HEAT 1 Heat cont Heat Consist of the



















- Slides: 47

HEAT 1

Heat cont… Heat Consist of the following (i) Thermometry (Thermometers) (ii) Heat Transfer -Thermal Conduction -Thermal Convection -Thermal Radiation (iii) Thermodynamics 2

Heat cont… • Definition: Heat is the form of energy which can flow from one point to another depending to the change of temperature. • INTRODUCTION Heat is the form of energy or we simply call thermal energy which can flow in a given system depending to fundamental quantity called Temperature • TEMPERATURE The temperature is a quantity of the degree of hotness or coldness being common to two systems being in thermal dynamics equilibrium. The dimension of temperature is Q • Temperature is described by a number (chosen Scale such that when two bodies are in Contact heat flows from higher temperature to lower temperature 3

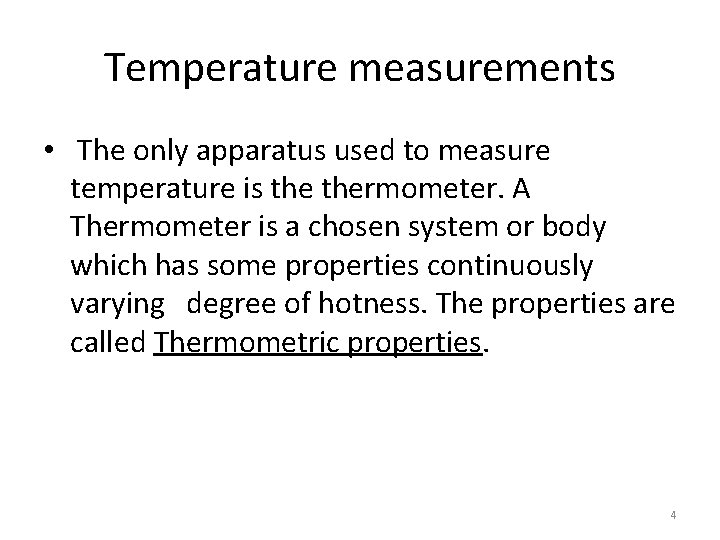
Temperature measurements • The only apparatus used to measure temperature is thermometer. A Thermometer is a chosen system or body which has some properties continuously varying degree of hotness. The properties are called Thermometric properties. 4

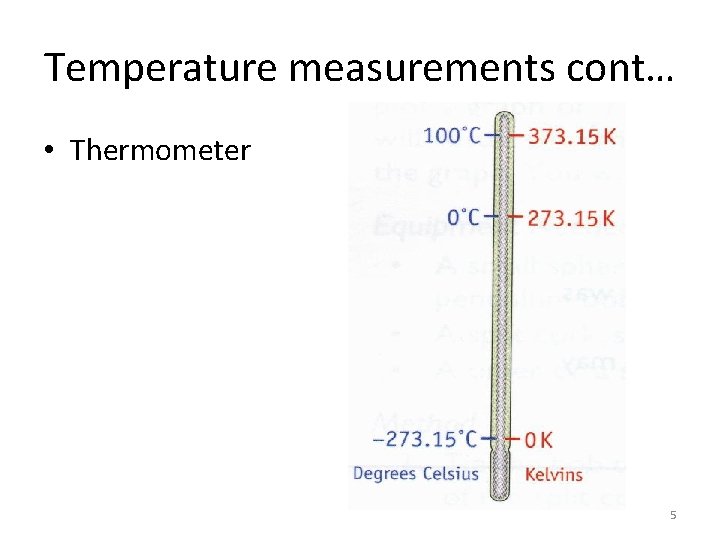
Temperature measurements cont… • Thermometer 5

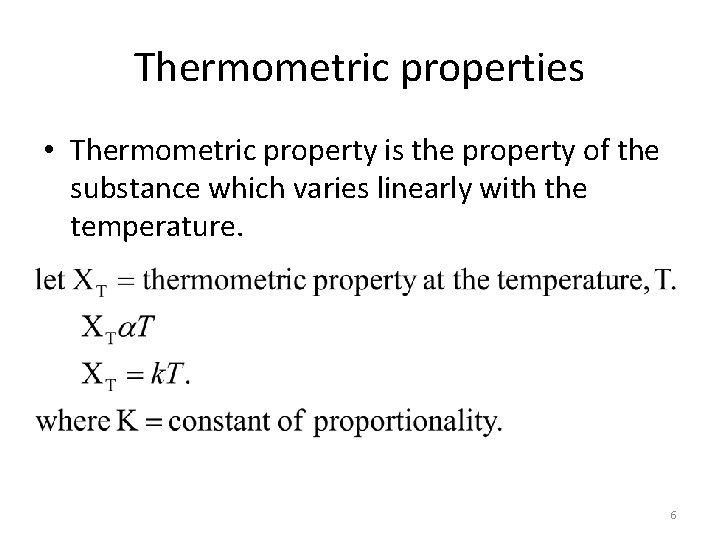
Thermometric properties • Thermometric property is the property of the substance which varies linearly with the temperature. 6

