Holes Human Anatomy and Physiology Eleventh Edition Shier







- Slides: 32

Hole’s Human Anatomy and Physiology Eleventh Edition Shier w Butler w Lewis Chapter 2 1 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

CHAPTER 2 CHEMICAL BASIS OF LIFE Why study chemistry in an Anatomy and Physiology class? - body functions depend on cellular functions - cellular functions result from chemical changes - biochemistry helps to explain physiological processes, and develop new drugs and methods for treating diseases 2

STRUCTURE OF MATTER Matter – anything that takes up space and has weight; composed of elements Elements – composed of chemically identical atoms • bulk elements – required by the body in large amounts • trace elements – required by the body in small amounts Atoms – smallest particle of an element 3

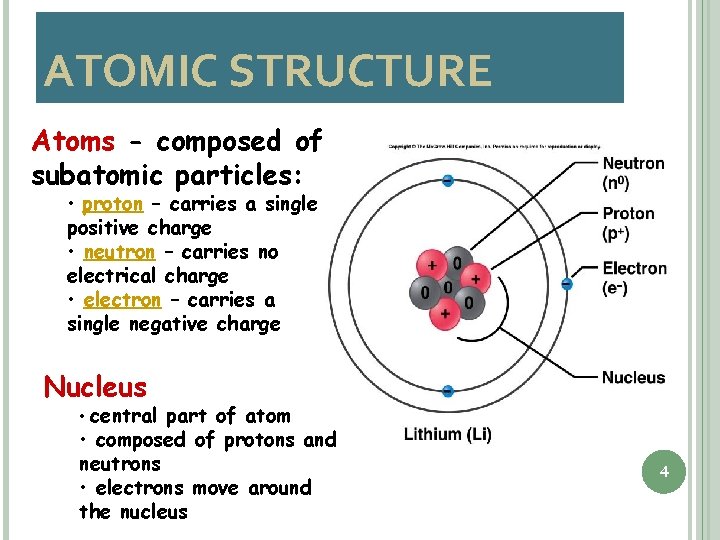
ATOMIC STRUCTURE Atoms - composed of subatomic particles: • proton – carries a single positive charge • neutron – carries no electrical charge • electron – carries a single negative charge Nucleus • central part of atom • composed of protons and neutrons • electrons move around the nucleus 4

ATOMIC NUMBER AND ATOMIC WEIGHT Atomic Number • number of protons in the nucleus of one atom • each element has a unique atomic number • equals the number of electrons in the atom Atomic Weight • the number of protons plus the number of neutrons in one atom • electrons do not contribute to the weight of the atom 5

ISOTOPES Isotopes • atoms with the same atomic numbers but with different atomic weights • atoms with the same number of protons and electrons but a different number of neutrons • oxygen often forms isotopes (O 16, O 17, O 18) • unstable isotopes are radioactive; they emit energy or atomic fragments 6

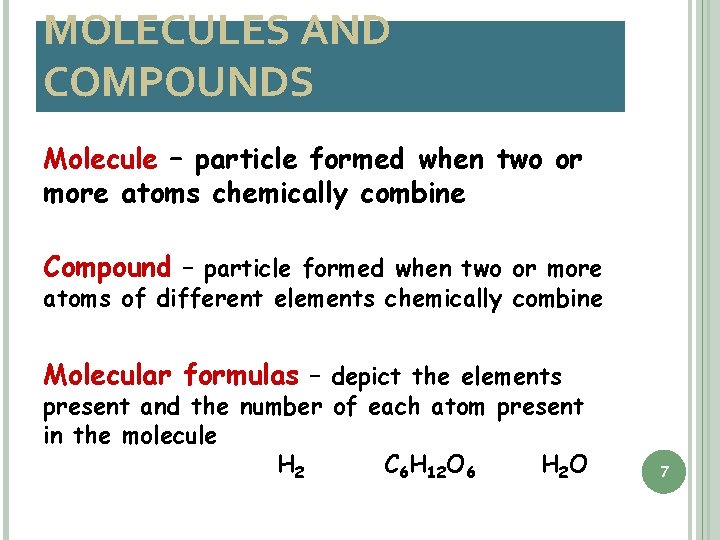
MOLECULES AND COMPOUNDS Molecule – particle formed when two or more atoms chemically combine Compound – particle formed when two or more atoms of different elements chemically combine Molecular formulas – depict the elements present and the number of each atom present in the molecule H 2 C 6 H 12 O 6 H 2 O 7

