Holes Human Anatomy and Physiology 12 th Edition


- Slides: 16

Hole’s Human Anatomy and Physiology 12 th Edition Chapter 2, pp. 51 -55 Introduction to Chemistry, part I Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. 1 -1

Chemical Basis of Life Why study chemistry in an Anatomy and Physiology class? 2 -2

Matter • takes up space + has weight • solids, liquids and gases • atom = smallest stable unit of matter • different types of atoms form from different elements 2 -3

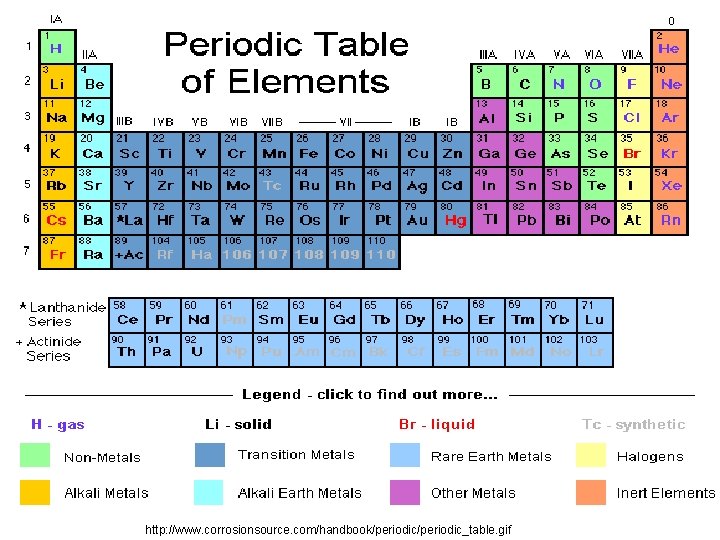
Elements • 92 naturally occurring elements • additional created by man • form all matter, both living and non-living • 26 elements found in humans • each type of element is composed of chemically identical atoms • an atom is the smallest particle of an element 2 -3

http: //www. corrosionsource. com/handbook/periodic_table. gif


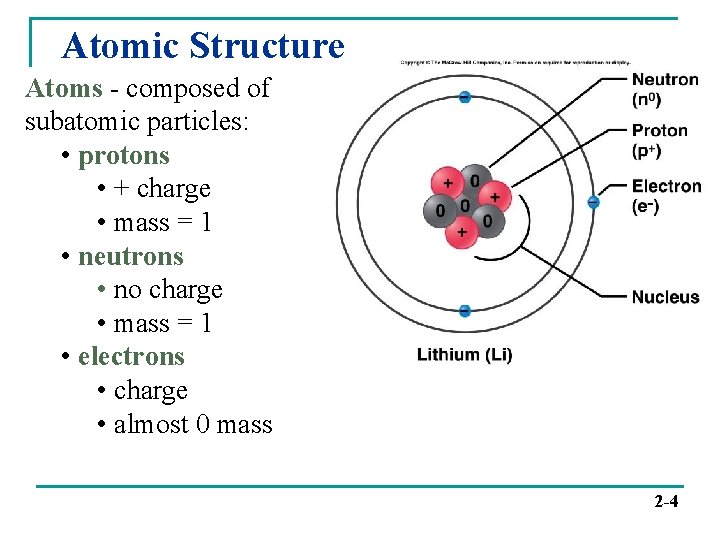
Atomic Structure Atoms - composed of subatomic particles: • protons • + charge • mass = 1 • neutrons • no charge • mass = 1 • electrons • charge • almost 0 mass 2 -4

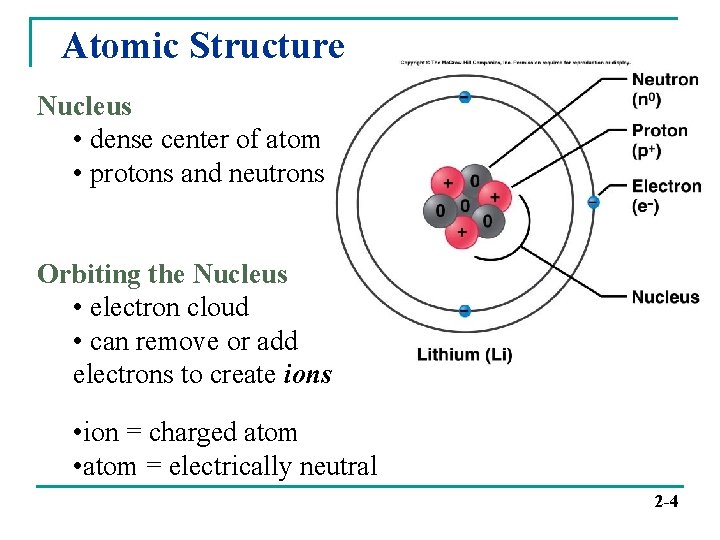
Atomic Structure Nucleus • dense center of atom • protons and neutrons Orbiting the Nucleus • electron cloud • can remove or add electrons to create ions • ion = charged atom • atom = electrically neutral 2 -4