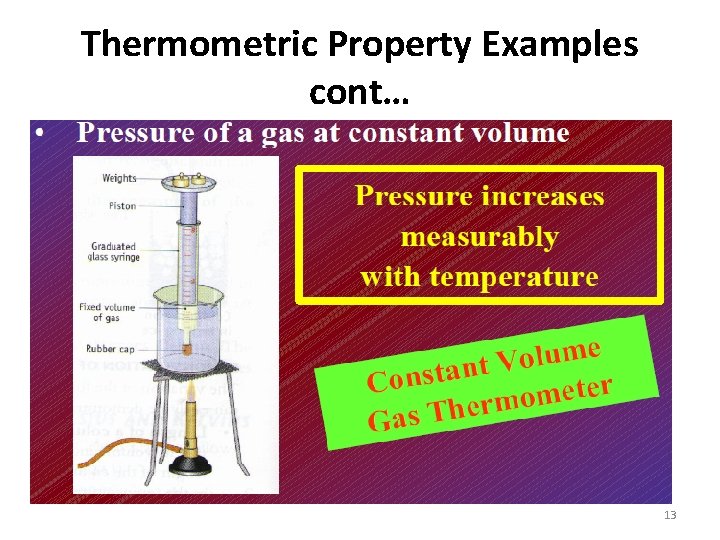
Thermometric property examples i. The length of liquid column in a glass to be ( This is for mercury in –glass Thermometer) ii. The e. m. f of a Thermo couple (for thermocouple Thermometer) iii. The electrical resistance of platinum wire wound in to a Coil. (for platinum resistance thermometer) iv. The pressure of a gas whose volume is kept constant (for constant volume gas Thermometer) 7

Thermometric property examples cont… (v) Volume of the gas at constant pressure. (for constant pressure thermometer) (vi) Colour of radiation emitted by hot body 8

Thermometric Property Examples cont… (i) Length of a liquid column L • Mercury in glass thermometer • Alcohol in glass thermometer • Laboratory thermometer • Clinical thermometer • (Barometer) / Thermometer 9

Thermometric Property Examples cont… 10

Thermometric Property Examples cont… (ii) Electromotive force (e. m. f) of a thermocouple. 11

Thermometric Prop. Examples cont… (iii) resistance of the wire 12

Thermometric Property Examples cont… 13

Thermometric Prop. Examples cont… 14

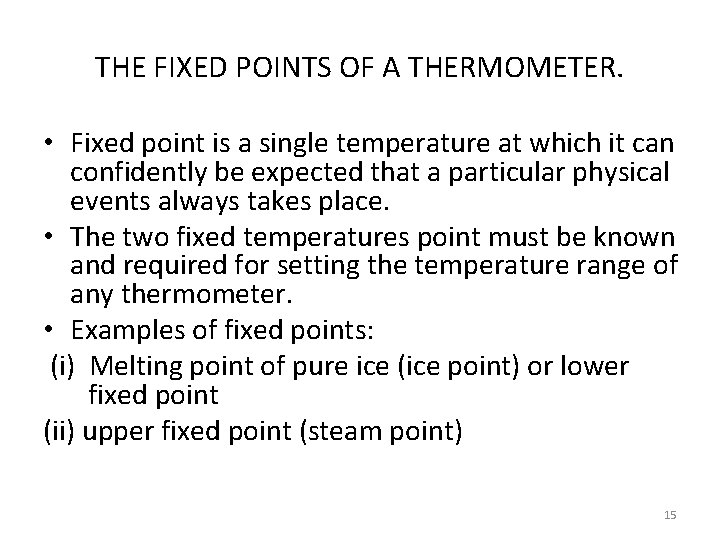
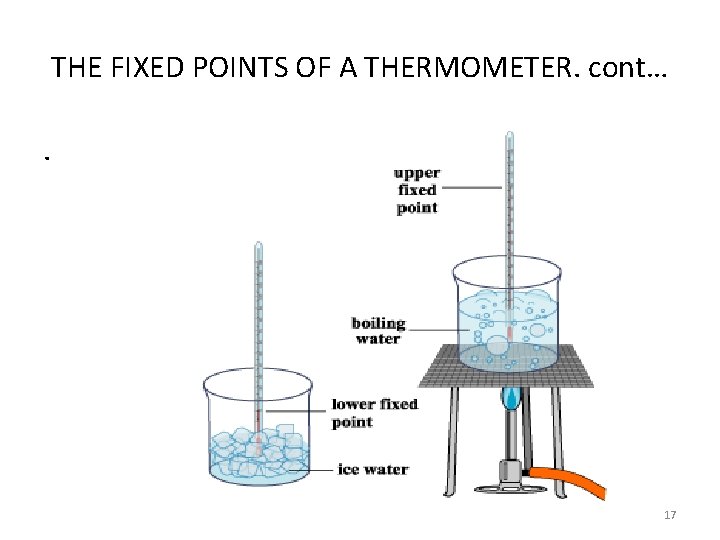
THE FIXED POINTS OF A THERMOMETER. • Fixed point is a single temperature at which it can confidently be expected that a particular physical events always takes place. • The two fixed temperatures point must be known and required for setting the temperature range of any thermometer. • Examples of fixed points: (i) Melting point of pure ice (ice point) or lower fixed point (ii) upper fixed point (steam point) 15