BONDING OF ATOMS • lower energy levels are filled first • if the outermost level has 8 electrons, the atom is stable 8

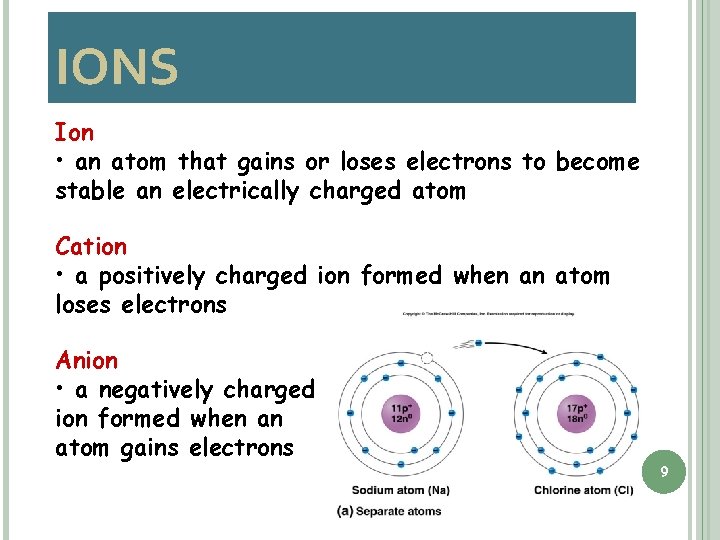
IONS Ion • an atom that gains or loses electrons to become stable an electrically charged atom Cation • a positively charged ion formed when an atom loses electrons Anion • a negatively charged ion formed when an atom gains electrons 9

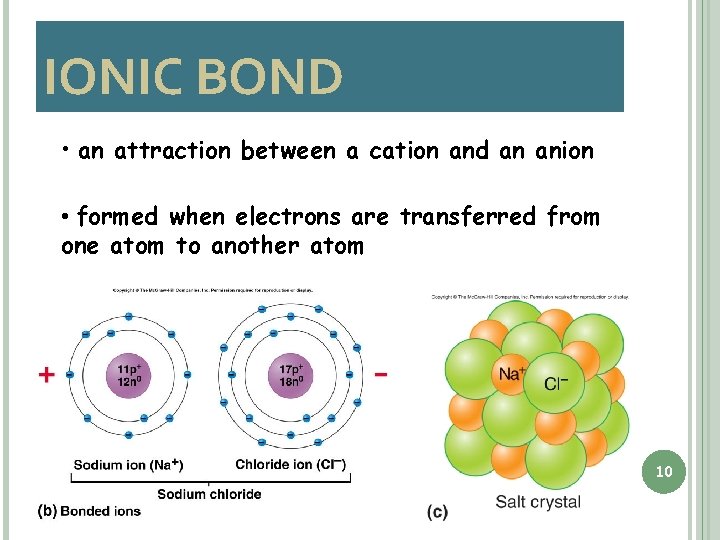
IONIC BOND • an attraction between a cation and an anion • formed when electrons are transferred from one atom to another atom 10

COVALENT BOND Formed when atoms share electrons • Hydrogen atoms form single bonds • Oxygen atoms form two bonds • Nitrogen atoms form three bonds • Carbon atoms form four bonds H O N O ― H = O ≡ N = C = O 11

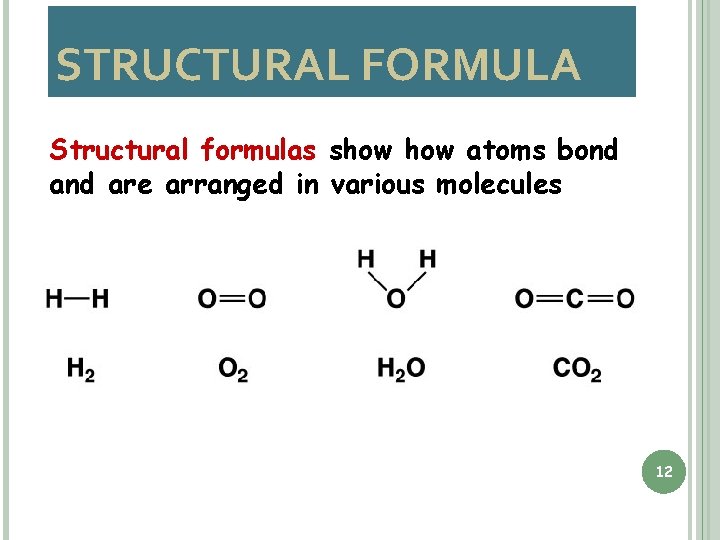
STRUCTURAL FORMULA Structural formulas show atoms bond are arranged in various molecules 12

