Thermochemistry The study of energy Chapter 6 1



























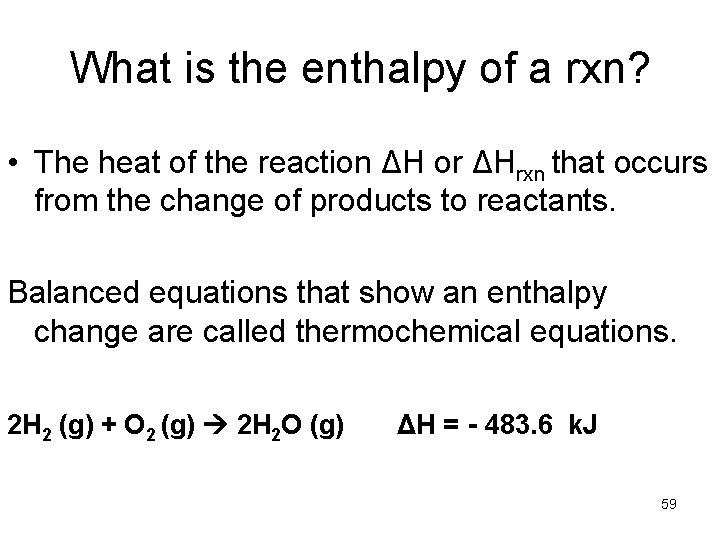





















- Slides: 101

Thermochemistry The study of energy Chapter 6 1

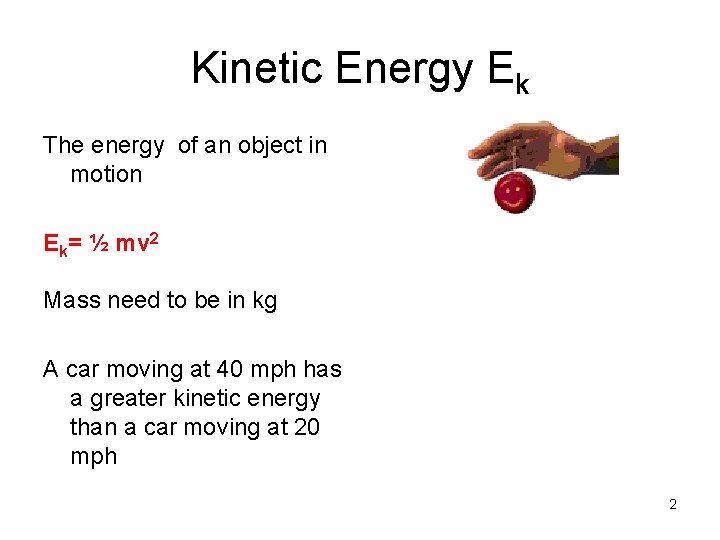
Kinetic Energy Ek The energy of an object in motion Ek= ½ mv 2 Mass need to be in kg A car moving at 40 mph has a greater kinetic energy than a car moving at 20 mph 2

Types of kinetic energy • Work: energy used to cause an object with mass to move • Heat: energy used to cause the temperature of an object to increase 3

Potential Energy • Stored energy in an object by virtue of its position. • Units of energy: Joule J, 1 Calorie Cal = 4. 184 J 4

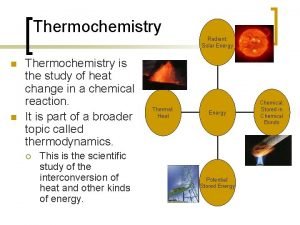
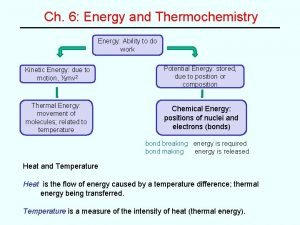
5. 1 The Nature of Energy • Energy is capacity to do work or to produce heat. • Thermochemistry is the study of heat change in chemical reactions. 5

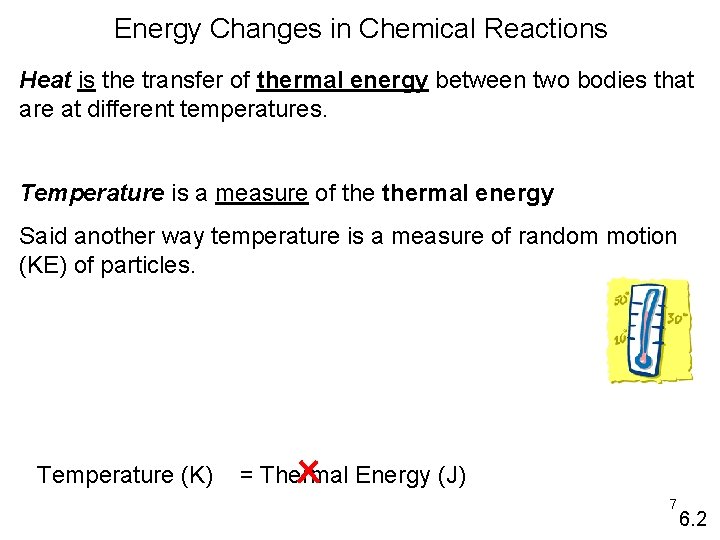
Energy Changes in Chemical Reactions Heat is the transfer of thermal energy between two bodies that are at different temperatures. Temperature is a measure of thermal energy Said another way temperature is a measure of random motion (KE) of particles. Temperature (K) = Thermal Energy (J) 7 6. 2

Physics vs. Chemistry Chemical energy: is the energy stored within the bonds of chemical substances (potential) Thermal energy: is the energy associated with the random motion of atoms and molecules (Kinetic molecular theory) (kinetic) In this chapter we will discuss the transfer of these types of energy. 8

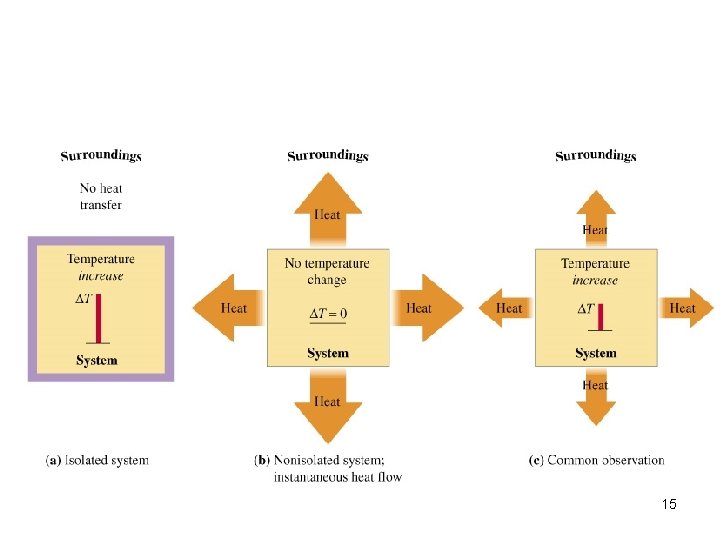
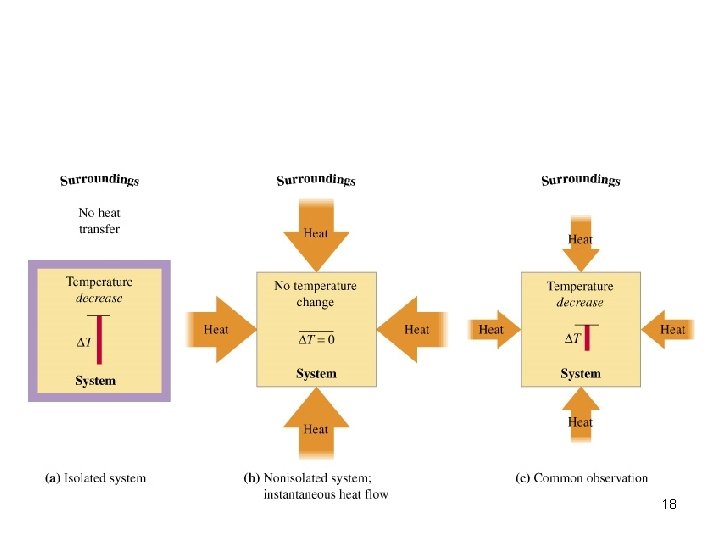
Energy Systems System: portion we single out to study typically the chemical Ex: reactants products. Surroundings: everything else Ex: Reaction vessel, environment 9

10

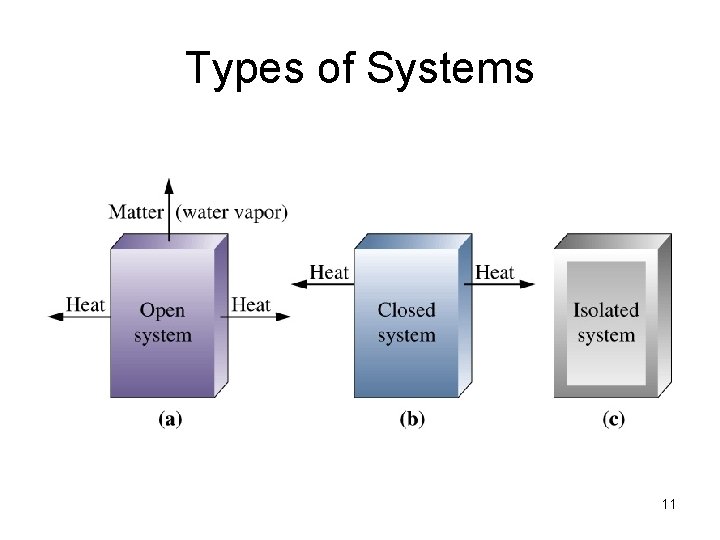
Types of Systems 11

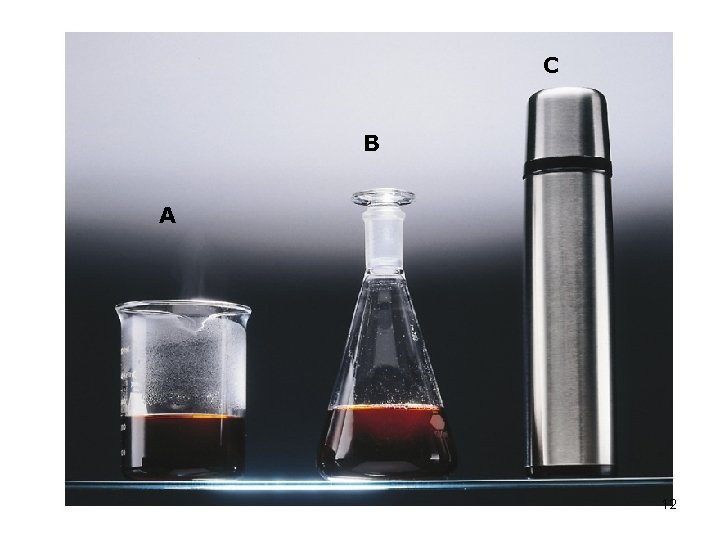
C B A 12

Exothermic A reaction that results in the evolution of heat. Thus heat flows out of the system Exo = out = heat loss from system = energy loss = -heat 13