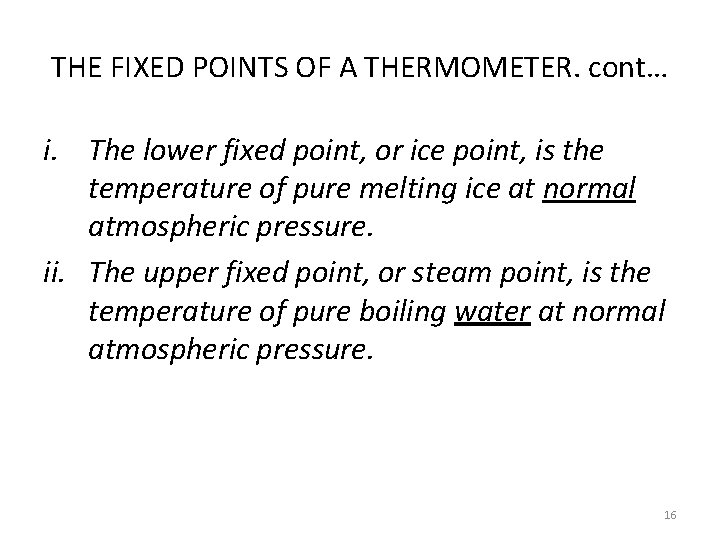
THE FIXED POINTS OF A THERMOMETER. cont… i. The lower fixed point, or ice point, is the temperature of pure melting ice at normal atmospheric pressure. ii. The upper fixed point, or steam point, is the temperature of pure boiling water at normal atmospheric pressure. 16

THE FIXED POINTS OF A THERMOMETER. cont…. 17

THE FIXED POINTS OF A THERMOMETER. cont…. 18

HOW TO DEFINE A TEMPERETURE SCALE. • Essential steps are needed. a) Choose thermometric material b) Select thermometric property of the material c) Select the two fixed point (lower and upper) d) To allocate a value such as tm to degree of material. 19

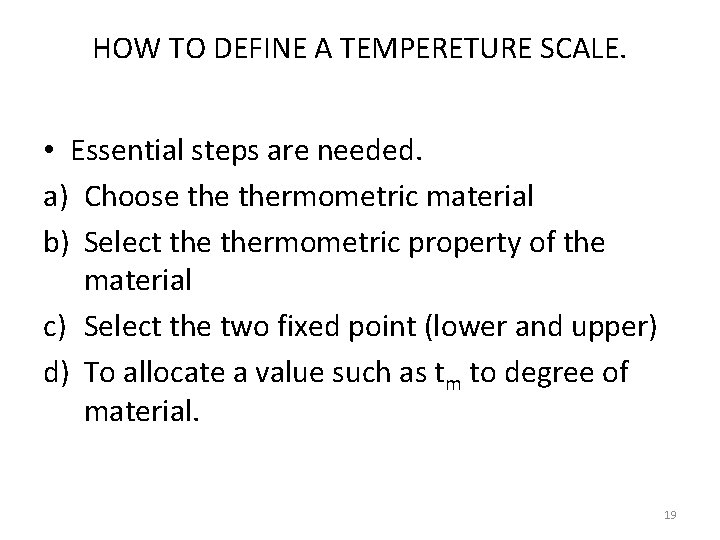
Determination of temperature by using thermometric property. . 20