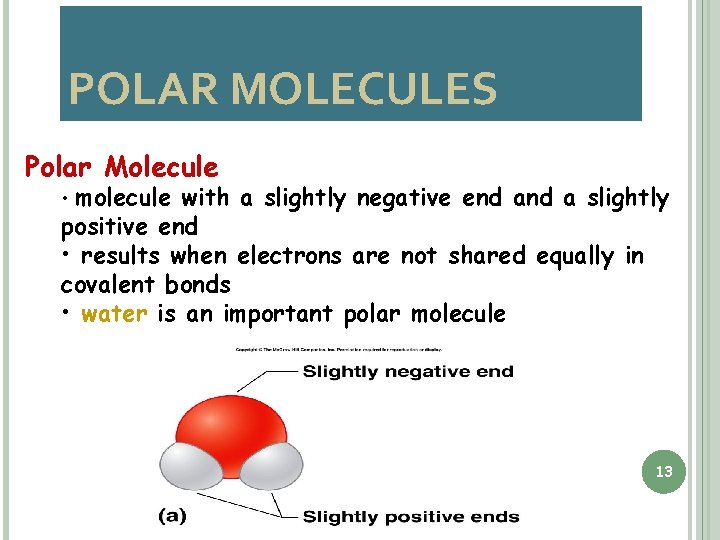
POLAR MOLECULES Polar Molecule • molecule with a slightly negative end a slightly positive end • results when electrons are not shared equally in covalent bonds • water is an important polar molecule 13

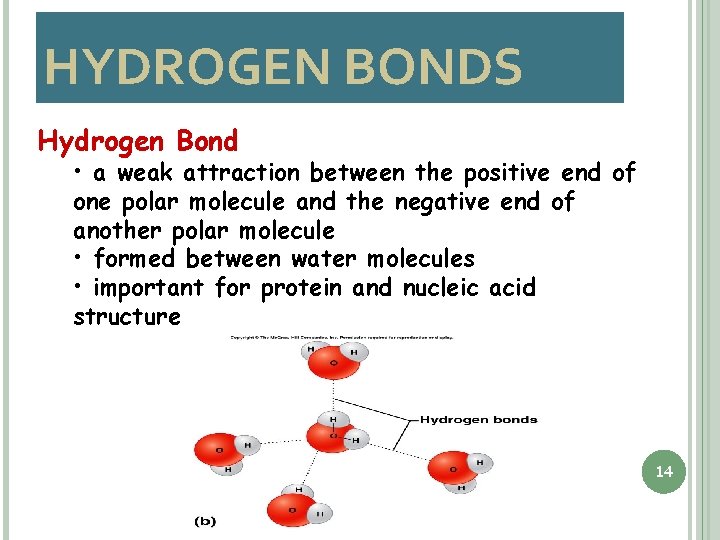
HYDROGEN BONDS Hydrogen Bond • a weak attraction between the positive end of one polar molecule and the negative end of another polar molecule • formed between water molecules • important for protein and nucleic acid structure 14

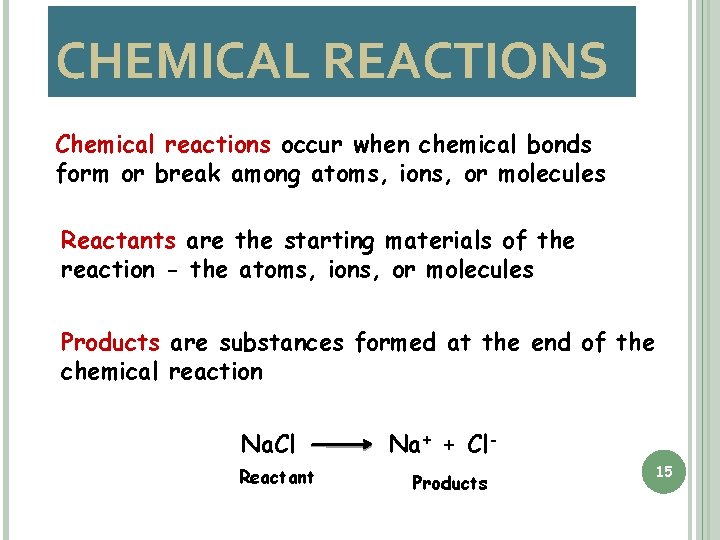
CHEMICAL REACTIONS Chemical reactions occur when chemical bonds form or break among atoms, ions, or molecules Reactants are the starting materials of the reaction - the atoms, ions, or molecules Products are substances formed at the end of the chemical reaction Na. Cl Reactant Na+ + Cl. Products 15