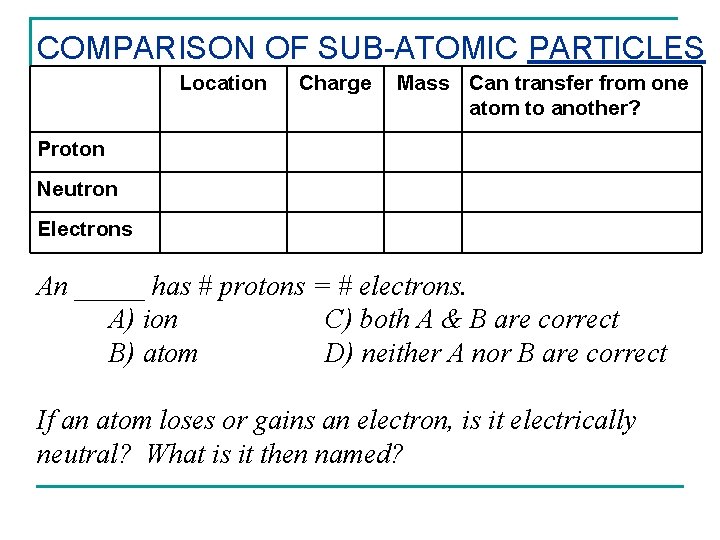
COMPARISON OF SUB-ATOMIC PARTICLES Location Charge Mass Can transfer from one atom to another? Proton Neutron Electrons An _____ has # protons = # electrons. A) ion C) both A & B are correct B) atom D) neither A nor B are correct If an atom loses or gains an electron, is it electrically neutral? What is it then named?

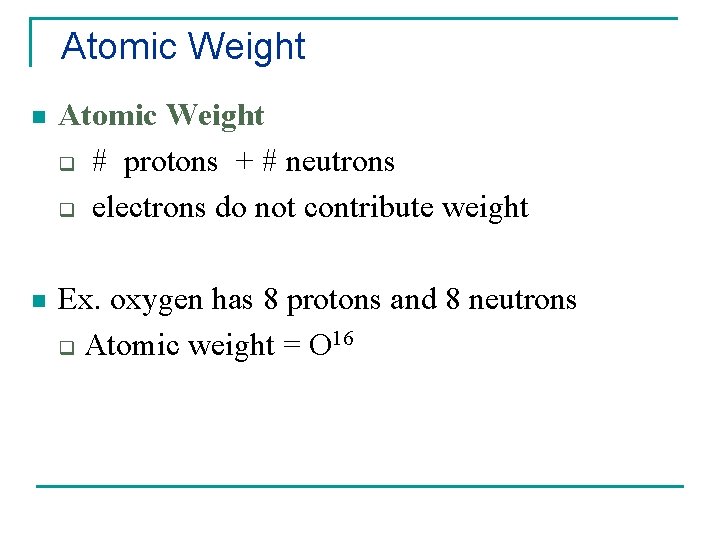
Atomic Weight n Atomic Weight q # protons + # neutrons q electrons do not contribute weight n Ex. oxygen has 8 protons and 8 neutrons q Atomic weight = O 16

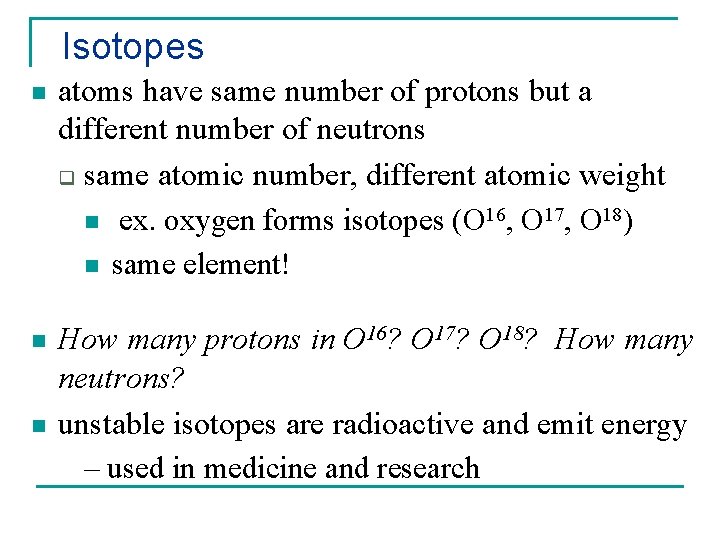
Isotopes n atoms have same number of protons but a different number of neutrons q same atomic number, different atomic weight n ex. oxygen forms isotopes (O 16, O 17, O 18) n same element! n How many protons in O 16? O 17? O 18? How many neutrons? unstable isotopes are radioactive and emit energy – used in medicine and research n