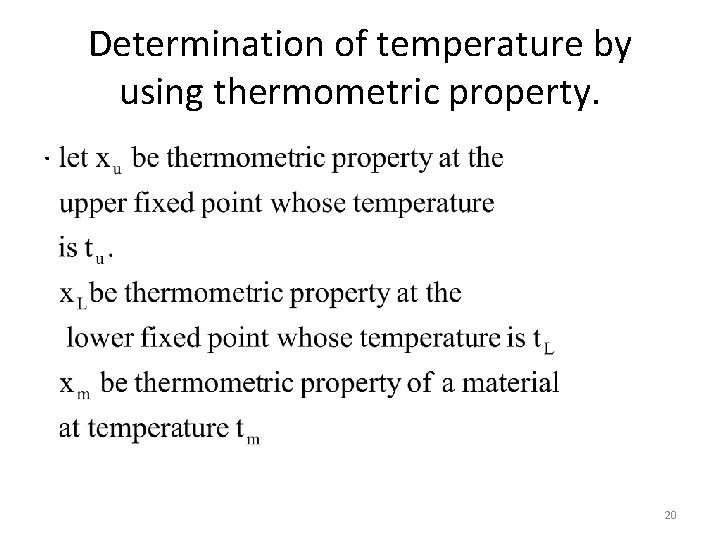
Determination of temperature by using thermometric property. cont… 21

Determination of temperature by using thermometric property. cont…. 22

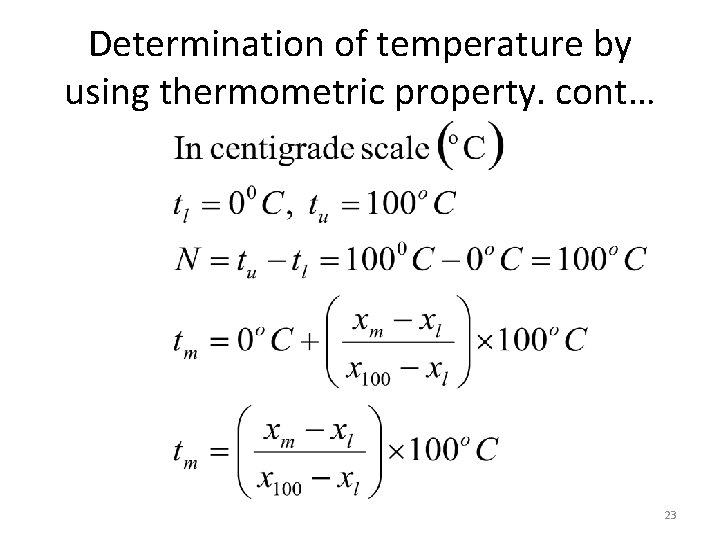
Determination of temperature by using thermometric property. cont… 23

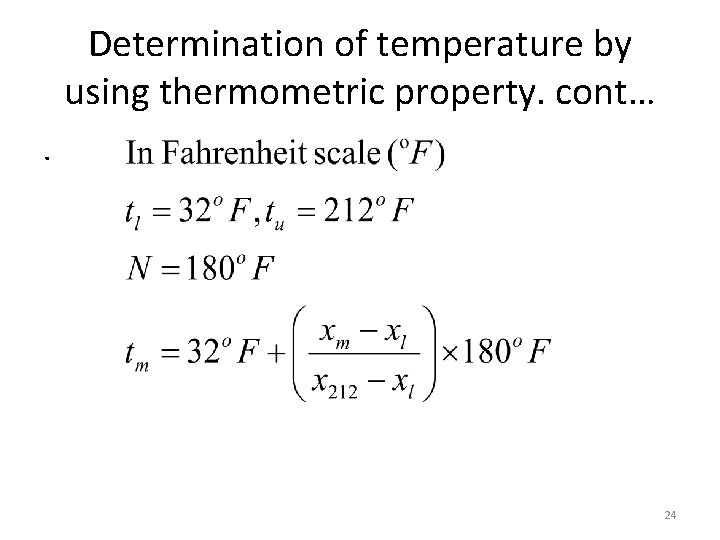
Determination of temperature by using thermometric property. cont…. 24

