How much wood U W Q Evaluating heat





- Slides: 21

How much wood… ? U -W Q

Evaluating heat engines with PV diagrams

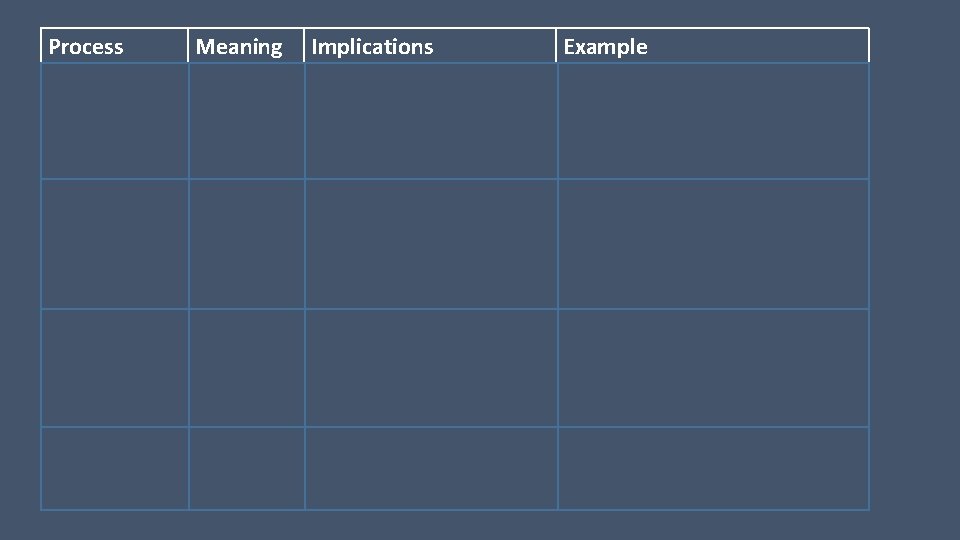
Process Meaning Isobaric P=0 Isovolumetric V=0 Isothermal T=0 Adiabatic Q=0 Implications Example

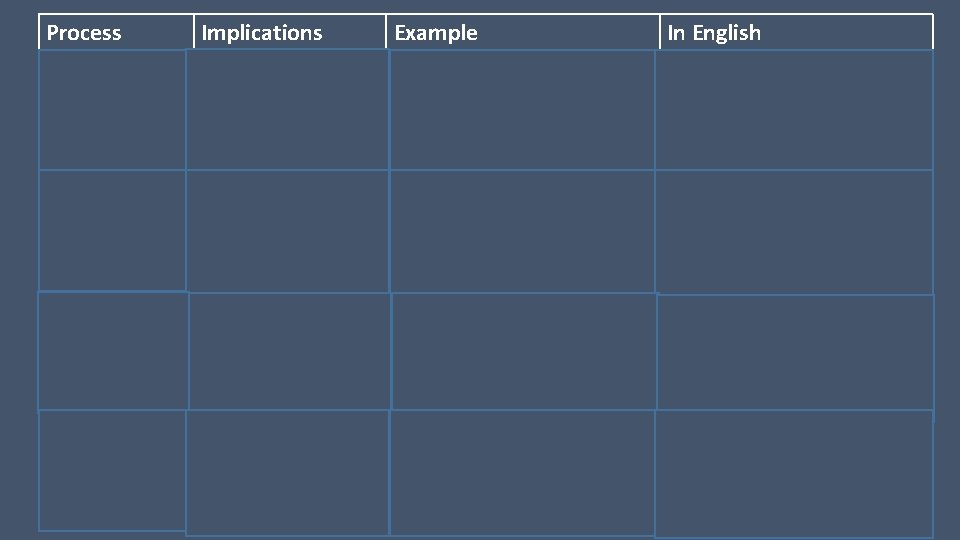
Process Implications Example In English Isobaric To expand (contract) a gas at constant pressure, heat must be added (taken away). Isovolumetric Adding (subtracting) heat to a gas at constant volume increases (decreases) pressure. Isothermal When energy is added to a gas at constant temperature, the gas will expand its pressure will drop. Adiabatic Doing work on a gas decreases its volume, and increases its pressure and temperature.

