Heat capacity of the lattice General Theories Thermodynamics

- Slides: 16

Heat capacity of the lattice

General Theories Thermodynamics for no numerical value at finite T Classical Statistical Mechanics Dulong-Petit law, numerical value independent of T For details see Mandl

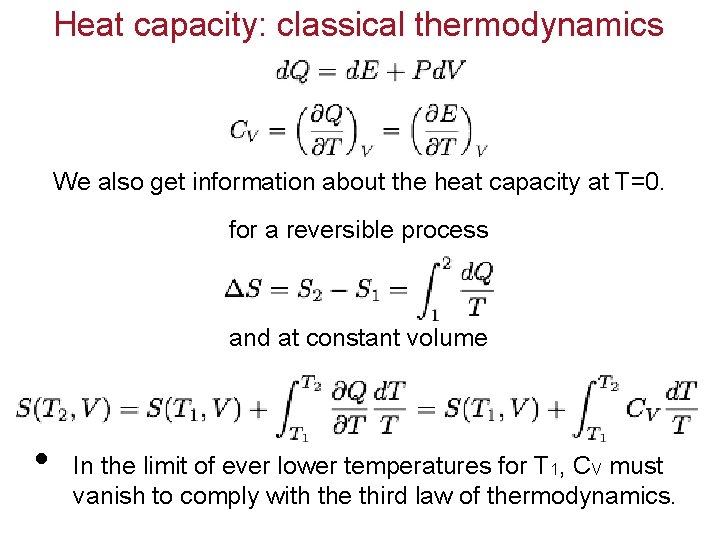
Heat capacity: classical thermodynamics We also get information about the heat capacity at T=0. for a reversible process and at constant volume • In the limit of ever lower temperatures for T 1, CV must vanish to comply with the third law of thermodynamics.

Heat capacity: classical thermodynamics • So classical thermodynamics does not tell us anything about the heat capacity of solids at finite temperature but we know that it must vanish at zero temperature.

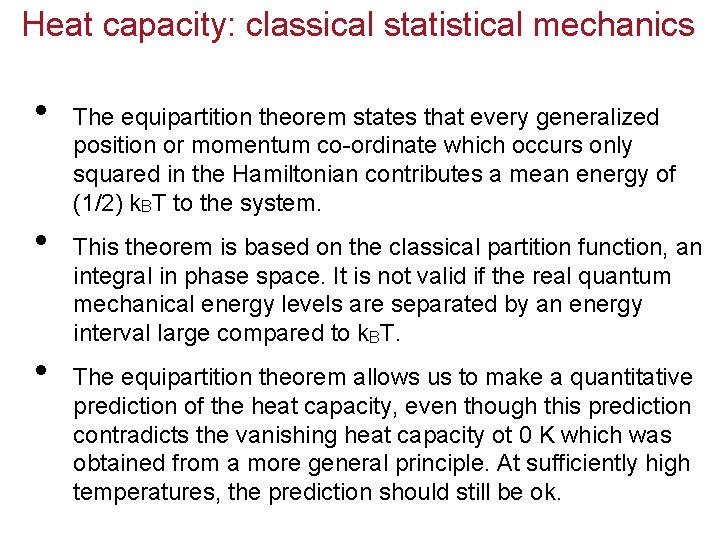
Heat capacity: classical statistical mechanics • • • The equipartition theorem states that every generalized position or momentum co-ordinate which occurs only squared in the Hamiltonian contributes a mean energy of (1/2) k. BT to the system. This theorem is based on the classical partition function, an integral in phase space. It is not valid if the real quantum mechanical energy levels are separated by an energy interval large compared to k. BT. The equipartition theorem allows us to make a quantitative prediction of the heat capacity, even though this prediction contradicts the vanishing heat capacity ot 0 K which was obtained from a more general principle. At sufficiently high temperatures, the prediction should still be ok.