Examples of thermometric property. 25

Examples of thermometric property. Cont…. 26

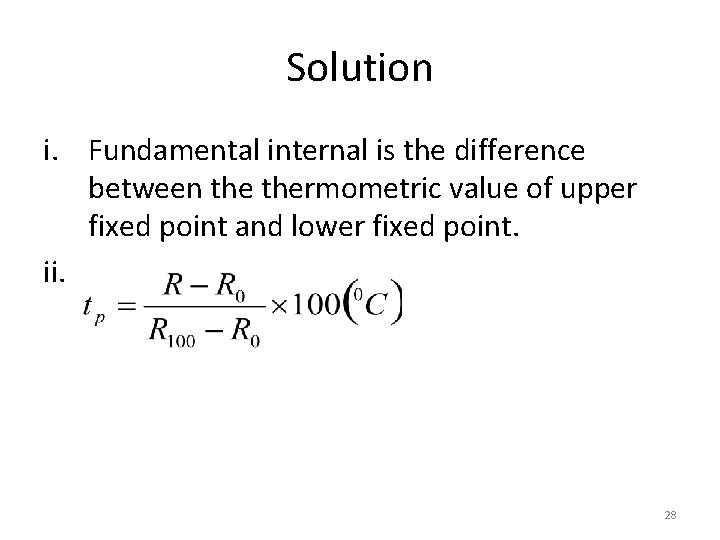
Example 1 • A given platinum has resistance Ro and R 100 at the ice point and steam point respectively i. What is its fundamental interval? ii. If it has resistance R at unknown temperature tp. what is the expression for tp? • Solution 27

Solution i. Fundamental internal is the difference between thermometric value of upper fixed point and lower fixed point. ii. 28

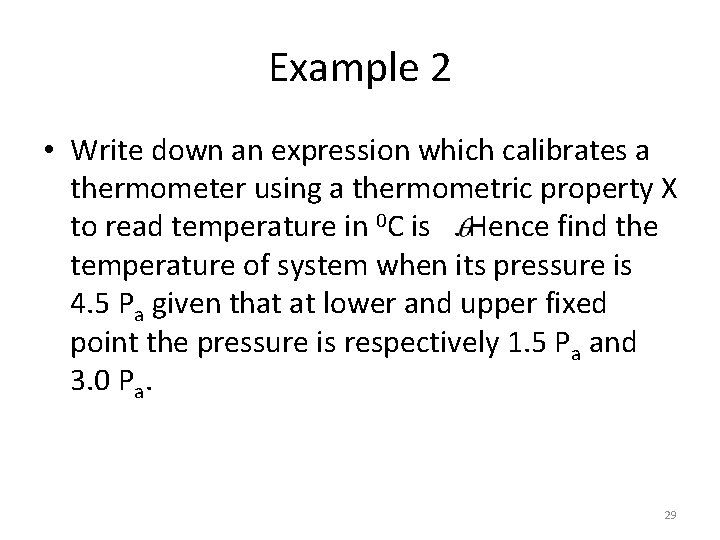
Example 2 • Write down an expression which calibrates a thermometer using a thermometric property X to read temperature in 0 C is . Hence find the temperature of system when its pressure is 4. 5 Pa given that at lower and upper fixed point the pressure is respectively 1. 5 Pa and 3. 0 Pa. 29

Solution. NB: If the scale of the degree of hotness is to be in k, then tm =T, t =Ok, N=273. 16 k. 100 30

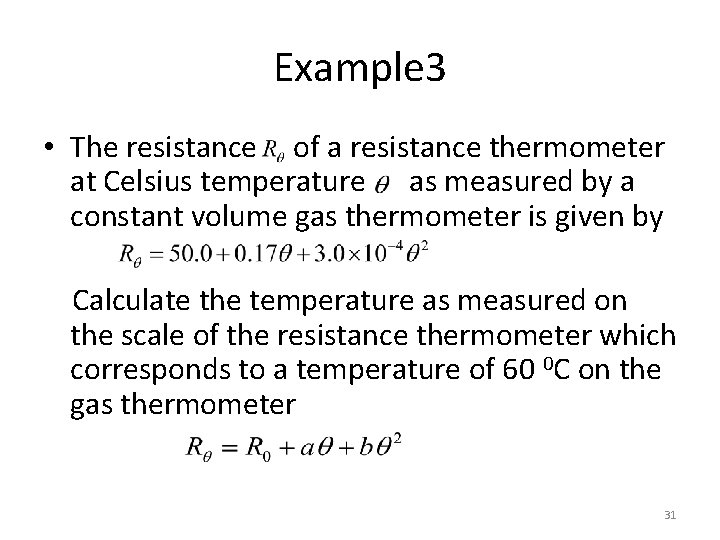
Example 3 • The resistance of a resistance thermometer at Celsius temperature as measured by a constant volume gas thermometer is given by Calculate the temperature as measured on the scale of the resistance thermometer which corresponds to a temperature of 60 0 C on the gas thermometer 31