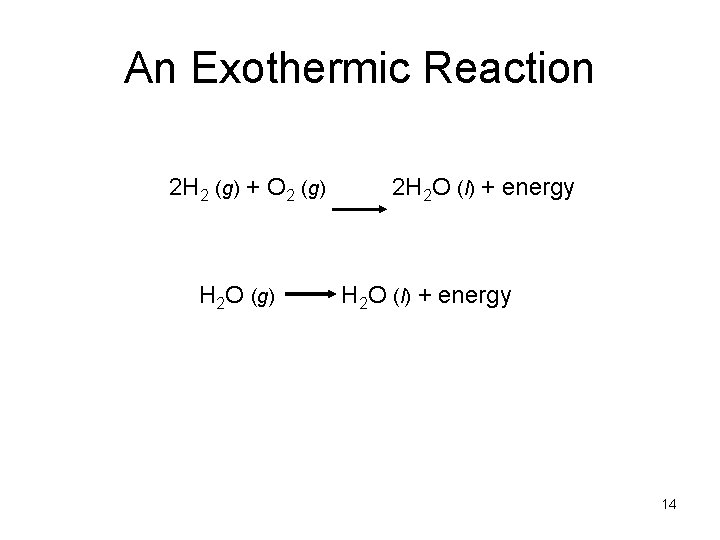
An Exothermic Reaction 2 H 2 (g) + O 2 (g) H 2 O (g) 2 H 2 O (l) + energy 14

15

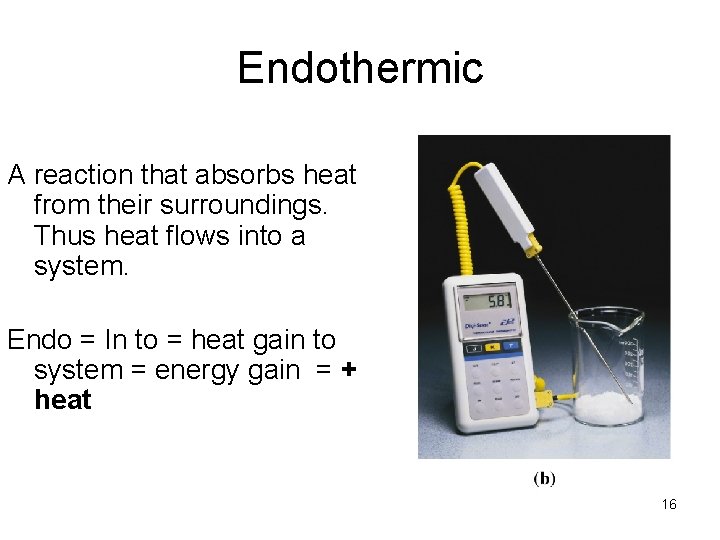
Endothermic A reaction that absorbs heat from their surroundings. Thus heat flows into a system. Endo = In to = heat gain to system = energy gain = + heat 16

An Endothermic Reaction energy + 2 Hg. O (s) 2 Hg (l) + O 2 (g) energy + H 2 O (s) H 2 O (l) 17

18

Work, work • Recall that energy Can be transferred in the form of motion and heat Work = force x distance Units f = Newton, N d = meter, m W = N*m or Joule, J 19

• Force is any kind of push or pull exerted on an object. – Ex: gravity, mechanical, electrostatic • Distance is how far the object moved as a result of the force applied. 20

Work in Biology • Think about water moving from the ground up the trunk of a tree. What part of the system if any undergoes a change in potential energy? Is work done in the process? 21

Guided Questions What is changing location ? Does this change involve potential energy? 22

• Water moves up the trunk against the force of gravity. Thus the potential energy of the water does change • Work is movement of a mass over a distance against an opposing force. 23

24

Homework Chang: pg 255 1, 9, 10 ****BL: Pg 188 • 1, 2, 3, 6, 9, 11, 25

5. 2 First law of thermodynamics Aka: The law of conservation of energy Energy is neither created nor destroyed, thus energy is converted from one form to another. Thus the energy of the universe is constant Universe = System + Surroundings 26

Chemical energy lost by combustion = Energy gained by the surroundings system surroundings 27

Internal Energy • The energy (E) of a system can be defined as the sum of the kinetic and potential energies of all of the particles in a system. • Internal energy can be changed by a flow of HEAT, WORK, or BOTH 29

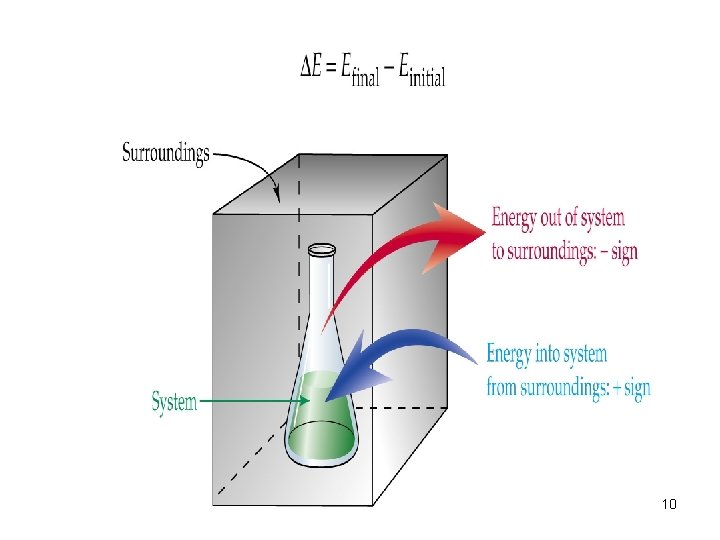
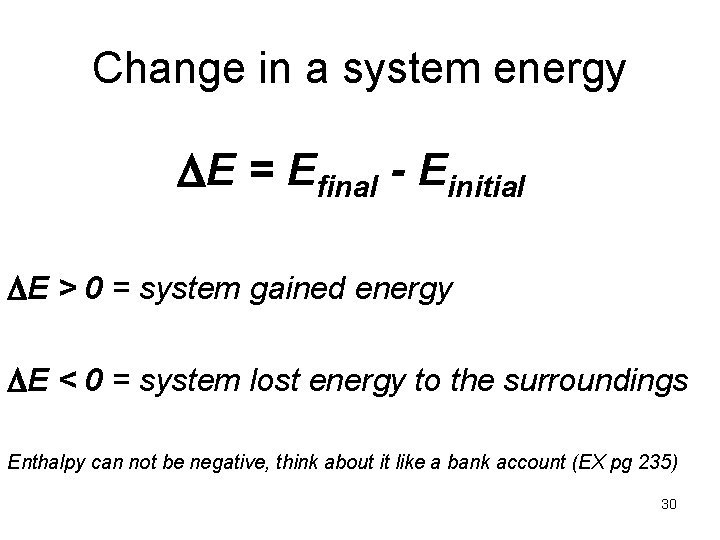
Change in a system energy E = Efinal - Einitial E > 0 = system gained energy E < 0 = system lost energy to the surroundings Enthalpy can not be negative, think about it like a bank account (EX pg 235) 30

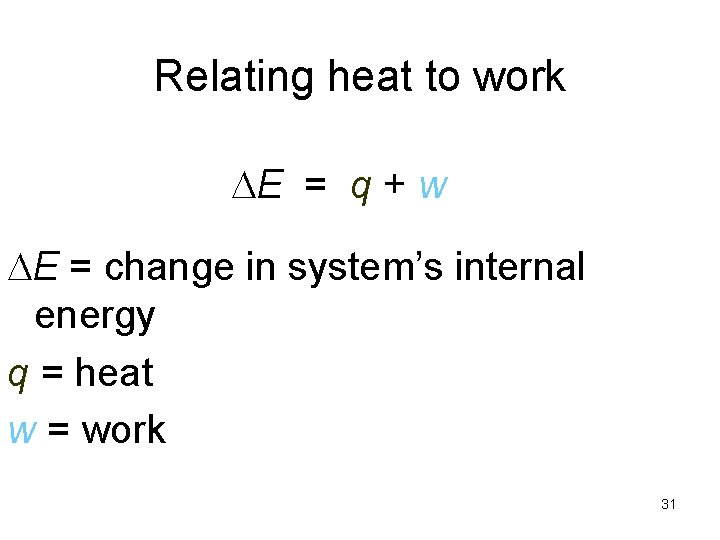
Relating heat to work E = q + w E = change in system’s internal energy q = heat w = work 31