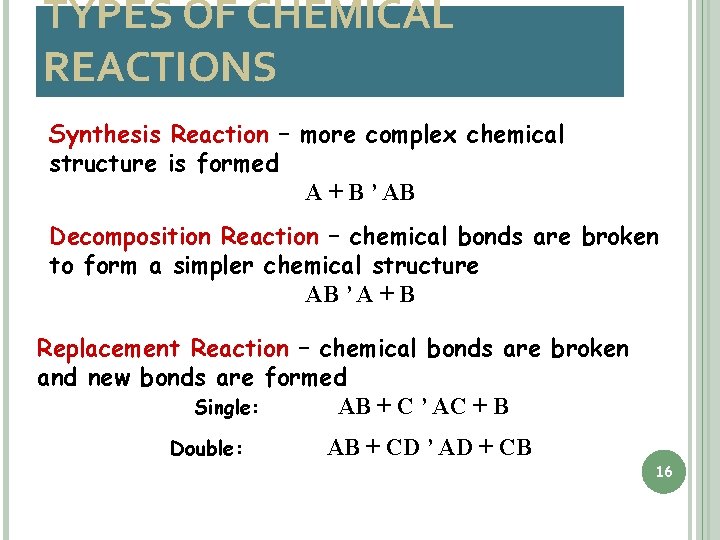
TYPES OF CHEMICAL REACTIONS Synthesis Reaction – more complex chemical structure is formed A + B ’ AB Decomposition Reaction – chemical bonds are broken to form a simpler chemical structure AB ’ A + B Replacement Reaction – chemical bonds are broken and new bonds are formed Single: AB + C ’ AC + B Double: AB + CD ’ AD + CB 16

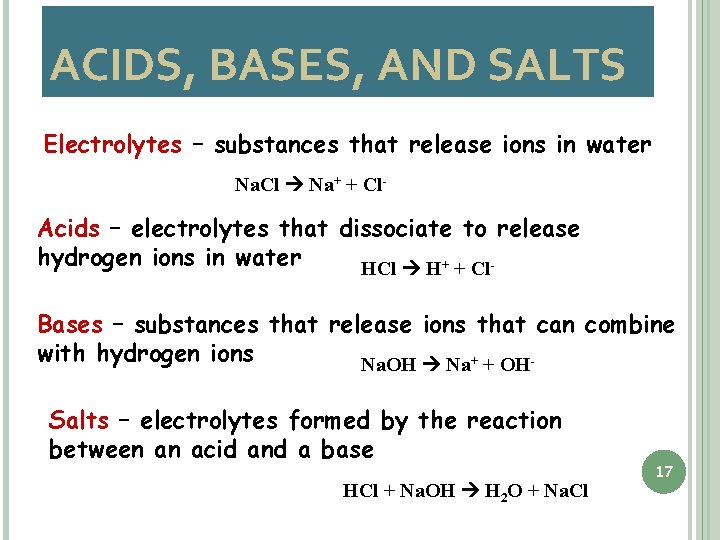
ACIDS, BASES, AND SALTS Electrolytes – substances that release ions in water Na. Cl Na+ + Cl- Acids – electrolytes that dissociate to release hydrogen ions in water HCl H+ + Cl. Bases – substances that release ions that can combine with hydrogen ions Na. OH Na+ + OHSalts – electrolytes formed by the reaction between an acid and a base HCl + Na. OH H 2 O + Na. Cl 17

ACID AND BASE CONCENTRATIONS p. H scale - indicates the concentration of hydrogen ions in solution Neutral – p. H 7; indicates equal concentrations of H+ and OHAcidic – p. H less than 7; indicates a greater concentration of H+ Basic or alkaline – p. H greater than 7; indicates a greater concentration of OH- 18