Solution . 32

Solution. Cont…. 33

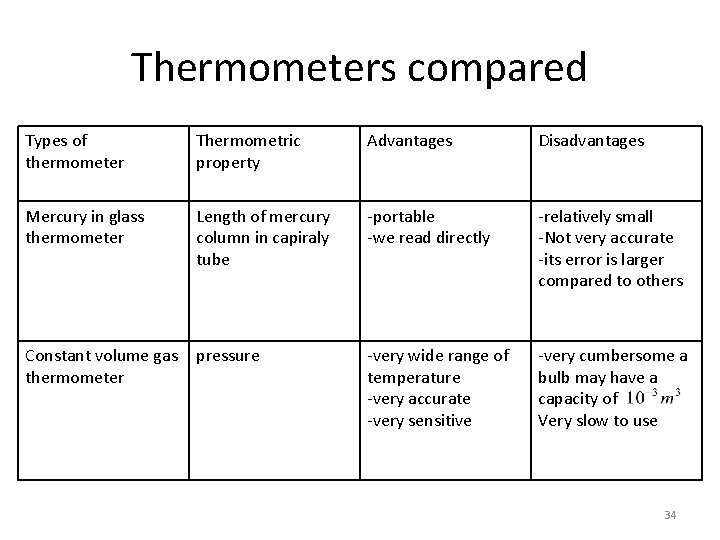
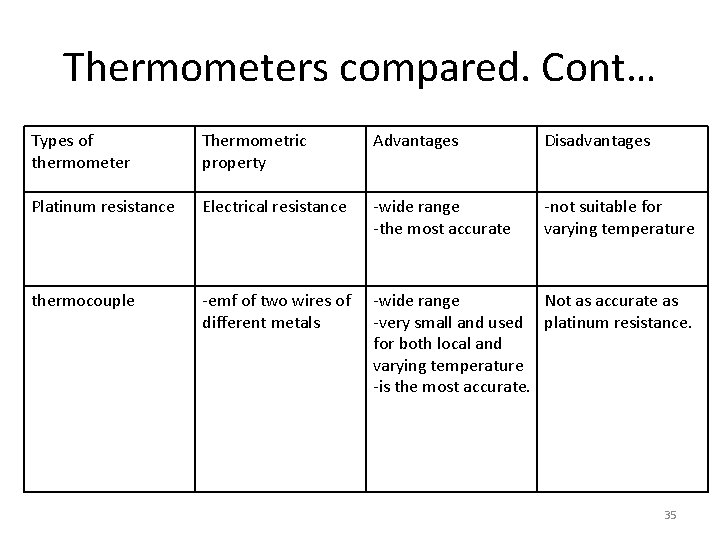
Thermometers compared Types of thermometer Thermometric property Advantages Disadvantages Mercury in glass thermometer Length of mercury column in capiraly tube -portable -we read directly -relatively small -Not very accurate -its error is larger compared to others -very wide range of temperature -very accurate -very sensitive -very cumbersome a bulb may have a capacity of Very slow to use Constant volume gas pressure thermometer 34

Thermometers compared. Cont… Types of thermometer Thermometric property Advantages Disadvantages Platinum resistance Electrical resistance -wide range -the most accurate -not suitable for varying temperature thermocouple -emf of two wires of different metals -wide range Not as accurate as -very small and used platinum resistance. for both local and varying temperature -is the most accurate. 35

HEAT TRANSFER • Heat may be transferred from one point to another depending in the difference in temperature between the two points. • When heat travels it involves some molecular vibrations or complete movement • Therefore Conduction of heat can be explained in terms of the kinetic theory of matter. • 36

CONDUCTION • In conduction a temperature difference causes the transfer of energy from one region hot body to another region of the same body which is at a lower temperature. The flow tends to equalize the temperature within the body. • Conduction takes place in solids, liquids and gases. 37

In solids and Liquids • Free electrons in the lattice structure of solids or liquids move randomly as molecules in the gas. These transfer energy from the hot region to the cold region of the body. • In solids the ions are coupled (joined together to form a shape of solid or liquids. The atoms in a heated region vibrate with high K. E to the coupling of atoms they easily transfer this energy to neighboring atoms. 38