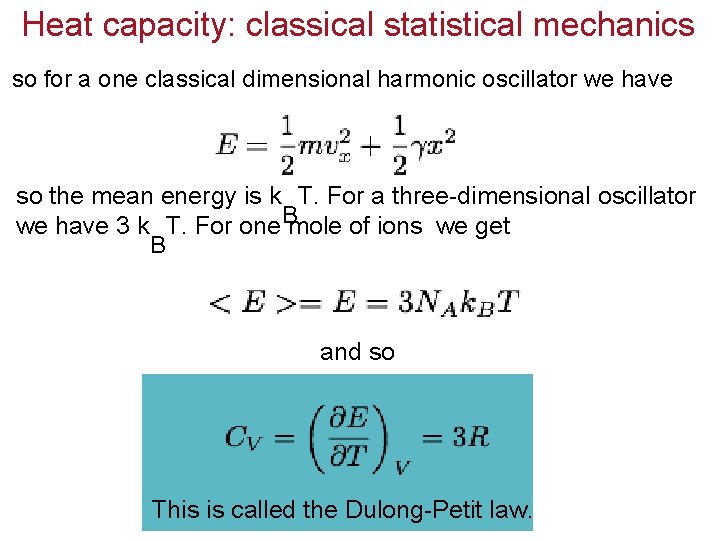
Heat capacity: classical statistical mechanics so for a one classical dimensional harmonic oscillator we have so the mean energy is k T. For a three-dimensional oscillator we have 3 k T. For one. Bmole of ions we get B and so This is called the Dulong-Petit law.

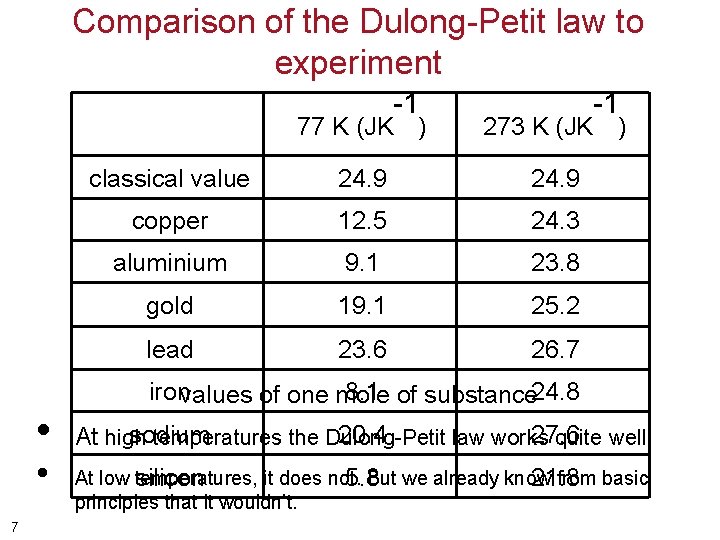
Comparison of the Dulong-Petit law to experiment 77 K (JK • • 7 -1 ) 273 K (JK classical value 24. 9 copper 12. 5 24. 3 aluminium 9. 1 23. 8 gold 19. 1 25. 2 lead 23. 6 26. 7 -1 ) ironvalues of one mole 8. 1 of substance 24. 8 sodium 20. 4 27. 6 At high temperatures the Dulong-Petit law works quite well. At low temperatures, it does not. But we already know from basic silicon 5. 8 21. 8 principles that it wouldn’t.