How much work is done to gas? How much does internal energy change? How much heat is added? Conventions: W+ = work done TO gas by environment • i. e. , gas is compressed, final volume is smaller W- = work done BY gas on environment • i. e. , gas expands, final volume is larger Q+ = heat flows INTO gas Q- = heat flows FROM gas

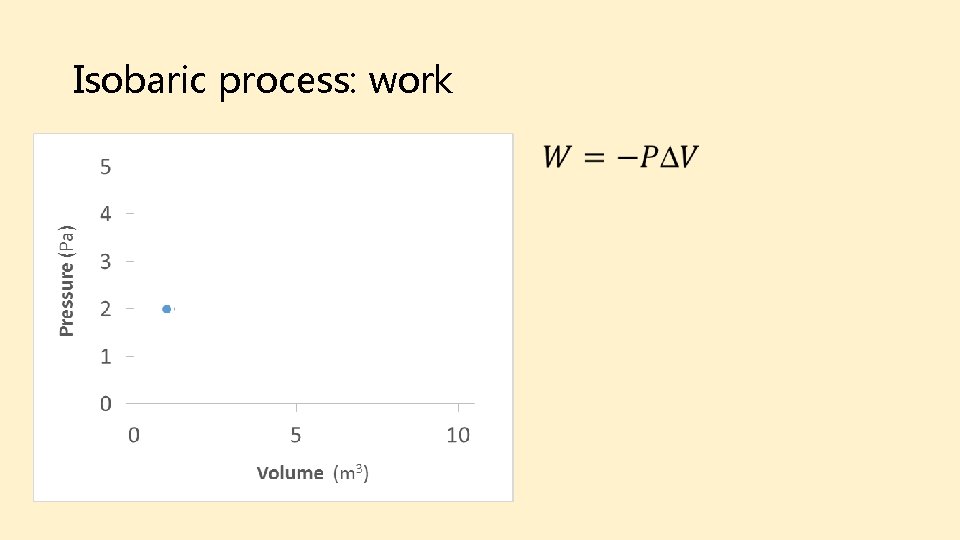
Isobaric process: work Final Initial

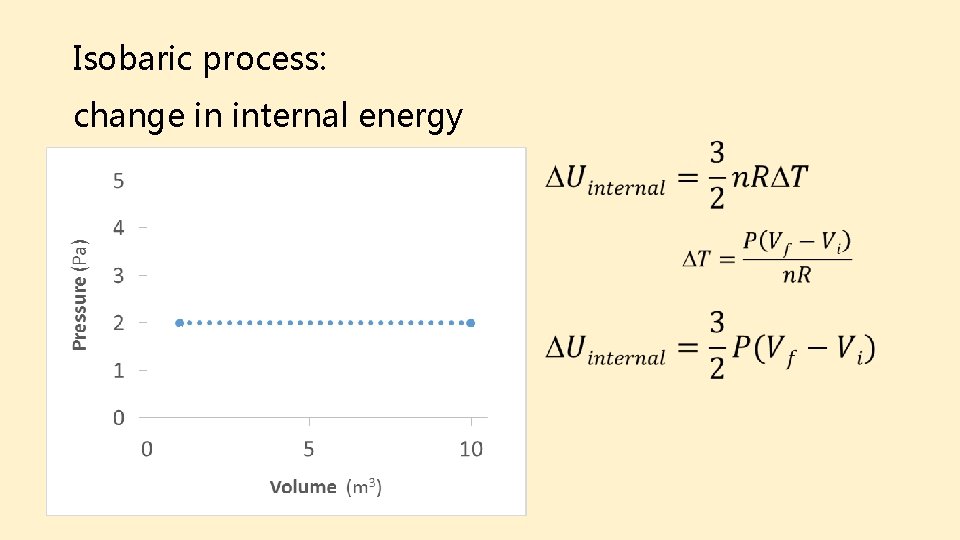
Isobaric process: change in internal energy Final Initial