In gases • It is are suit of collision of fast moving molecules and slow moving molecules during a collision gain K. E • From kinetic theory the fast moving molecules are from the heated point. 39

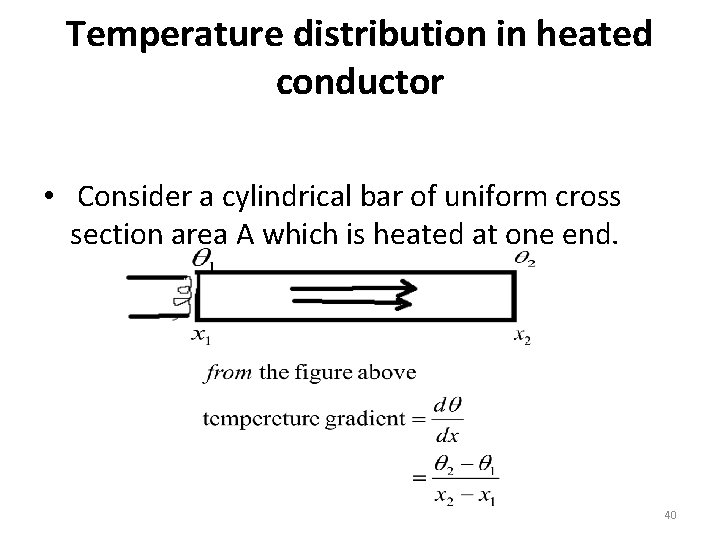
Temperature distribution in heated conductor • Consider a cylindrical bar of uniform cross section area A which is heated at one end. 40

Temperature distribution in heated conductor cont… NB: • Temperature gradient may be constant or continuously changing depending on the heat loss to the surrounding before it reaches the other end. 41

Temperature distribution in heated conductor cont… 42

Temperature distribution in heated conductor cont… 43

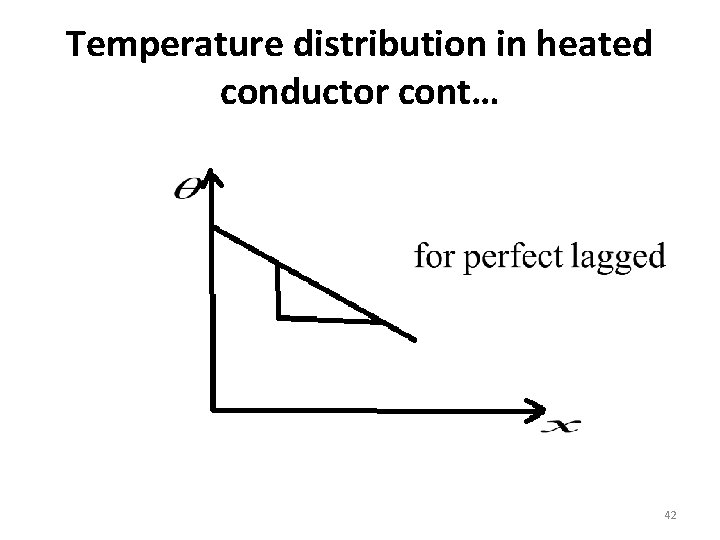
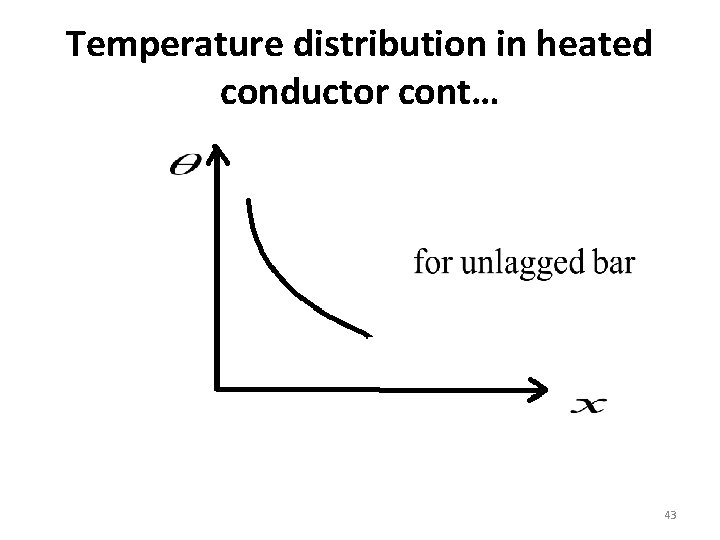
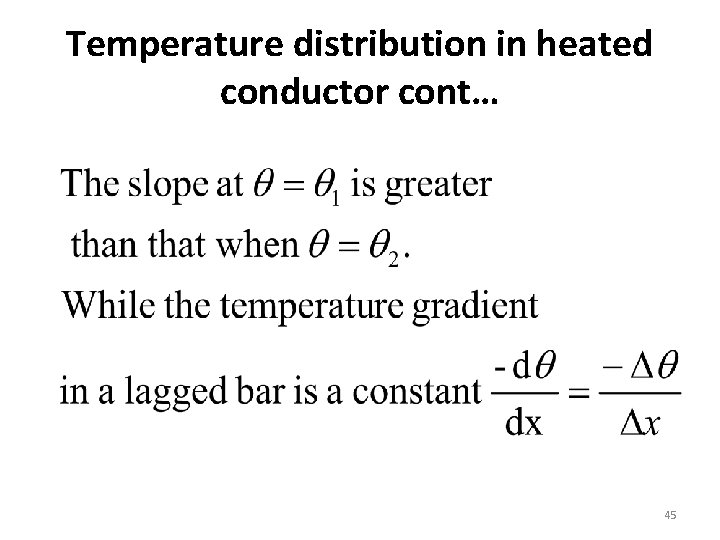
Temperature distribution in heated conductor cont… • If the bar is lagged no loss of heat by any way –conduction, convection or radiation. • Temperature gradient determines the rate of flow of heat in a body. This indicates that the rate of flow of heat is constant the lines of flow of heat are parallel. • The temperature gradient along the un lagged bar is not a constant, it decreases with distance from the heated end 44

