence of celiac disease , demonstrating that a number of patients who receive a diagnosis of celiac disease on the basis of negative specific serum markers and minimal alteration of mucosa at histology are unlikely to have celiac disease. • In our hands, TG 2–Ig. A deposits had high accuracy only when analyses were limited to patients who were not on GFD

• According to previous studies, full appraisal of the immunological response to gliadin should be conducted analyzed for a number of markers for up to 24 h. On the basis of our findings, we suggest a gliadin challenge with testing of HLA-DR alone and only for 3 h of culture with gliadin. • This simplified in vitro gliadin challenge might be less an expensive procedure than repeated endoscopies and biopsies. Although in this simplified form the gliadin challenge should be performed strictly limited to cases in which a GFD or non-concordant tests interfere with a correct diagnosis of celiac disease. • Further investigation is needed on the in vitro gliadin challenge in non-celiac gluten-sensitive individuals.

Conclusion • The in vitro gliadin challenge is not only a model for studying the mechanisms of the mucosal immune response in celiac disease but also a useful tool for reaching a final diagnosis in “difficult” cases of celiac disease, in which sensitivity to gluten is suspected but undemonstrated by standard diagnostic tests.

Thank you for your attention
 Omega-5-gliadin
Omega-5-gliadin Budget constraint
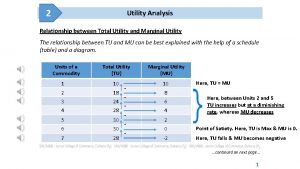
Budget constraint Relation between marginal utility and total utility
Relation between marginal utility and total utility La digestion in vitro du pain par l amylase salivaire
La digestion in vitro du pain par l amylase salivaire Procedure for isolation of cell for in vitro culture
Procedure for isolation of cell for in vitro culture University of pittsburgh
University of pittsburgh Wetboek familierecht
Wetboek familierecht Tumacenje ige
Tumacenje ige Sap netweaver portal vitro
Sap netweaver portal vitro Felice shieh
Felice shieh Fallon sherrock smoking
Fallon sherrock smoking Fluency def
Fluency def Chemistry definition of accuracy
Chemistry definition of accuracy Difference accuracy and precision
Difference accuracy and precision Accuracy, precision, and significant figures worksheet
Accuracy, precision, and significant figures worksheet Absolute uncertainty multiplication
Absolute uncertainty multiplication Perceptual speed and accuracy assessment
Perceptual speed and accuracy assessment Fountas and pinnell benchmark assessment forms
Fountas and pinnell benchmark assessment forms Micro and macro components of accuracy in gis
Micro and macro components of accuracy in gis Poor accuracy good precision
Poor accuracy good precision Quick lab accuracy and precision answers
Quick lab accuracy and precision answers Precision vs uncertainty
Precision vs uncertainty Currency of an article
Currency of an article Three primary privacy issues are accuracy property and
Three primary privacy issues are accuracy property and Credibility accuracy reasonableness support
Credibility accuracy reasonableness support Brainpop precision and accuracy quiz answers
Brainpop precision and accuracy quiz answers Lesson 1-3 reporting with precision and accuracy
Lesson 1-3 reporting with precision and accuracy Active and passive instruments pdf
Active and passive instruments pdf Precision and accuracy
Precision and accuracy