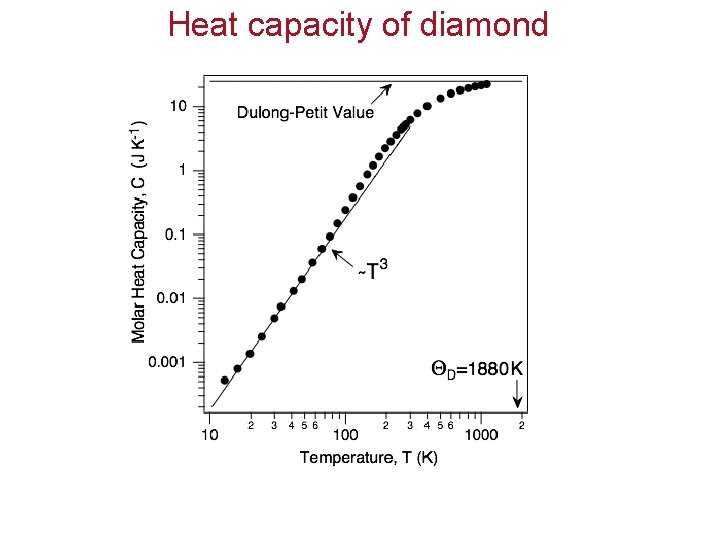
Heat capacity of diamond

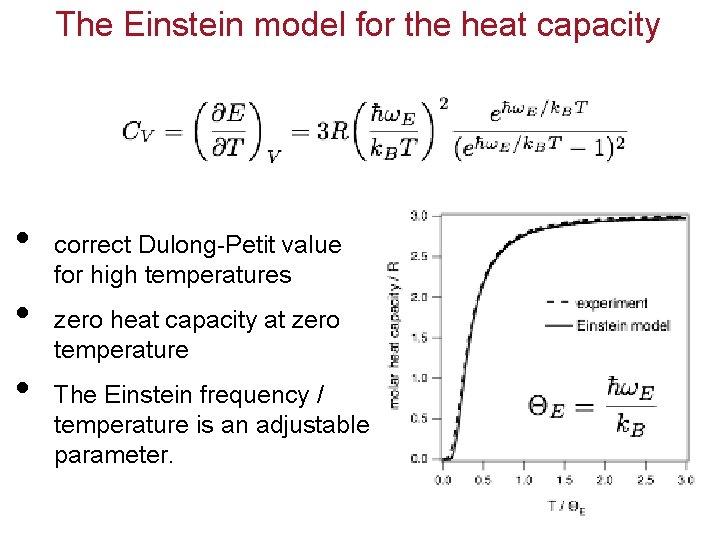
The Einstein model for the heat capacity • • • correct Dulong-Petit value for high temperatures zero heat capacity at zero temperature The Einstein frequency / temperature is an adjustable parameter.

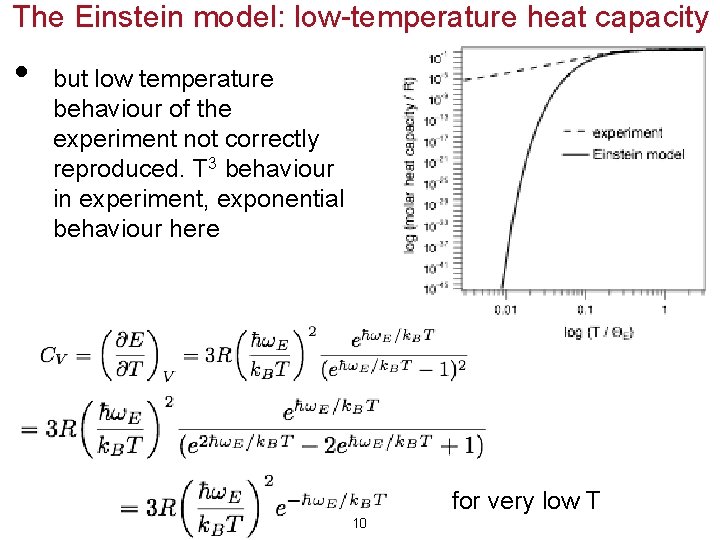
The Einstein model: low-temperature heat capacity • but low temperature behaviour of the experiment not correctly reproduced. T 3 behaviour in experiment, exponential behaviour here for very low T 10

Why does the Einstein model work at high T? Why does it fail at low T? • • • At high T the small spacing between the energy levels is irrelevant. At sufficiently low temperature, the energy level separation is much bigger than k T. B Eventually all the oscillators are “frozen” in the ground state. Increasing T a little does not change this, i. e. it does not change the energy.