Signs signs every where are signs 32

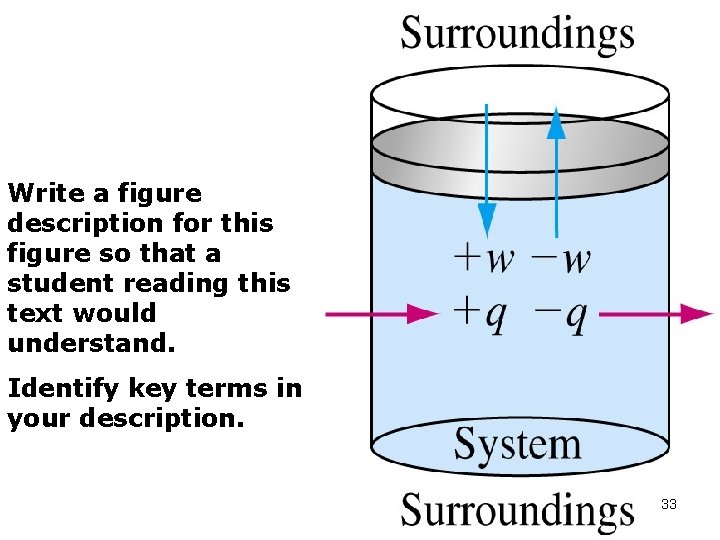
Write a figure description for this figure so that a student reading this text would understand. Identify key terms in your description. 33

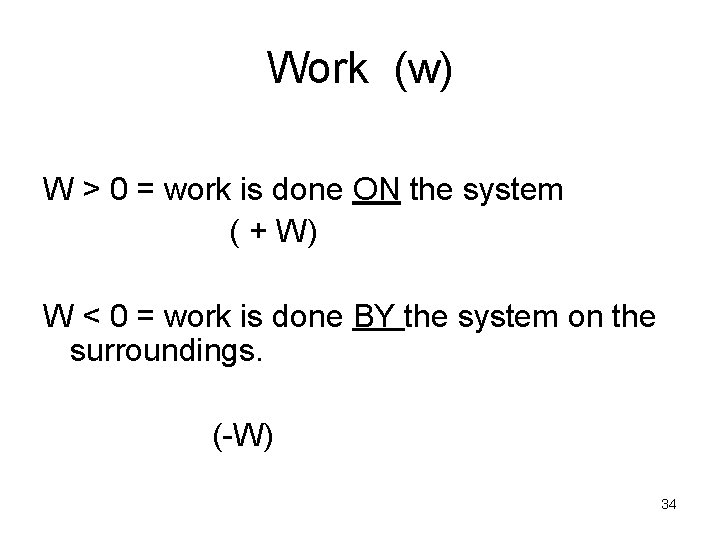
Work (w) W > 0 = work is done ON the system ( + W) W < 0 = work is done BY the system on the surroundings. (-W) 34

Prove it…. • Write an example that illustrates work being done by a system Then write a sentence illustrating work being done on a system 35

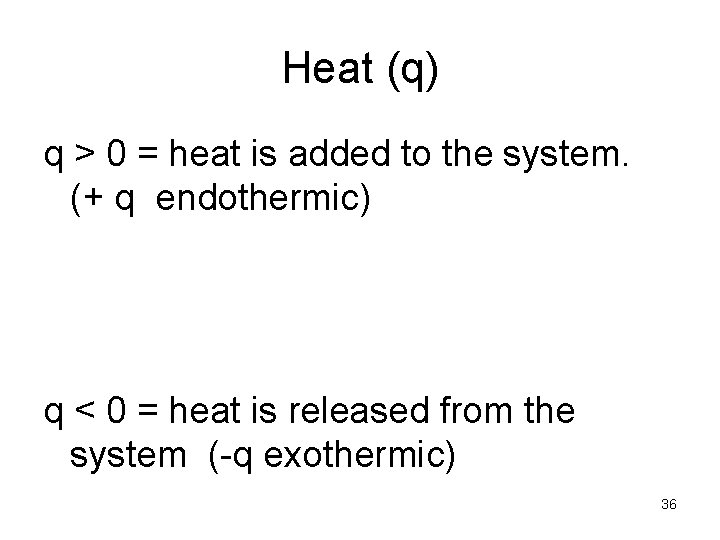
Heat (q) q > 0 = heat is added to the system. (+ q endothermic) q < 0 = heat is released from the system (-q exothermic) 36

Prove it …. • Write an example that illustrates heat being added to a system Then write a sentence illustrating heat being released form a system 37

Example: Calculate E for a system undergoing an endothermic process in which 15. 6 k. J of heat flows and where 1. 4 k. J of work is done by the system. 38

Answer q = + 15. 6 (endothermic) W = - 1. 4 k. J (work is done by the system) E = q + w E = 15. 6 + (- 1. 4) = 14. 2 39

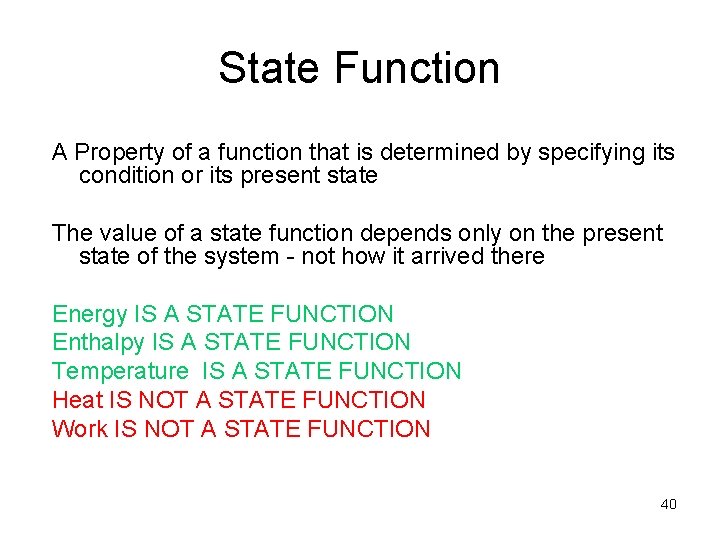
State Function A Property of a function that is determined by specifying its condition or its present state The value of a state function depends only on the present state of the system - not how it arrived there Energy IS A STATE FUNCTION Enthalpy IS A STATE FUNCTION Temperature IS A STATE FUNCTION Heat IS NOT A STATE FUNCTION Work IS NOT A STATE FUNCTION 40

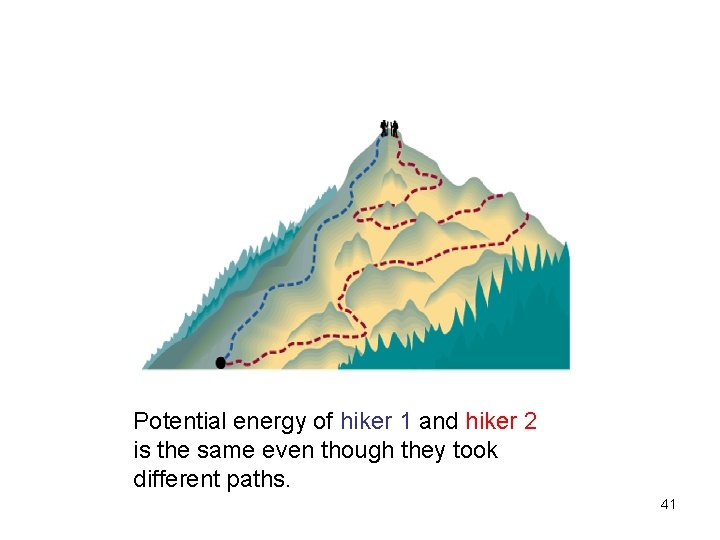
Potential energy of hiker 1 and hiker 2 is the same even though they took different paths. 41

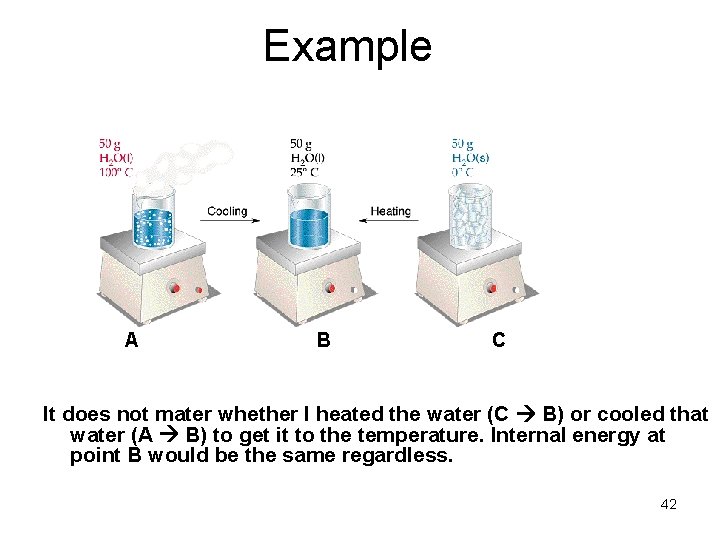
Example A B C It does not mater whether I heated the water (C B) or cooled that water (A B) to get it to the temperature. Internal energy at point B would be the same regardless. 42

Change in energy ΔE • ΔE is a state function because it is independent of pathway. (all we care about is energy at Ei and energy at Ef. Heat (q) and work (w) are not state functions!!!!! 43

Example 44

5. 2 Homework Chang: pg 255 11, 12, 17, 18 ***BL: Pg 189 17, 19, 21, 22, 25, 26 45