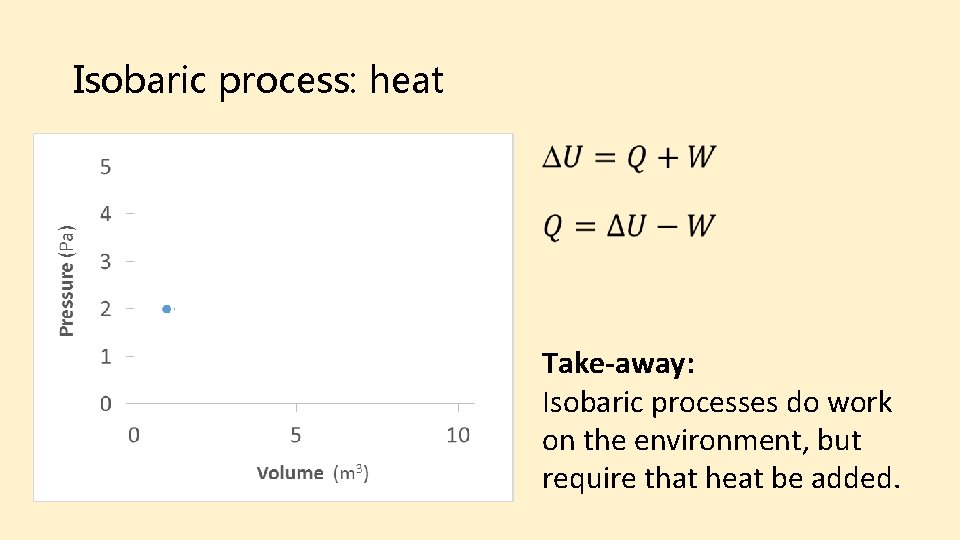
Isobaric process: heat Final Initial Take-away: Isobaric processes do work on the environment, but require that heat be added.

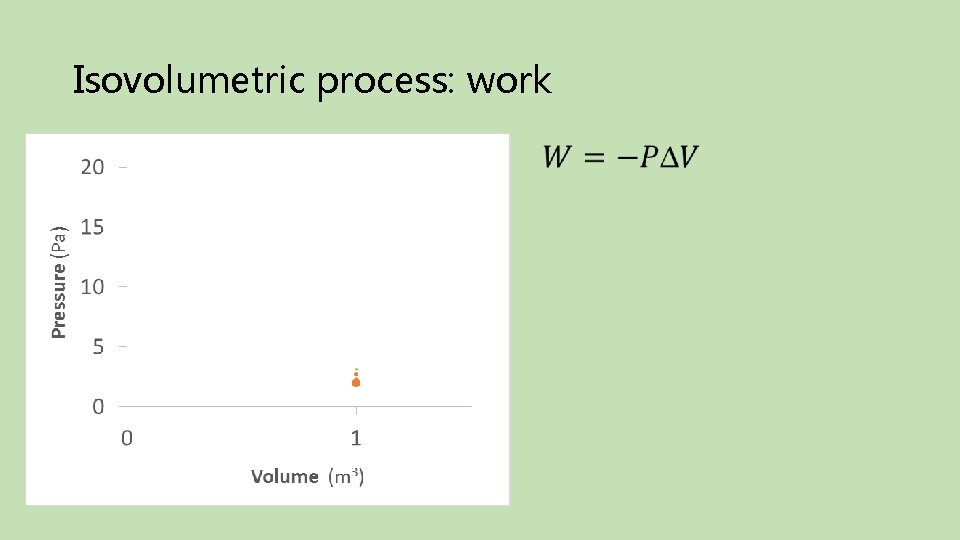
Isovolumetric process: work Final Initial