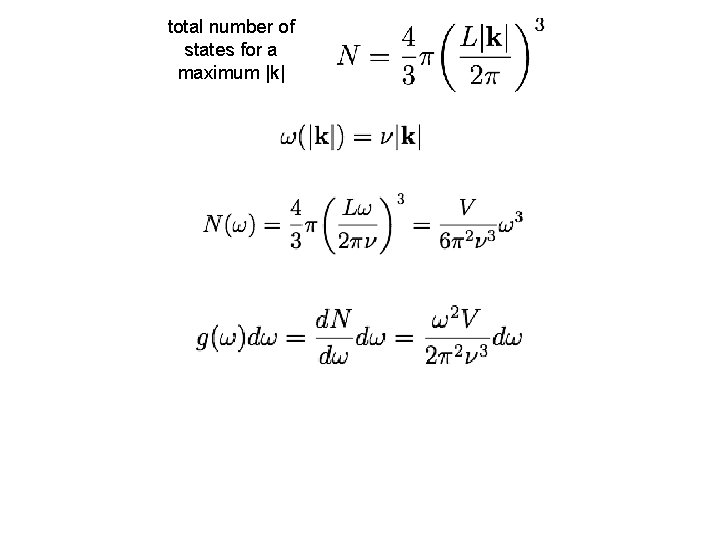
total number of states for a maximum |k|

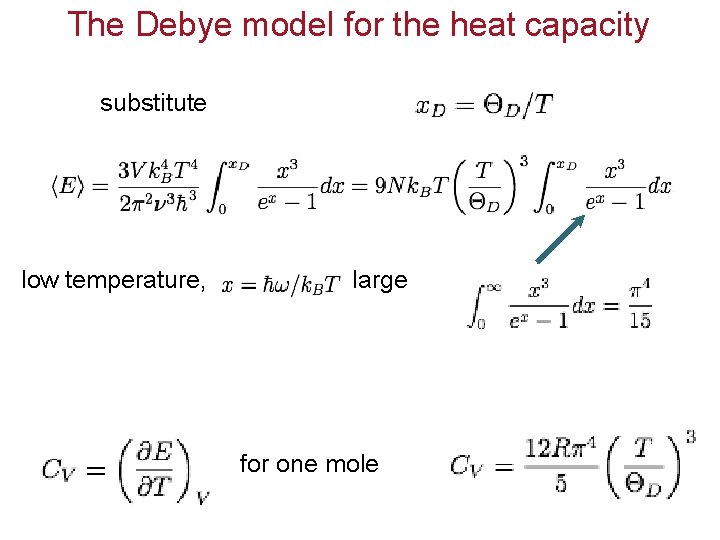
The Debye model for the heat capacity substitute low temperature, large for one mole