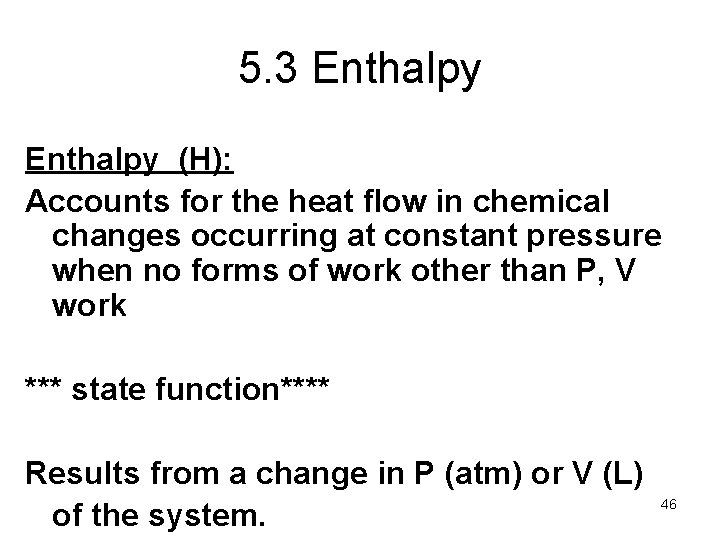
5. 3 Enthalpy (H): Accounts for the heat flow in chemical changes occurring at constant pressure when no forms of work other than P, V work *** state function**** Results from a change in P (atm) or V (L) of the system. 46

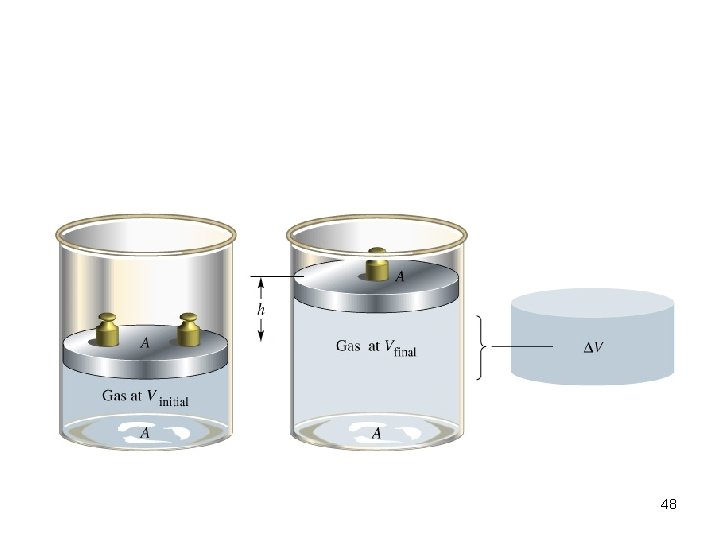
What is Pressure volume work Build up of gas that causes a piston to lift against the force of gravity. Work = -P ΔV 47

48

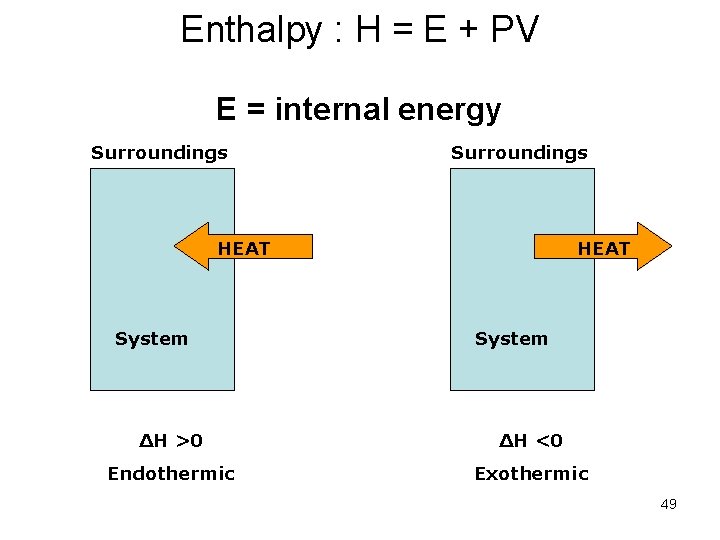
Enthalpy : H = E + PV E = internal energy Surroundings HEAT System ΔH >0 ΔH <0 Endothermic Exothermic 49

Don’t forget you can manipulate these equations Enthalpy = H = E + PV E = H P V H = E + P V 50

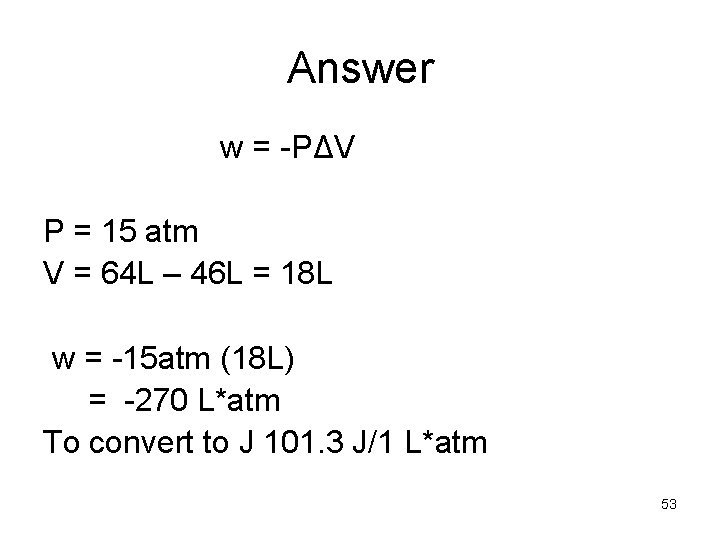
Example: • Calculate the work associated with the expansion of gas from 46 L to 64 L at a constant external pressure of 15 atm. W = -PΔV 52

Answer w = -PΔV P = 15 atm V = 64 L – 46 L = 18 L w = -15 atm (18 L) = -270 L*atm To convert to J 101. 3 J/1 L*atm 53

5. 3 Homework Chang: 15, 16, BL: Pg 190 • #’s 27 A and C only, 29 skip A do B, 30 • HINT ON #30 write a balance chemical reaction first, don’t over think it. 55

5. 4 Enthalpy Changes • Enthalpy: The heat content of a chemical system is called the enthalpy (symbol: H) • The enthalpy change (Δ H) is the amount of heat released or absorbed when a chemical reaction occurs at constant pressure. 56

More enthalpy • Enthalpy, along with the pressure and volume of a system, is a state function (property of a system that depends only on its state, not how it arrived at its present state). 57

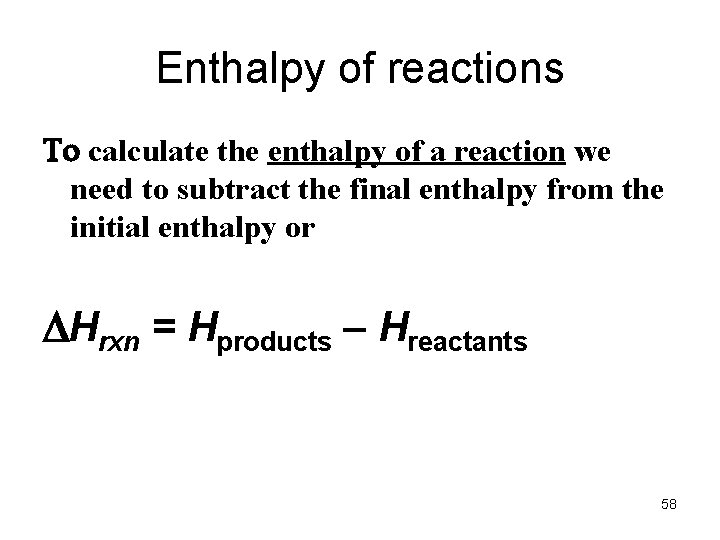
Enthalpy of reactions To calculate the enthalpy of a reaction we need to subtract the final enthalpy from the initial enthalpy or Hrxn = Hproducts – Hreactants 58
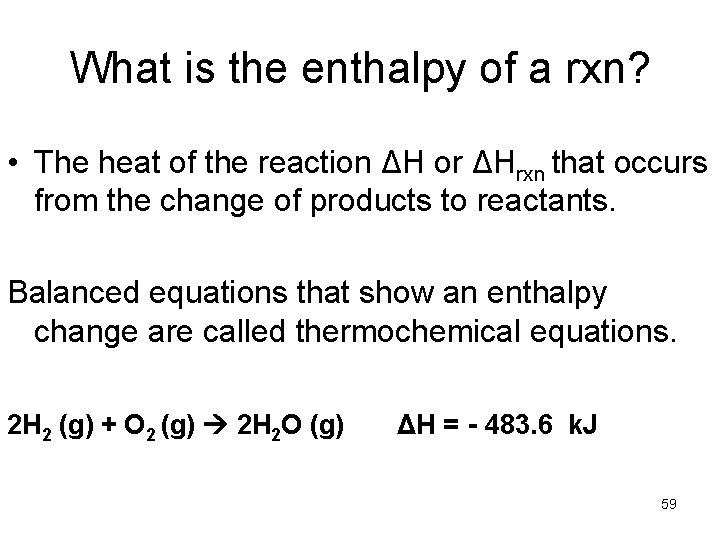
What is the enthalpy of a rxn? • The heat of the reaction ΔH or ΔHrxn that occurs from the change of products to reactants. Balanced equations that show an enthalpy change are called thermochemical equations. 2 H 2 (g) + O 2 (g) 2 H 2 O (g) ΔH = - 483. 6 k. J 59