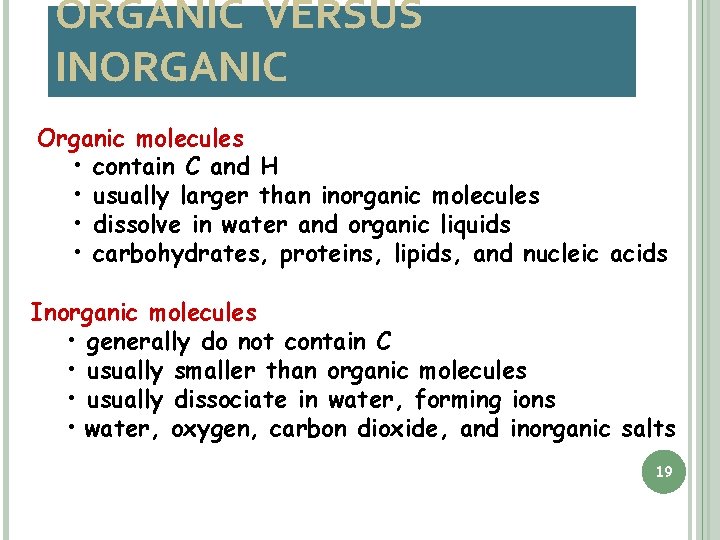
ORGANIC VERSUS INORGANIC Organic molecules • contain C and H • usually larger than inorganic molecules • dissolve in water and organic liquids • carbohydrates, proteins, lipids, and nucleic acids Inorganic molecules • generally do not contain C • usually smaller than organic molecules • usually dissociate in water, forming ions • water, oxygen, carbon dioxide, and inorganic salts 19

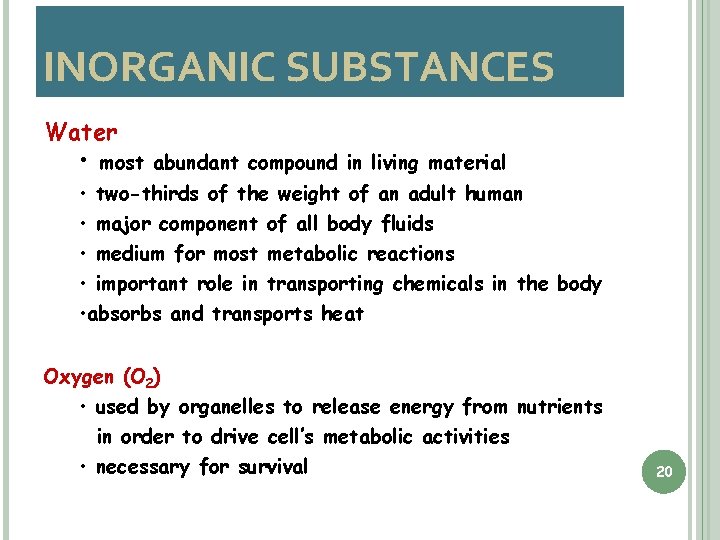
INORGANIC SUBSTANCES Water • most abundant compound in living material • two-thirds of the weight of an adult human • major component of all body fluids • medium for most metabolic reactions • important role in transporting chemicals in the body • absorbs and transports heat Oxygen (O 2) • used by organelles to release energy from nutrients in order to drive cell’s metabolic activities • necessary for survival 20

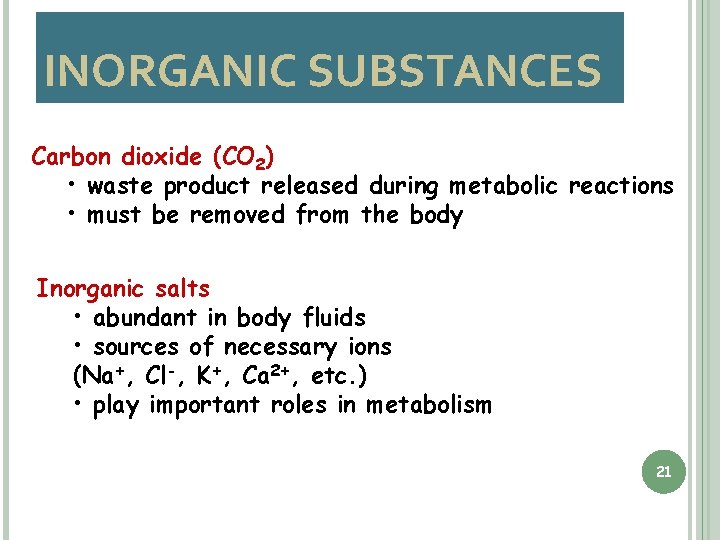
INORGANIC SUBSTANCES Carbon dioxide (CO 2) • waste product released during metabolic reactions • must be removed from the body Inorganic salts • abundant in body fluids • sources of necessary ions (Na+, Cl-, K+, Ca 2+, etc. ) • play important roles in metabolism 21

ORGANIC SUBSTANCES • 4 different compounds • Carbohydrates – C, H, O • Lipids – C, H, O , & often P • Proteins – C, H, O, N & often P & sometimes S • Nucleic Acids – C, H, O, N, P 22