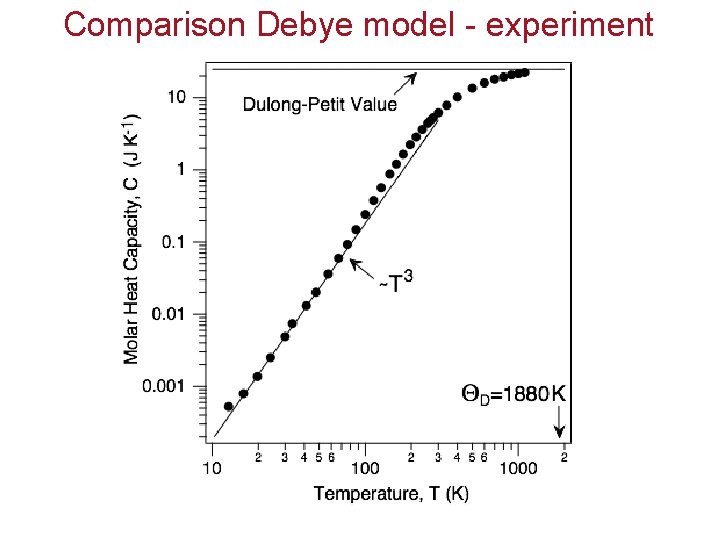
Comparison Debye model - experiment

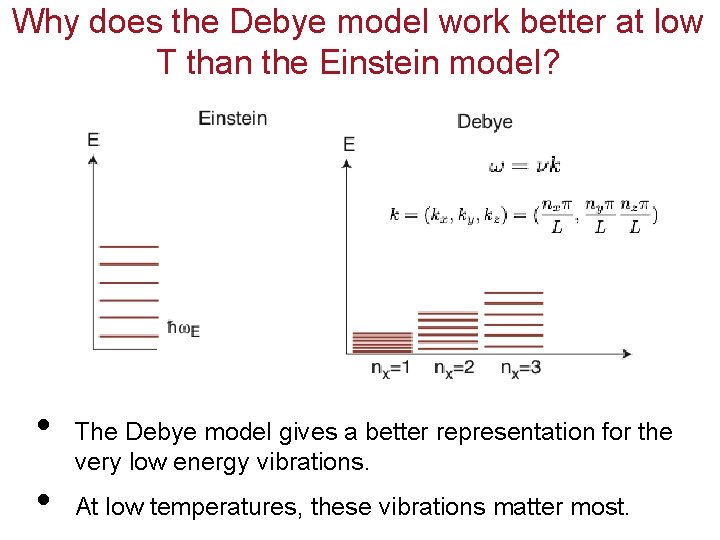
Why does the Debye model work better at low T than the Einstein model? • • The Debye model gives a better representation for the very low energy vibrations. At low temperatures, these vibrations matter most.

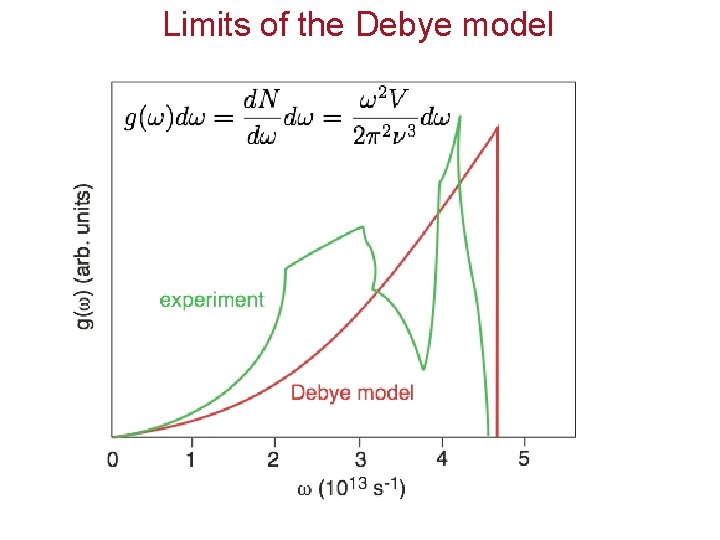
Limits of the Debye model
 Lattice heat capacity
Lattice heat capacity Site:slidetodoc.com
Site:slidetodoc.com Lattice heat capacity ppt
Lattice heat capacity ppt Lattice heat capacity ppt
Lattice heat capacity ppt Heat capacity vs specific heat
Heat capacity vs specific heat Lattice poset
Lattice poset Design capacity and effective capacity examples
Design capacity and effective capacity examples Heat capacity units
Heat capacity units What happens when you heat sugar
What happens when you heat sugar Specific heat capacity of ice cream
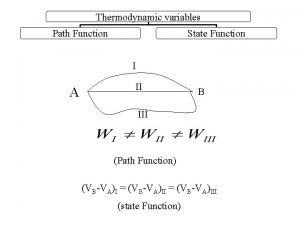
Specific heat capacity of ice cream State function and path function in thermodynamics
State function and path function in thermodynamics How to calculate heat capacity
How to calculate heat capacity Specific latent heat definition
Specific latent heat definition Heat calculations
Heat calculations What is latent heat capacity
What is latent heat capacity How to solve specific heat
How to solve specific heat Q=mct
Q=mct