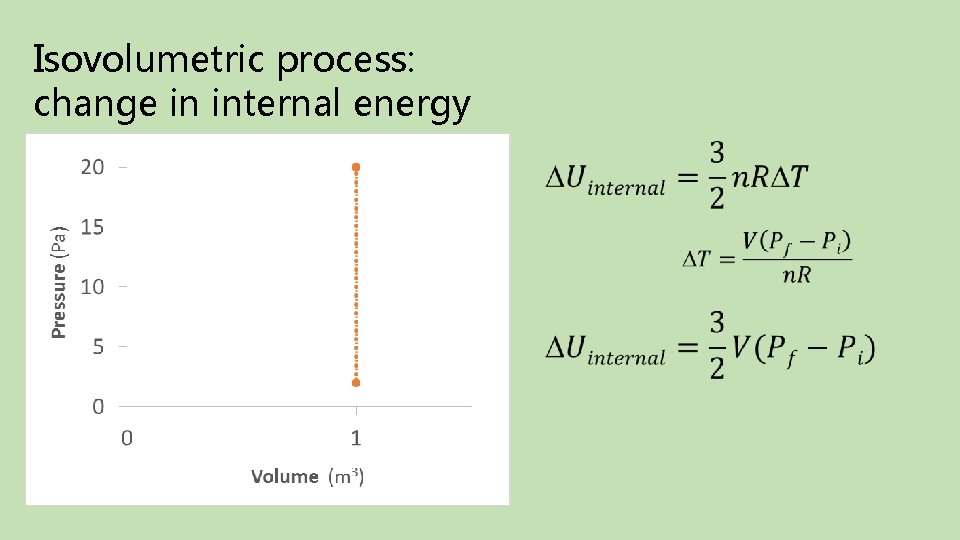
Isovolumetric process: change in internal energy Final Initial

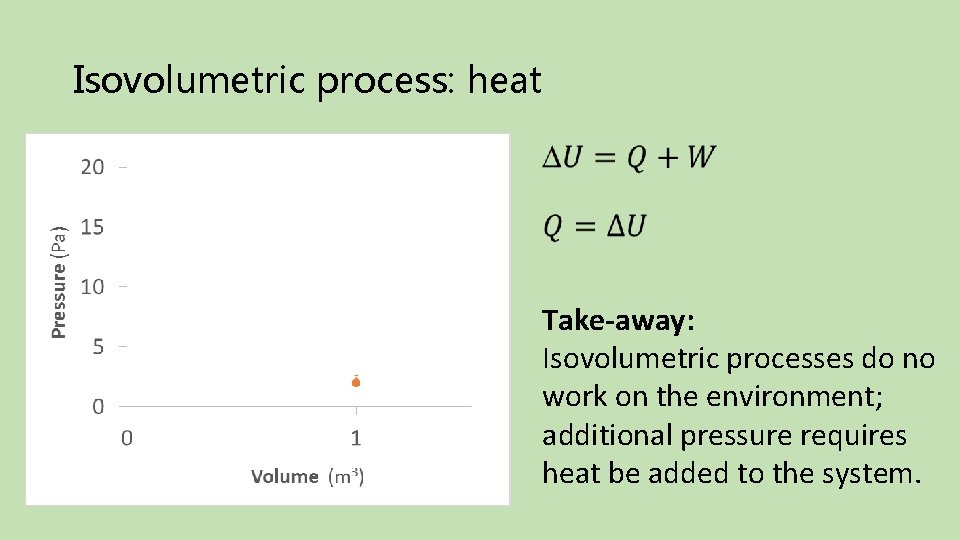
Isovolumetric process: heat Final Initial Take-away: Isovolumetric processes do no work on the environment; additional pressure requires heat be added to the system.

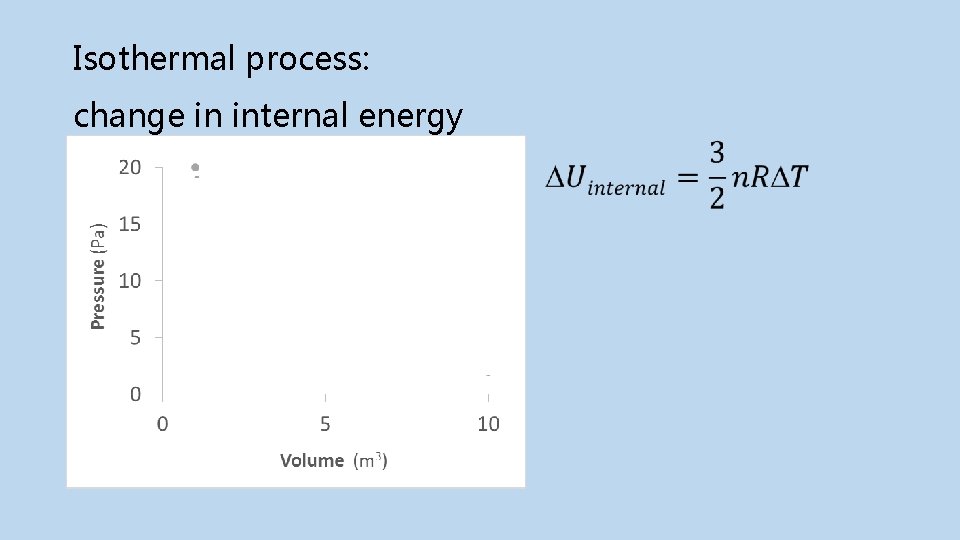
Isothermal process: change in internal energy Final Initial