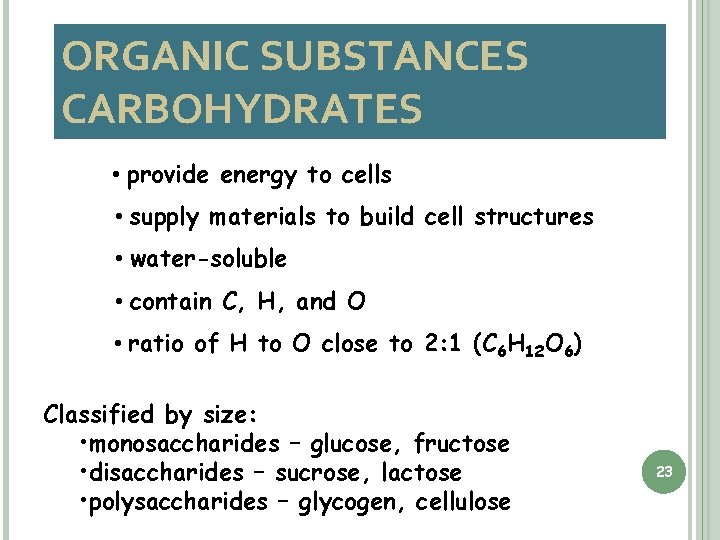
ORGANIC SUBSTANCES CARBOHYDRATES • provide energy to cells • supply materials to build cell structures • water-soluble • contain C, H, and O • ratio of H to O close to 2: 1 (C 6 H 12 O 6) Classified by size: • monosaccharides – glucose, fructose • disaccharides – sucrose, lactose • polysaccharides – glycogen, cellulose 23

ORGANIC SUBSTANCES CARBOHYDRATES 24

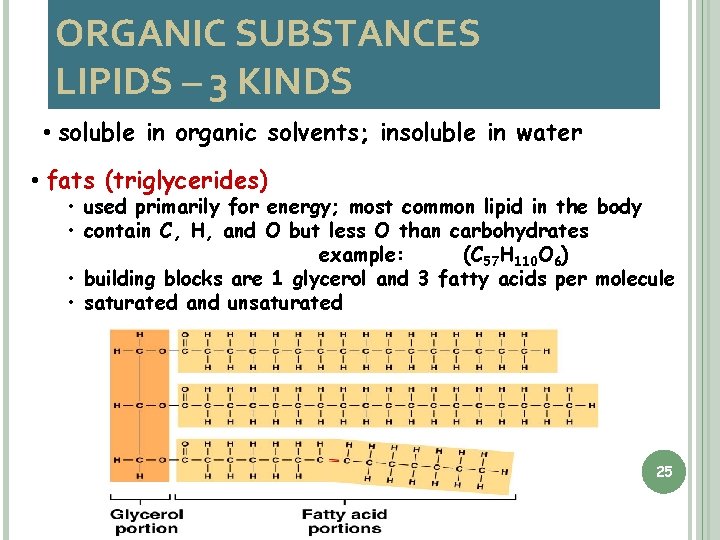
ORGANIC SUBSTANCES LIPIDS – 3 KINDS • soluble in organic solvents; insoluble in water • fats (triglycerides) • used primarily for energy; most common lipid in the body • contain C, H, and O but less O than carbohydrates example: (C 57 H 110 O 6) • building blocks are 1 glycerol and 3 fatty acids per molecule • saturated and unsaturated 25

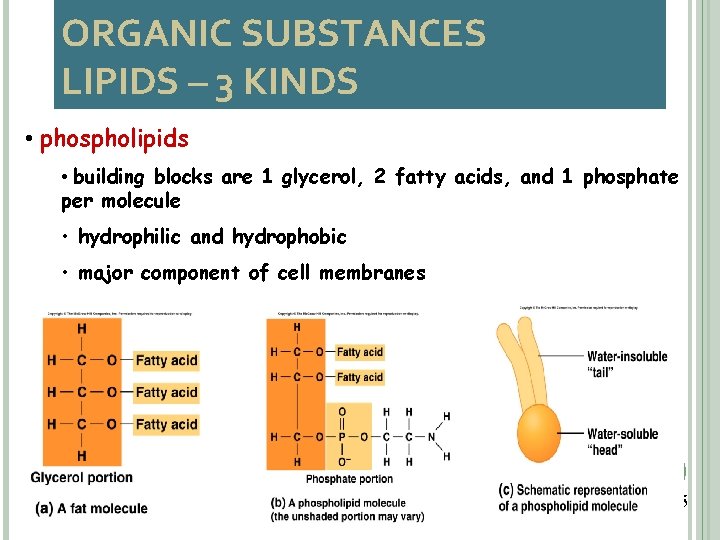
ORGANIC SUBSTANCES LIPIDS – 3 KINDS • phospholipids • building blocks are 1 glycerol, 2 fatty acids, and 1 phosphate per molecule • hydrophilic and hydrophobic • major component of cell membranes 26 2 -25