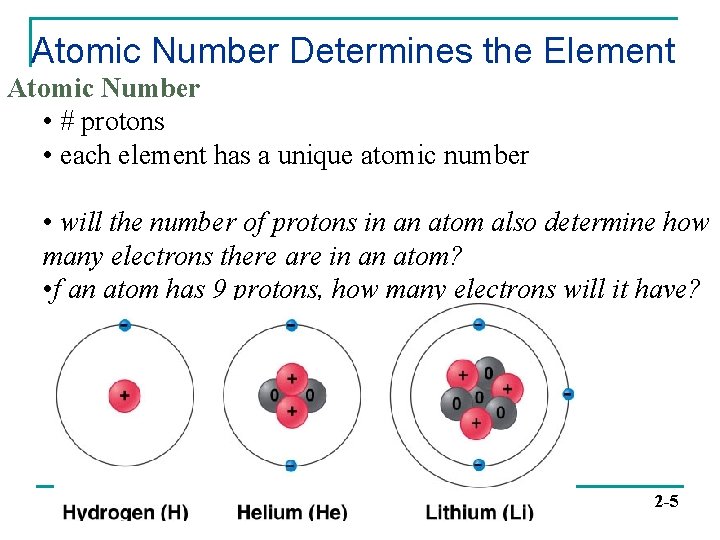
Atomic Number Determines the Element Atomic Number • # protons • each element has a unique atomic number • will the number of protons in an atom also determine how many electrons there are in an atom? • f an atom has 9 protons, how many electrons will it have? 2 -5

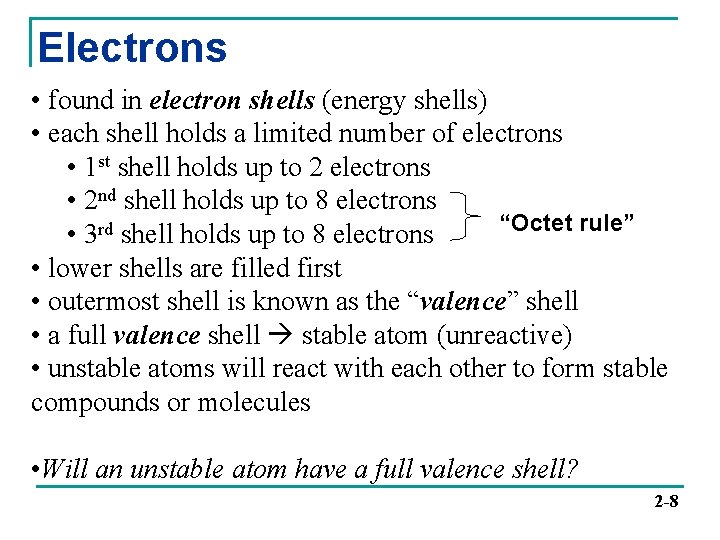
Electrons • found in electron shells (energy shells) • each shell holds a limited number of electrons • 1 st shell holds up to 2 electrons • 2 nd shell holds up to 8 electrons “Octet rule” • 3 rd shell holds up to 8 electrons • lower shells are filled first • outermost shell is known as the “valence” shell • a full valence shell stable atom (unreactive) • unstable atoms will react with each other to form stable compounds or molecules • Will an unstable atom have a full valence shell? 2 -8

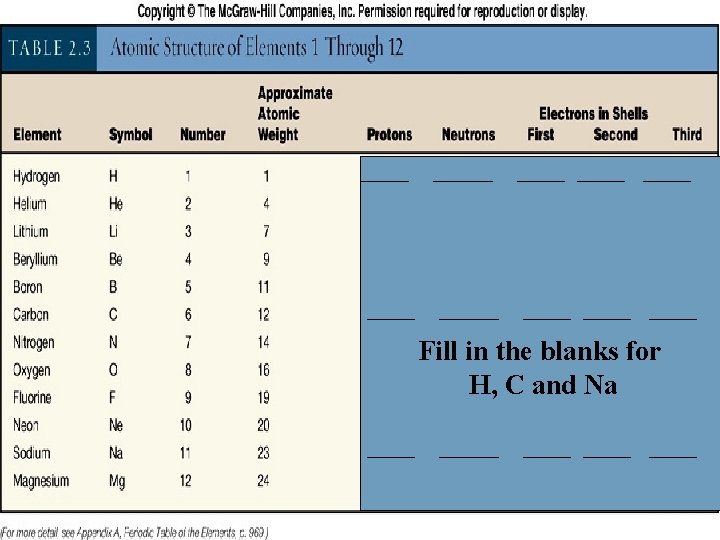
_____ ____ ____ Fill in the blanks for H, C and Na _____ ____