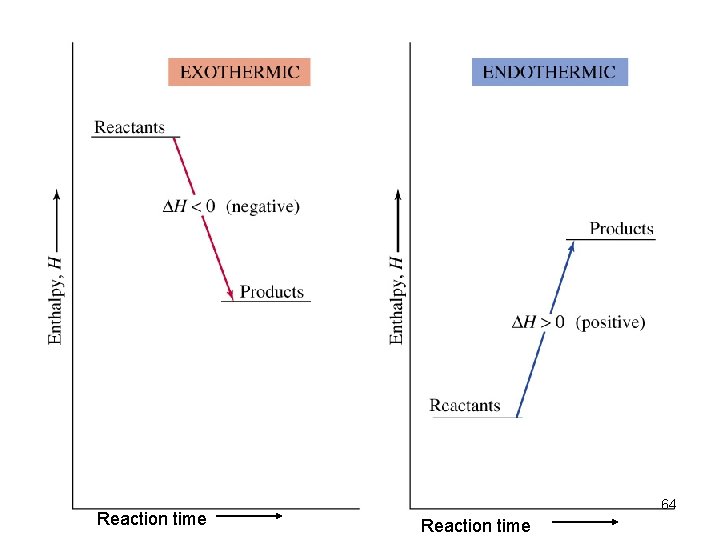
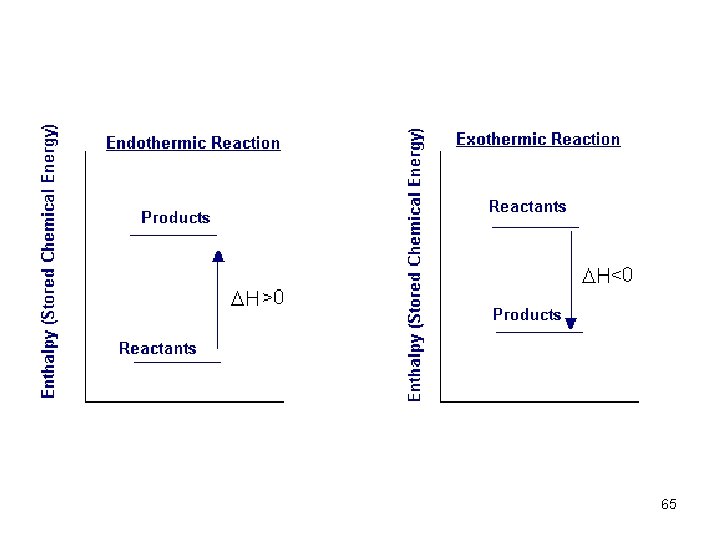
Enthalpy Diagrams • We can use diagrams to illustrate the change in enthalpy of a reaction. Exothermic = - ΔH value Endothermic = + ΔH value 60

Thermochemical Equations Is H negative or positive? System absorbs heat Endothermic H > 0 6. 01 k. J are absorbed for every 1 mole of ice that melts at 00 C and 1 atm. H 2 O (s) H 2 O (l) H = 6. 01 k. J 61 6. 4

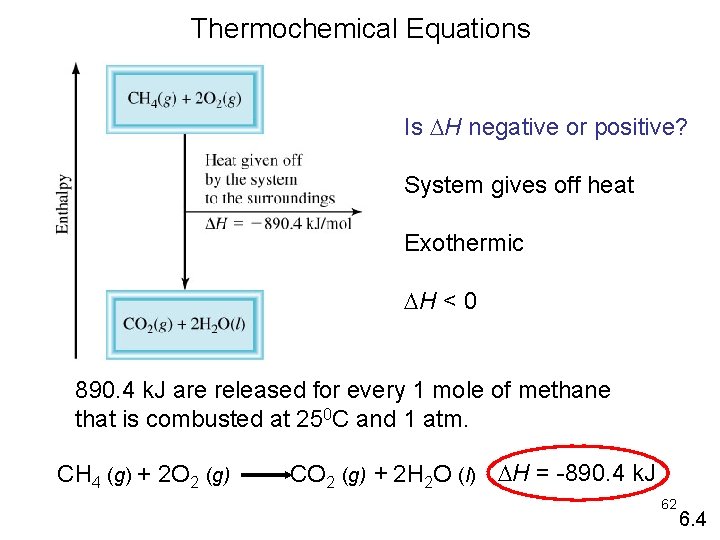
Thermochemical Equations Is H negative or positive? System gives off heat Exothermic H < 0 890. 4 k. J are released for every 1 mole of methane that is combusted at 250 C and 1 atm. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (l) H = -890. 4 k. J 62 6. 4

Reaction time 64 Reaction time

65

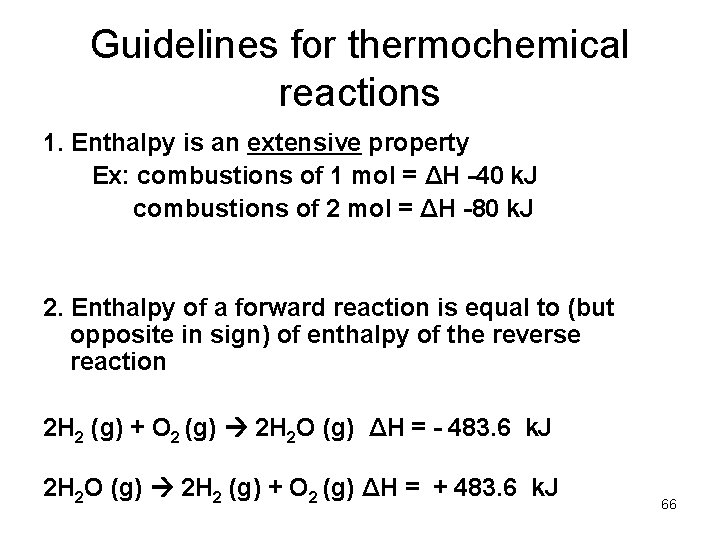
Guidelines for thermochemical reactions 1. Enthalpy is an extensive property Ex: combustions of 1 mol = ΔH -40 k. J combustions of 2 mol = ΔH -80 k. J 2. Enthalpy of a forward reaction is equal to (but opposite in sign) of enthalpy of the reverse reaction 2 H 2 (g) + O 2 (g) 2 H 2 O (g) ΔH = - 483. 6 k. J 2 H 2 O (g) 2 H 2 (g) + O 2 (g) ΔH = + 483. 6 k. J 66

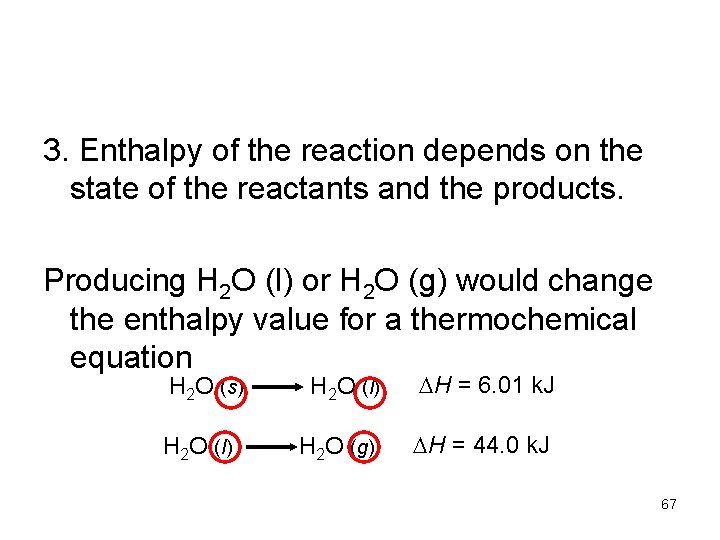
3. Enthalpy of the reaction depends on the state of the reactants and the products. Producing H 2 O (l) or H 2 O (g) would change the enthalpy value for a thermochemical equation H 2 O (s) H 2 O (l) H = 6. 01 k. J H 2 O (g) H = 44. 0 k. J 67

Steps CH 4 (g) + 2 O 2(g) CO 2 (g) + 2 H 2 O (l) ΔH = -890 k. J 1. Use mole ratios to establish a proportion for every 1 mol CH 4 (g) burned -890 k. J of heat is produced 2. Use dimensional analysis to convert g to mol to heat. (since we know heat production from 1 mole of CH 4) 4. 50 g CH 4 1 mol CH 4 16. 0 g CH 4 -890 k. J 1 mol CH 4 = -250 k. J 69

Homework Chang: BL: Pg: 190 #’s 31, 33, 36, 37, 40 Read 5. 5 Calorimetry 70

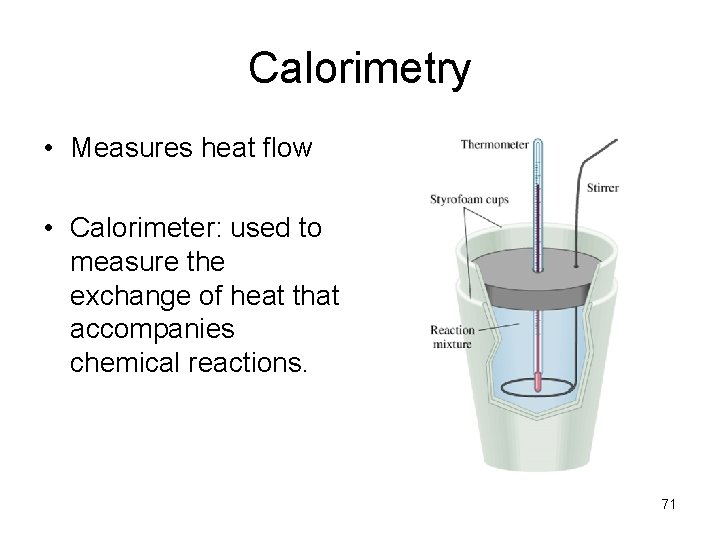
Calorimetry • Measures heat flow • Calorimeter: used to measure the exchange of heat that accompanies chemical reactions. 71

How it works • Reaction using known quantities of reactants is conducted in an insulated vessel that is submerge in a known qty of water. • The heat created from the reaction will increase the temperature of the water surrounding the vessel. • The amount of heat emitted can be calculated using the total heat capacity of the calorimeter and its contents. 72