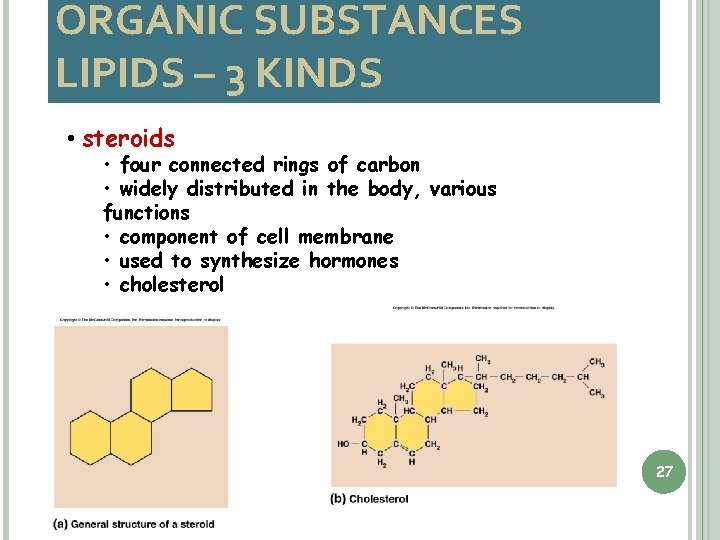
ORGANIC SUBSTANCES LIPIDS – 3 KINDS • steroids • four connected rings of carbon • widely distributed in the body, various functions • component of cell membrane • used to synthesize hormones • cholesterol 27

ORGANIC SUBSTANCES PROTEINS • structural material • energy source • hormones • receptors • enzymes • antibodies • amino acids held together with peptide bonds • building blocks are amino acids 28 2 -27

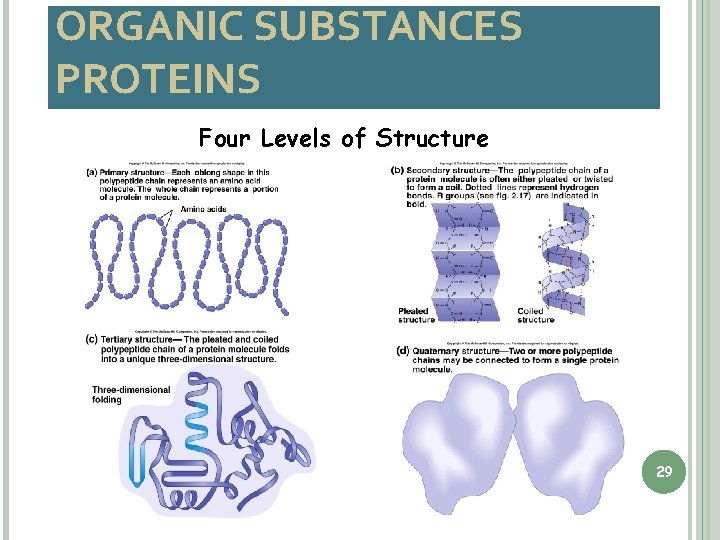
ORGANIC SUBSTANCES PROTEINS Four Levels of Structure 29