Temperature distribution in heated conductor cont… 45

Temperature distribution in heated conductor cont… NB: • In both cases, the temperature gradient is negative indicating that the heat flows in the direction of increasing distance between decreasing temp. • For un lagged bar the lines of flow of heat are diverged 46

THERMAL CONDUCTIVITY 47
 Debriefing report
Debriefing report Cont or cont'd
Cont or cont'd What does britain consist of
What does britain consist of Food web with at least 10 organisms
Food web with at least 10 organisms Great britain countries
Great britain countries Chorionic villus
Chorionic villus Different types of emulsion
Different types of emulsion Reseller markets consist mainly of
Reseller markets consist mainly of Gear terminology
Gear terminology Advantages and disadvantages of relocating loader
Advantages and disadvantages of relocating loader Worksheet formulas consist of two components: operands and
Worksheet formulas consist of two components: operands and What does the united kingdom consist of
What does the united kingdom consist of Brand elements example
Brand elements example What does an ict system consist of
What does an ict system consist of Consist of your most important targeted or segmented groups
Consist of your most important targeted or segmented groups White blood chapter 14
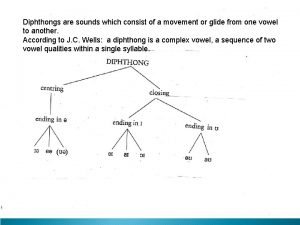
White blood chapter 14 How are diphthongs classified
How are diphthongs classified Servlet maintain session in mcq
Servlet maintain session in mcq Iron triangle example
Iron triangle example What does the united kingdom consist of
What does the united kingdom consist of Direct view storage tube in computer graphics
Direct view storage tube in computer graphics Carbohydrates consist of
Carbohydrates consist of Readily accessible nec
Readily accessible nec Sample food chain
Sample food chain Problem analysis chart
Problem analysis chart Safety net drop tests must consist of a
Safety net drop tests must consist of a The three purities consist of
The three purities consist of Direct material cost
Direct material cost Cont'd artinya
Cont'd artinya First bank comisioane persoane juridice
First bank comisioane persoane juridice 4315 cont
4315 cont Ibc cont
Ibc cont Intermodal cont.
Intermodal cont. Palabras con tilde
Palabras con tilde Cont'n air
Cont'n air Corpus alienum vitreum
Corpus alienum vitreum Cont class
Cont class Caracter alfanumerico
Caracter alfanumerico System call in os
System call in os Cont 402
Cont 402 File sharing management system
File sharing management system Atm protokol
Atm protokol Mobile cont
Mobile cont Cont nn
Cont nn Celtic/cont
Celtic/cont Cont 2131
Cont 2131 Cont+z
Cont+z What is mst
What is mst