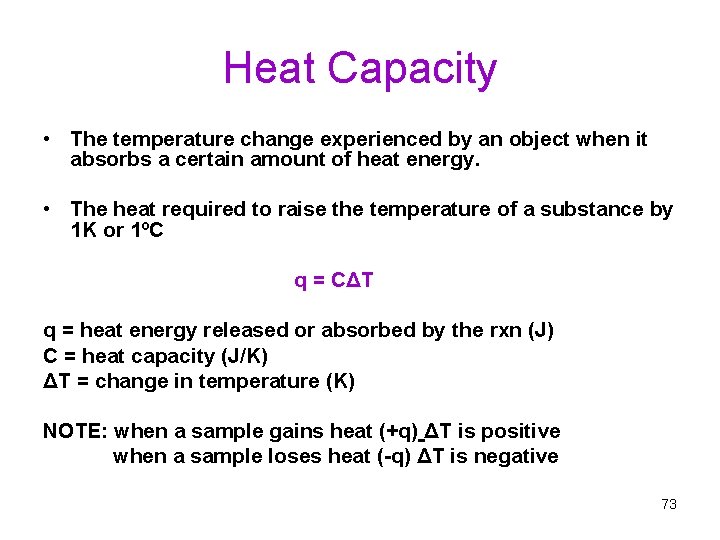
Heat Capacity • The temperature change experienced by an object when it absorbs a certain amount of heat energy. • The heat required to raise the temperature of a substance by 1 K or 1ºC q = CΔT q = heat energy released or absorbed by the rxn (J) C = heat capacity (J/K) ΔT = change in temperature (K) NOTE: when a sample gains heat (+q) ΔT is positive when a sample loses heat (-q) ΔT is negative 73

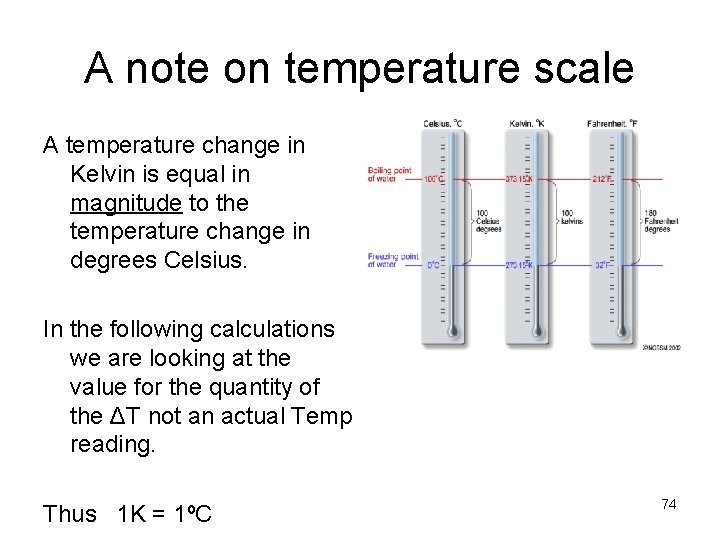
A note on temperature scale A temperature change in Kelvin is equal in magnitude to the temperature change in degrees Celsius. In the following calculations we are looking at the value for the quantity of the ΔT not an actual Temp reading. Thus 1 K = 1ºC 74

Molar Heat Capacity • The heat capacity of one mole of a substance is called its molar heat capacity. • If the molar heat capacity for 1 mol =20 J/mol*K Then the molar heat capacity for 10 moles = 20(10) 200 J/mol*K (20 J/mol*K for each mole present in the reaction) 75

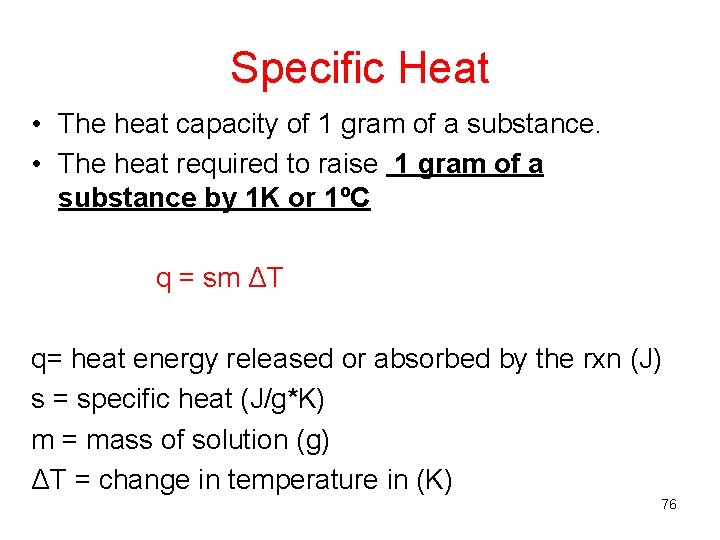
Specific Heat • The heat capacity of 1 gram of a substance. • The heat required to raise 1 gram of a substance by 1 K or 1ºC q = sm ΔT q= heat energy released or absorbed by the rxn (J) s = specific heat (J/g*K) m = mass of solution (g) ΔT = change in temperature in (K) 76

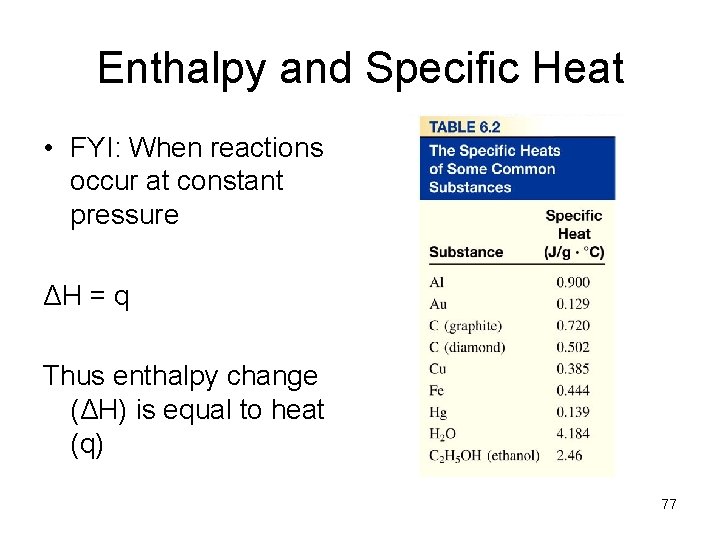
Enthalpy and Specific Heat • FYI: When reactions occur at constant pressure ΔH = q Thus enthalpy change (ΔH) is equal to heat (q) 77

How much heat is given off when an 869 g iron bar cools from 940 C to 50 C? s of Fe = 0. 444 J/g • 0 C t = tfinal – tinitial = 50 C – 940 C = -890 C q = ms t = 869 g x 0. 444 J/g • 0 C x – 890 C = -34, 000 J 78 6. 5

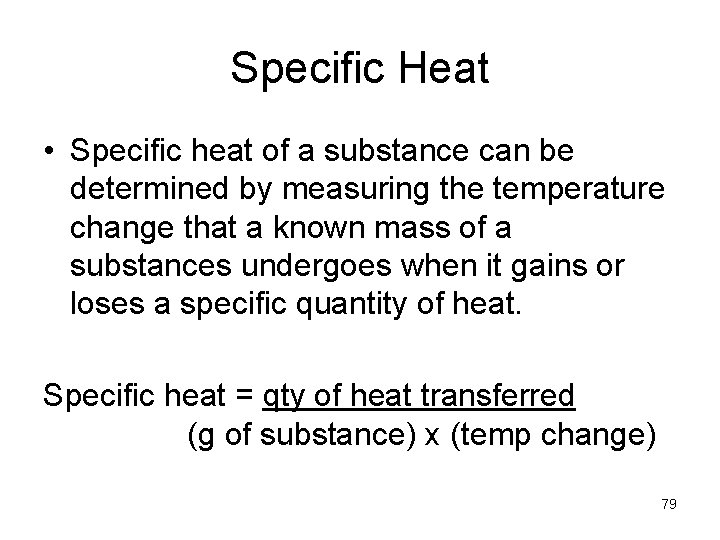
Specific Heat • Specific heat of a substance can be determined by measuring the temperature change that a known mass of a substances undergoes when it gains or loses a specific quantity of heat. Specific heat = qty of heat transferred (g of substance) x (temp change) 79

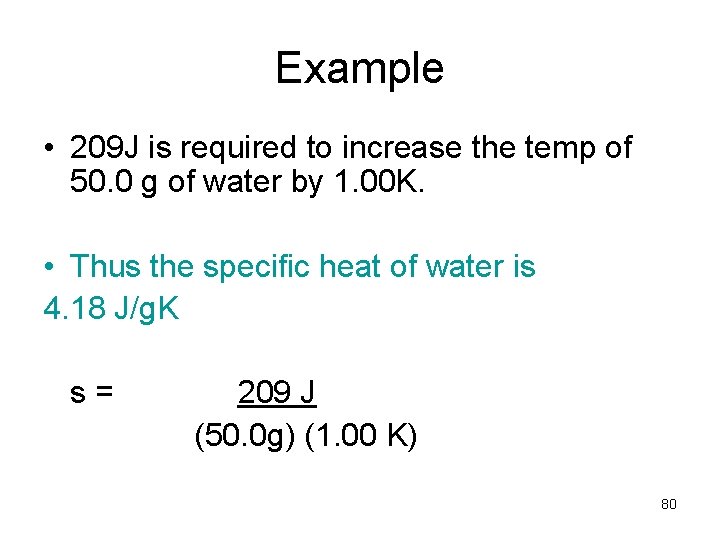
Example • 209 J is required to increase the temp of 50. 0 g of water by 1. 00 K. • Thus the specific heat of water is 4. 18 J/g. K s= 209 J (50. 0 g) (1. 00 K) 80