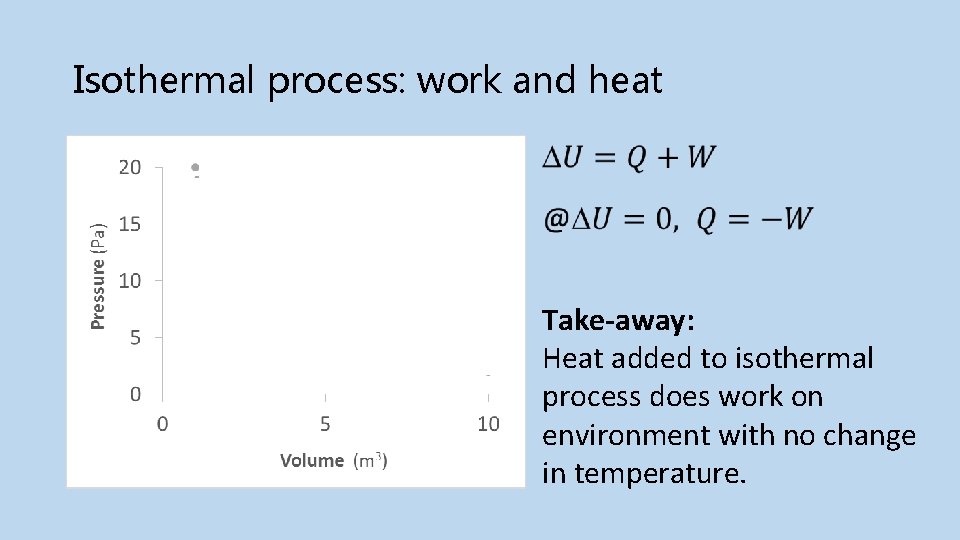
Isothermal process: work and heat Initial Final Take-away: Heat added to isothermal process does work on environment with no change in temperature.

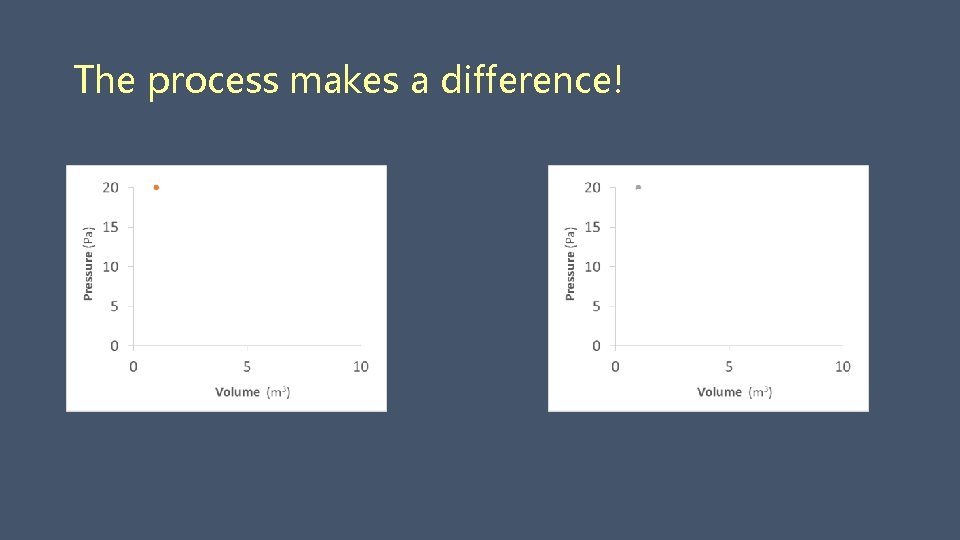
The process makes a difference! Initial Final