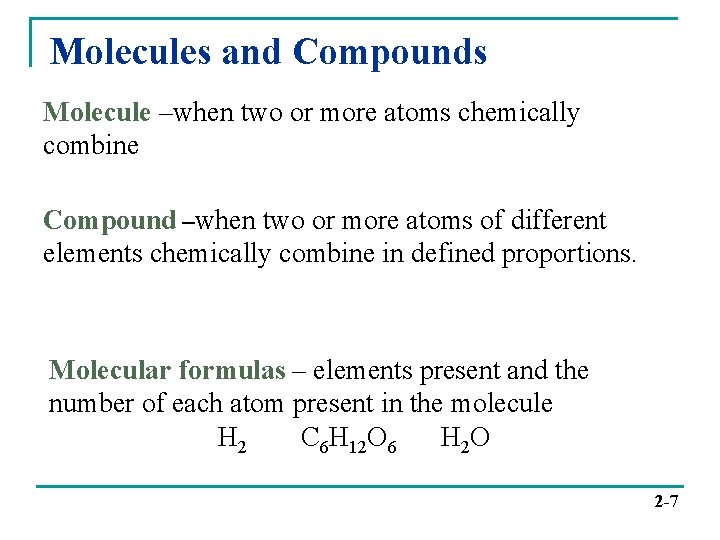
Molecules and Compounds Molecule –when two or more atoms chemically combine Compound –when two or more atoms of different elements chemically combine in defined proportions. Molecular formulas – elements present and the number of each atom present in the molecule H 2 C 6 H 12 O 6 H 2 O 2 -7

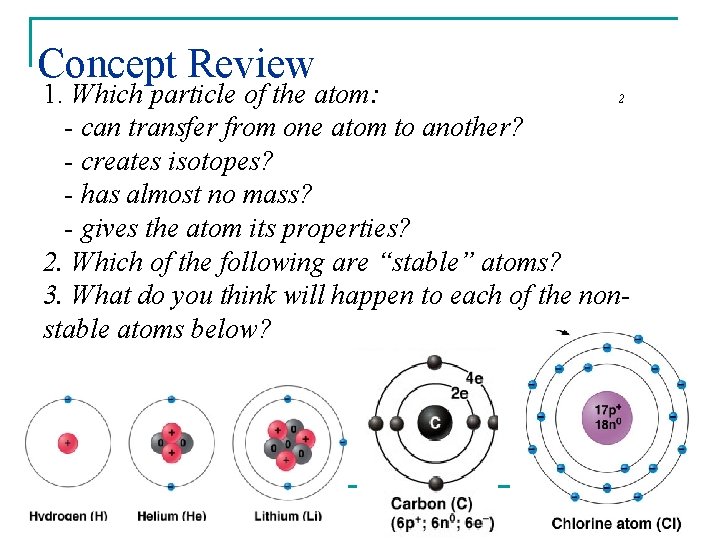
Concept Review 1. Which particle of the atom: 2 - can transfer from one atom to another? - creates isotopes? - has almost no mass? - gives the atom its properties? 2. Which of the following are “stable” atoms? 3. What do you think will happen to each of the nonstable atoms below?
 Holes essential of human anatomy and physiology
Holes essential of human anatomy and physiology 3 layers of muscle
3 layers of muscle Paratubular cyst
Paratubular cyst Holes anatomy and physiology chapter 1
Holes anatomy and physiology chapter 1 Anatomy and physiology edition 9
Anatomy and physiology edition 9 Waistline
Waistline Distal and proximal
Distal and proximal Chapter 2 human reproductive anatomy and physiology
Chapter 2 human reproductive anatomy and physiology Human anatomy fifth edition
Human anatomy fifth edition Human anatomy fifth edition
Human anatomy fifth edition Physiology of sport and exercise 5th edition
Physiology of sport and exercise 5th edition Upper airway anatomy
Upper airway anatomy Tattoo anatomy and physiology
Tattoo anatomy and physiology Science olympiad anatomy and physiology
Science olympiad anatomy and physiology Imperfect flowers examples
Imperfect flowers examples Anatomy and physiology bones
Anatomy and physiology bones Peptic ulcer anatomy
Peptic ulcer anatomy