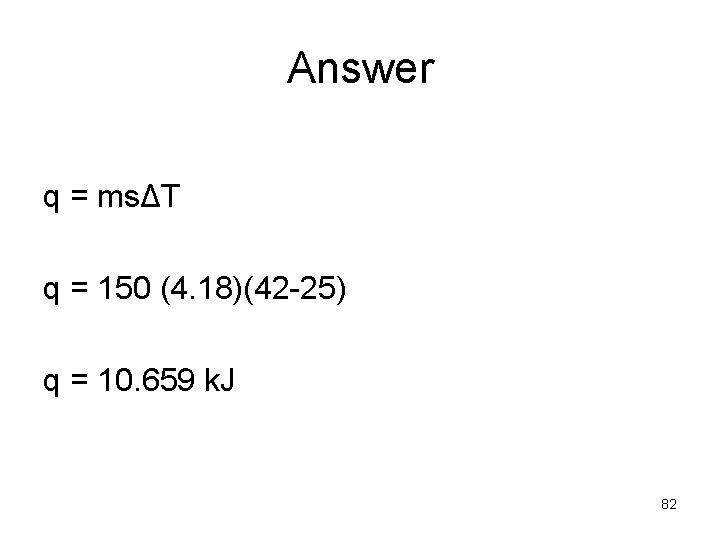
Example: • How much heat in k. J is required to increase the temperature if 150 g of water from 25°C to 42°C. Specific heat of water is 4. 18 J/g°C. (Memorize specific heat water) 81

Answer q = msΔT q = 150 (4. 18)(42 -25) q = 10. 659 k. J 82

Molar Heat Capacity Example • If you have 1 mol of water with a specific heat of 4. 18 J/g*K, the molar heat capacity of water is. • 4. 18 J/g*K (18. 0 g) = 75. 2 J/mol*K • 1 mol • What if you have 26 moles? 83

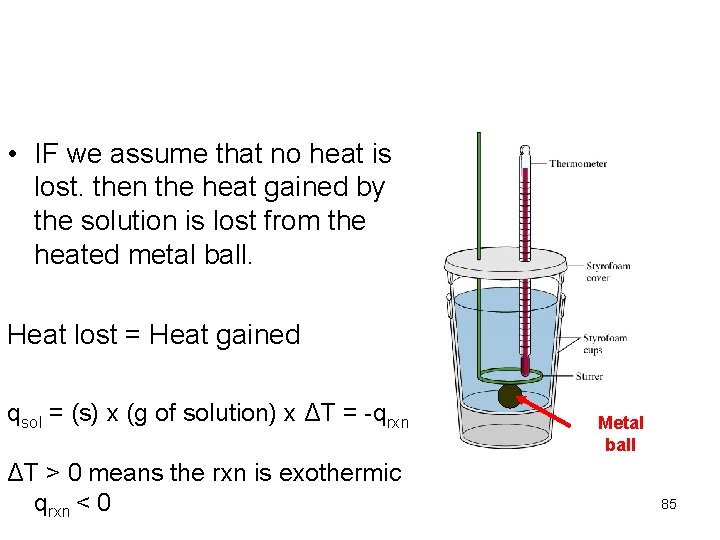
• IF we assume that no heat is lost. then the heat gained by the solution is lost from the heated metal ball. Heat lost = Heat gained qsol = (s) x (g of solution) x ΔT = -qrxn ΔT > 0 means the rxn is exothermic qrxn < 0 Metal ball 85

Example • When a student mixes 50 m. L of 1. 0 M HCl and 50 m. L of 1. 0 M Na. OH in a coffee cup calorimeter the temperature of the resulting 100 m. L solution increases from 21ºC to 27. 5 ºC. Calculate the enthalpy change for the reaction assuming that the calorimeter loses only a negligible amount of heat. The density of the solution is 1. 0 g/m. L with a specific heat of 4. 18 J/g*K 86

50 m. L 1. 0 M HCl + 50 m. L 1. 0 M Na. OH 100 m. L = q = smΔT ΔT = 27. 5 -21. 0 = 6. 5 S = 4. 18 J/g*K D = 1. 0 g/m. L m = 100 m. L (1. 0 g) = 100 g 1 m. L q = (4. 18 J/g. K)(100 g)(6. 5) = 2. 7 x 103 J Now we must go back to the problem and determine if the heat (q) is endothermic (+) or exothermic (-) Because the temperature increases heat is being released and the reaction is exothermic FINAL ANSWER q = = - 2. 7 x 103 J 87

One step further We solved for how much energy was produced from 100 g of solution… q = = - 2. 7 x 103 J What if the question asked for heat produced from 1 mol HCl? (0. 05 L HCl)(1. 0 mol HCl) = 0. 05 mol HCl in solution 1 L HCl -2. 7 103 J/ (0. 050 mol HCl) = -54000 J/mol of HCl 88

A coffee cup calorimeter initially contains 125 g of water at 24. 2°C. Potassium bromide (10. 5 g) also at 24. 2°C is added to the water, and after the KBr dissolves, the final temp is 21. 1°C. Calculate the enthalpy change for dissolving the salt in J. Assume that the specific heat capacity of the solution is 4. 184 J/°Cg and that no heat transferred to the surroundings or to the calorimeter. 89

q = msΔT (think m total) m = 125 g H 2 O + 10. 5 KBr = 135. 5 g total q= 135. 5 (4. 184)(21. 1 - 24. 2) q = -1757. 4 J 90

*******Example***** A 46. 2 g sample of copper is heated to 95. 4 ºC and then placed into a calorimeter containing 75. 0 g of water at 19. 6 ºC. The final temp of the metal and water was 21. 8 ºC. Calculate the specific heat of copper, assuming that all the heat lost by copper is gained by water. 91

Assume: Heat lost by copper = heat gained by the water qlost = 46. 2 g(s)(95. 4 -21. 8 ºC ) = 3400. 32(s) qgained = 75. 0 g(4. 18)(21. 8 -19. 6 ºC ) = 689. 7 3400. 32(s) = 689. 7 S = 0. 2 J/ºC *g 92

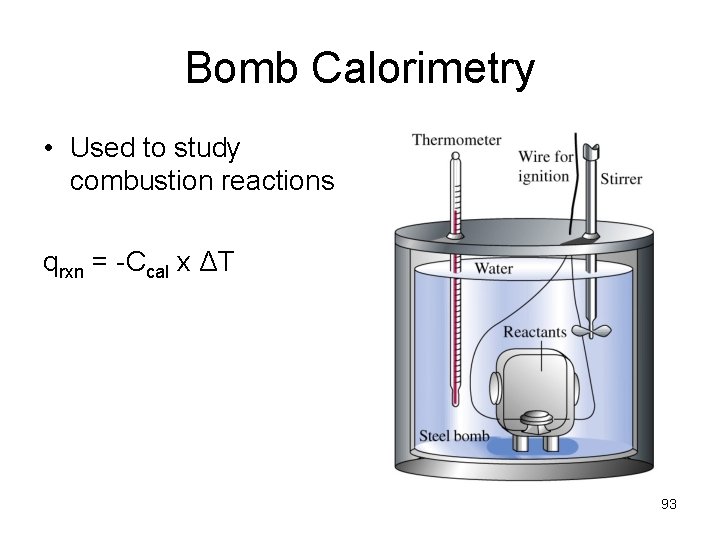
Bomb Calorimetry • Used to study combustion reactions qrxn = -Ccal x ΔT 93

Homework Brown Lemay Pg 191 -2 #’s Sp heat/ capacity 43, 44, 45, 46, 47, Calorimetry 49, 50 94

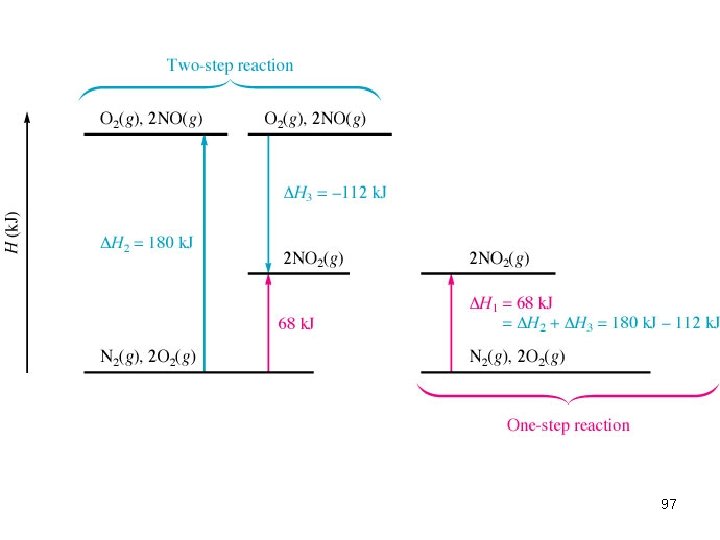
6. 3 Hess’s Law “Heat of summation” states that if reactions are carried out in a series of steps, ΔH for the reaction will be equal to the sum of the enthalpy changes by the individual steps. 95

Reactants Products The change in enthalpy is the same whether the reaction takes place in one step or a series of steps. Goal: manipulate the steps of the equation (multiple, reverse) to cancel out terms to isolate the final reaction and calculate the new enthalpy (ΔH ) 96

97

Calculations via Hess’s Law 1. If a reaction is reversed, H is also reversed. N 2(g) + O 2(g) 2 NO(g) N 2(g) + O 2(g) 2. H = 180 k. J If the coefficients of a reaction are multiplied by an integer, H is multiplied by that same integer. 2 NO(g) N 2(g) + O 2(g) 6 NO(g) 3 N 2(g) + 3 O 2(g) -180 x 3 = -540 H = 180 k. J H = 540 k. J 98

Solving Hess’s Law Problems Given the following data, calculate the ΔH for the reaction: H 2 S(g) + 2 O 2(g) H 2 SO 4(l) SO 3(g) + H 2 O(g) ΔH =-706. 5 KJ ΔH =184. 5 KJ H 2 O(g) H 2 O(l) ΔH=-99 KJ ___________________ SO 3(g) + H 2 O(l) H 2 S(g) + 2 O 2(g) ΔH= 99