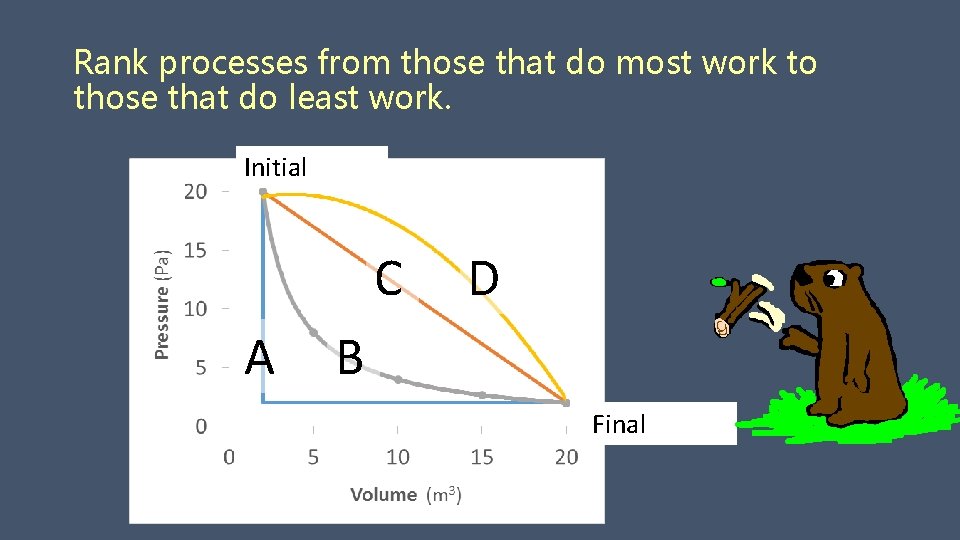
Rank processes from those that do most work to those that do least work. Initial C D A B Final

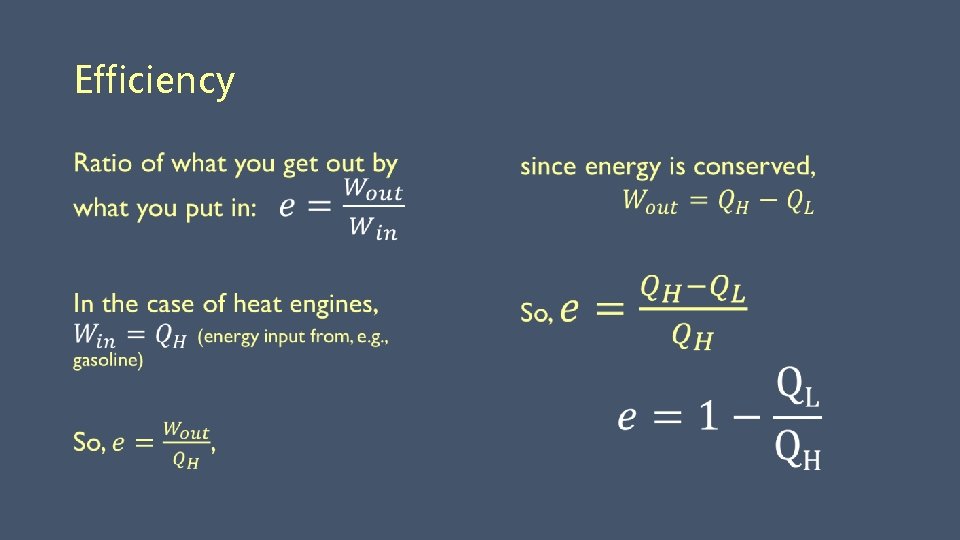
Efficiency

Example An automobile engine has an efficiency of 20% and produces an average of 23, 000 J of mechanical work per second. b) How much heat input is required?