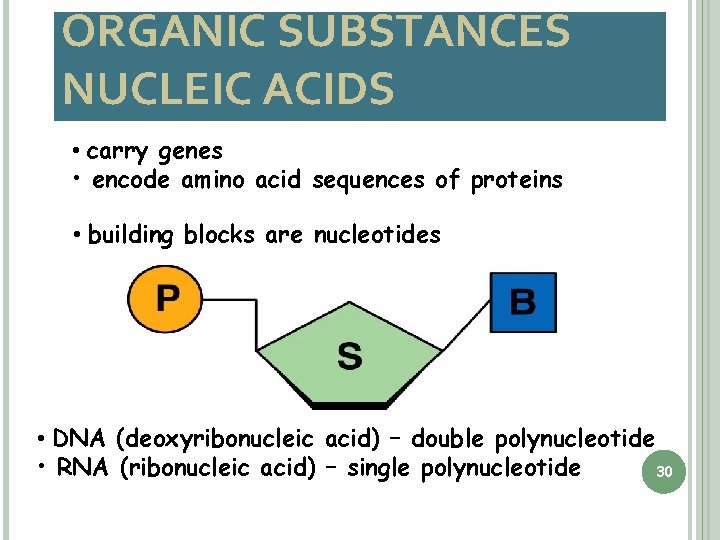
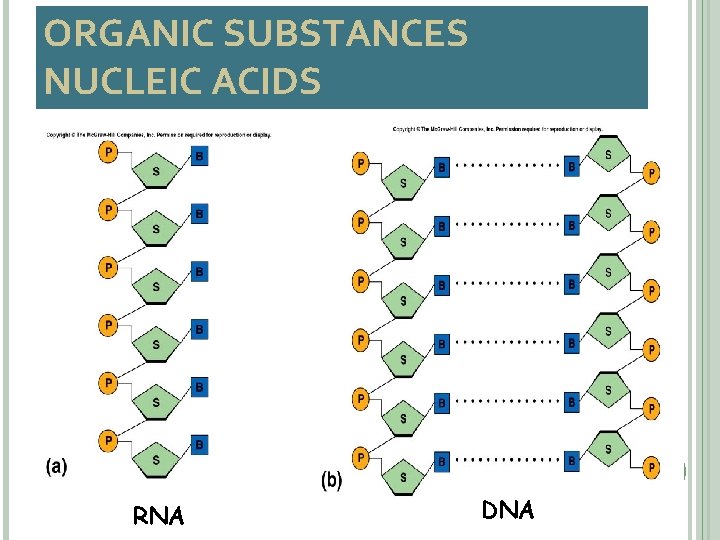
ORGANIC SUBSTANCES NUCLEIC ACIDS • carry genes • encode amino acid sequences of proteins • building blocks are nucleotides • DNA (deoxyribonucleic acid) – double polynucleotide • RNA (ribonucleic acid) – single polynucleotide 30

ORGANIC SUBSTANCES NUCLEIC ACIDS 31 RNA DNA

CLINICAL APPLICATIONS CT Scanning and PET Imaging • techniques used to give anatomical and physiological information • CT scanning uses X-ray emissions to provide 3 -D image of internal body parts • PET imaging used radioactive isotopes to detect biochemical activity in a specific body part 32
 Holes essential of human anatomy and physiology
Holes essential of human anatomy and physiology Human anatomy and physiology seventh edition marieb
Human anatomy and physiology seventh edition marieb Paratubular cyst
Paratubular cyst Holes anatomy and physiology chapter 1
Holes anatomy and physiology chapter 1 Anatomy and physiology edition 9
Anatomy and physiology edition 9 Eleventh edition management
Eleventh edition management Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition Chapter 1 introduction to human anatomy and physiology
Chapter 1 introduction to human anatomy and physiology Chapter 1 introduction to anatomy and physiology
Chapter 1 introduction to anatomy and physiology Chapter 2 human reproductive anatomy and physiology
Chapter 2 human reproductive anatomy and physiology Api testing
Api testing Escala de shier
Escala de shier степени сравнения shy
степени сравнения shy Human anatomy fifth edition
Human anatomy fifth edition Human anatomy fifth edition
Human anatomy fifth edition Physiology of sport and exercise 5th edition
Physiology of sport and exercise 5th edition Eleventh 5 year plan
Eleventh 5 year plan Eleventh 5 year plan
Eleventh 5 year plan Thfive
Thfive For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Respiratory physiology
Respiratory physiology Tattoo anatomy and physiology
Tattoo anatomy and physiology Science olympiad anatomy and physiology
Science olympiad anatomy and physiology Structure anatomy and physiology in agriculture
Structure anatomy and physiology in agriculture Anatomy and physiology bones
Anatomy and physiology bones Peptic ulcer disease anatomy
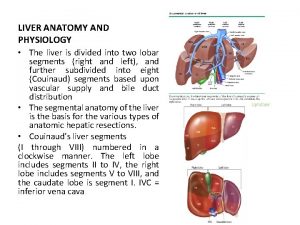
Peptic ulcer disease anatomy Sheep liver lobes
Sheep liver lobes Podbřišek
Podbřišek Wpigastric region
Wpigastric region Blood in anatomy and physiology
Blood in anatomy and physiology Chapter 14 anatomy and physiology
Chapter 14 anatomy and physiology