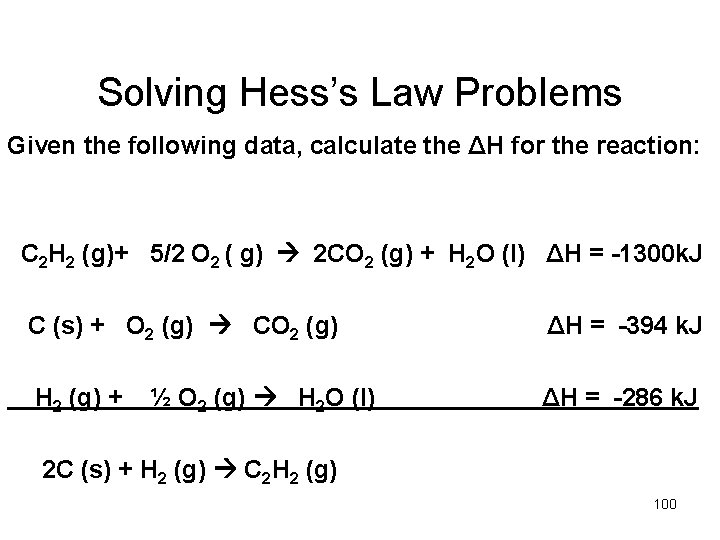
Solving Hess’s Law Problems Given the following data, calculate the ΔH for the reaction: C 2 H 2 (g)+ 5/2 O 2 ( g) 2 CO 2 (g) + H 2 O (l) ΔH = -1300 k. J C (s) + O 2 (g) CO 2 (g) ΔH = -394 k. J H 2 (g) + ΔH = -286 k. J ½ O 2 (g) H 2 O (l) 2 C (s) + H 2 (g) C 2 H 2 (g) 100

C 2 H 2 (g)+ 5/2 O 2 2 CO 2 (g) + H 2 O (l) ΔH = -1300 k. J C (s) + O 2 (g) CO 2 (g) ΔH = -394 k. J H 2 (g) + ΔH = -286 k. J ½ O 2 (g) H 2 O (l) _________________ 2 C (s) + H 2 (g) C 2 H 2 (g) ΔH = 101

Homework • BL packet • #’s 57, 59, 60, 61, 62 102

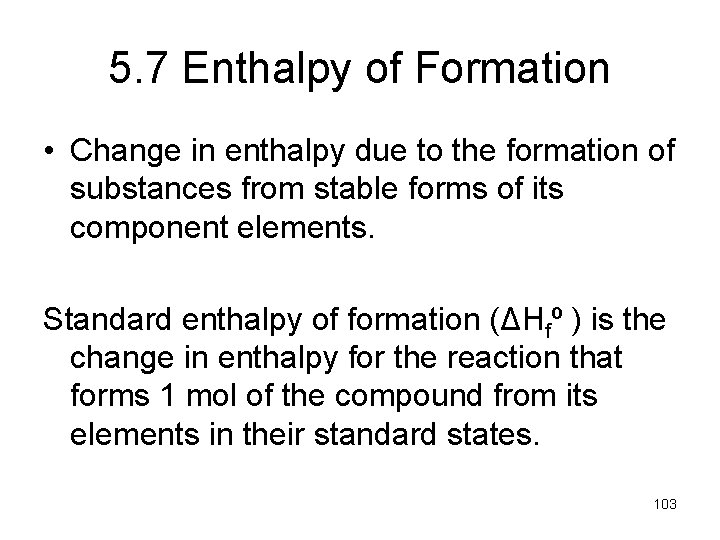
5. 7 Enthalpy of Formation • Change in enthalpy due to the formation of substances from stable forms of its component elements. Standard enthalpy of formation (ΔHfº ) is the change in enthalpy for the reaction that forms 1 mol of the compound from its elements in their standard states. 103

Where to Find ΔHfº Values • Appendix 3 pg A-8 • NOTE THE STATE OF YOUR MOLECULE!!! 105

ΔHrxn = H (products) – H (reactants) multiply any ∆H by its coefficient 106

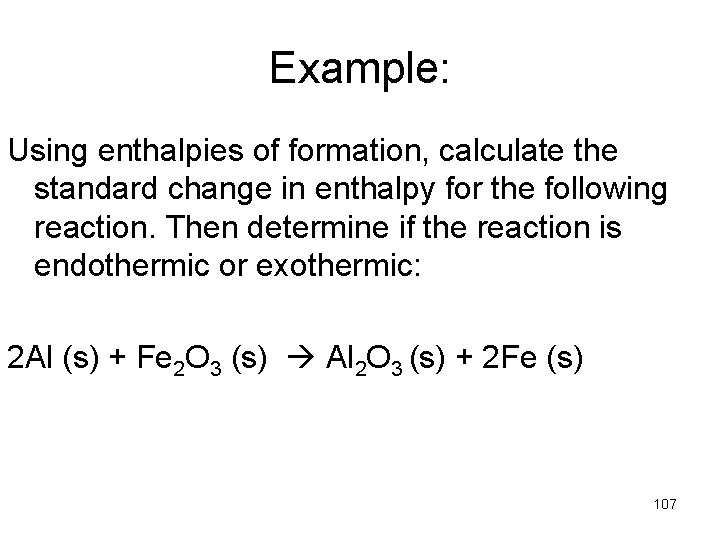
Example: Using enthalpies of formation, calculate the standard change in enthalpy for the following reaction. Then determine if the reaction is endothermic or exothermic: 2 Al (s) + Fe 2 O 3 (s) Al 2 O 3 (s) + 2 Fe (s) 107

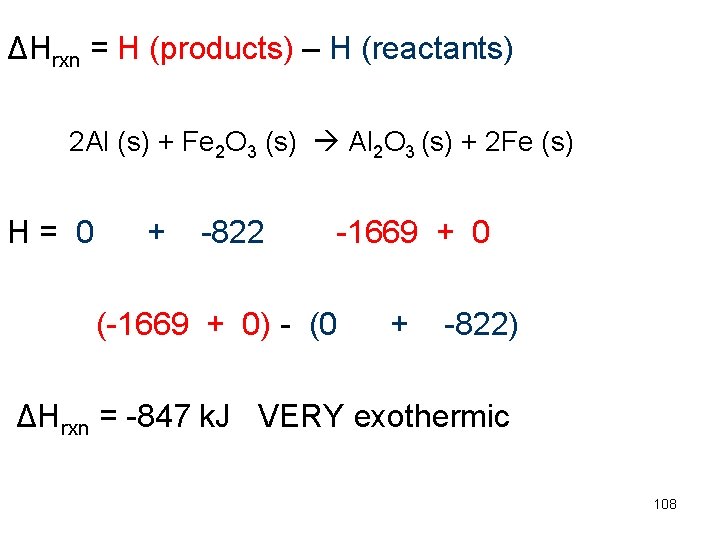
ΔHrxn = H (products) – H (reactants) 2 Al (s) + Fe 2 O 3 (s) Al 2 O 3 (s) + 2 Fe (s) H= 0 + -822 -1669 + 0 (-1669 + 0) - (0 + -822) ΔHrxn = -847 k. J VERY exothermic 108

Homework • #’s 67, 71, 72 (You will need to look up H in BL table) 109
 Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is study of: *
Thermochemistry is study of: * Thermochemistry is concerned with the study of
Thermochemistry is concerned with the study of Thermochemistry is the study of
Thermochemistry is the study of Study of energy and its transformations
Study of energy and its transformations Termokimia adalah cabang ilmu yang mempelajari
Termokimia adalah cabang ilmu yang mempelajari Thermochemistry is the study of
Thermochemistry is the study of Q = m x cp x ∆t
Q = m x cp x ∆t Energy diagram thermochemistry
Energy diagram thermochemistry Kinetic energy thermochemistry
Kinetic energy thermochemistry Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 17 thermochemistry answer key
Chapter 17 thermochemistry answer key Chapter 5 science form 3
Chapter 5 science form 3 Chapter 17 thermochemistry
Chapter 17 thermochemistry Chapter 6 thermochemistry
Chapter 6 thermochemistry Chapter 17 thermochemistry
Chapter 17 thermochemistry Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Chapter 12 thermal energy study guide answers
Chapter 12 thermal energy study guide answers Chapter 11 study guide energy and its conservation
Chapter 11 study guide energy and its conservation Thermochemistry
Thermochemistry Thermochemistry equations
Thermochemistry equations Introduction to thermochemistry
Introduction to thermochemistry Thermochemistry gaussian
Thermochemistry gaussian Thermochemical equation
Thermochemical equation Cartoon thermochemistry
Cartoon thermochemistry Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry equations
Thermochemistry equations Symbolic border one pager
Symbolic border one pager Thermochemical equation
Thermochemical equation Kirchhoff's law of thermochemistry
Kirchhoff's law of thermochemistry Thermochemistry equations
Thermochemistry equations Thermochemistry cartoon
Thermochemistry cartoon Ap chemistry thermodynamics
Ap chemistry thermodynamics Ap chemistry thermochemistry frq
Ap chemistry thermochemistry frq General chemistry thermochemistry
General chemistry thermochemistry Energy changes
Energy changes What is thermochemistry
What is thermochemistry Thermochemistry concepts
Thermochemistry concepts Unit 9 thermochemistry
Unit 9 thermochemistry Thermochemistry is concerned with the
Thermochemistry is concerned with the Thermochemistry
Thermochemistry A piece of metal is heated then submerged
A piece of metal is heated then submerged Bomb calorimetry equation
Bomb calorimetry equation Thermochemistry video
Thermochemistry video Thermochemistry conversions
Thermochemistry conversions Thermochemistry
Thermochemistry Thermochemistry exam review
Thermochemistry exam review Hess law constant heat summation
Hess law constant heat summation Now answer these questions
Now answer these questions Thermochemistry
Thermochemistry Why are chemists interested in studying thermochemistry?
Why are chemists interested in studying thermochemistry? Specific heat chem worksheet 16-1
Specific heat chem worksheet 16-1 Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Số nguyên là gì
Số nguyên là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Case series
Case series Retrospective cohort study vs prospective cohort study
Retrospective cohort study vs prospective cohort study Work study and method study
Work study and method study Marty lobdell study less study smart
Marty lobdell study less study smart Phytogeographical regions of india
Phytogeographical regions of india