Example An automobile engine has an efficiency of 20% and produces an average of 23, 000 J of mechanical work per second. a) How much energy is discharged as waste heat?

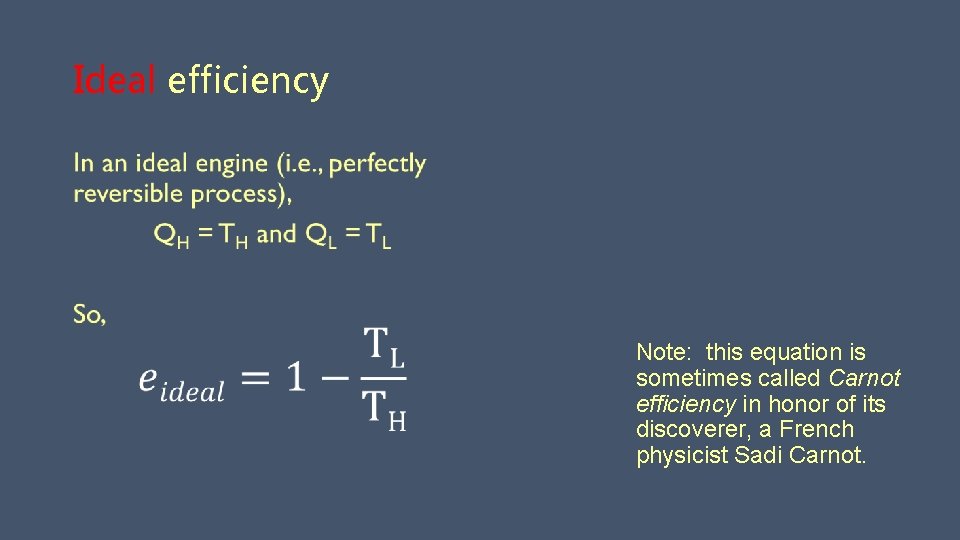
Ideal efficiency Note: this equation is sometimes called Carnot efficiency in honor of its discoverer, a French physicist Sadi Carnot.

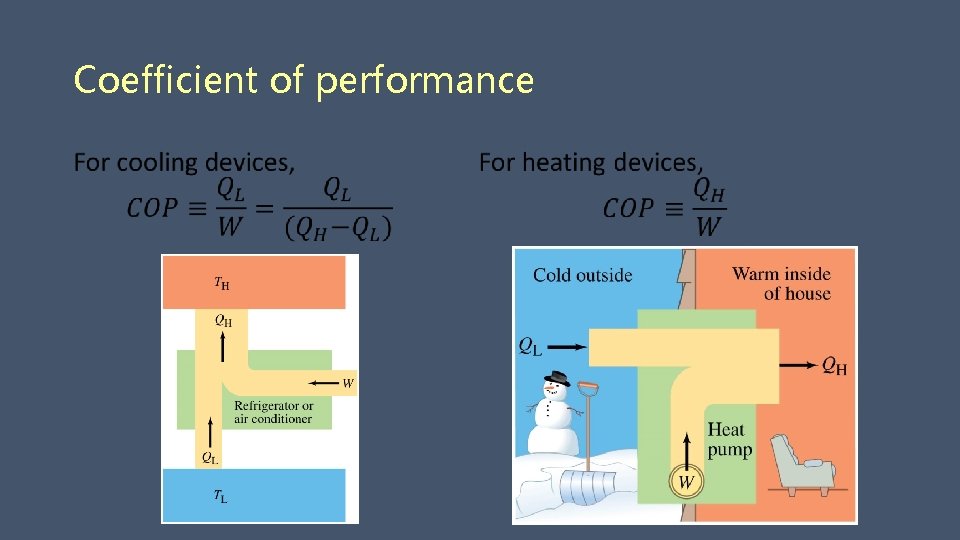
Coefficient of performance

Why heat pumps? a) How much thermal energy is delivered by 1500 -W electric heater? b) How much thermal energy is delivered by 1500 -W heat pump with a COP of 3. 0?