Section 4 4 Heat Capacity Specific Heat The






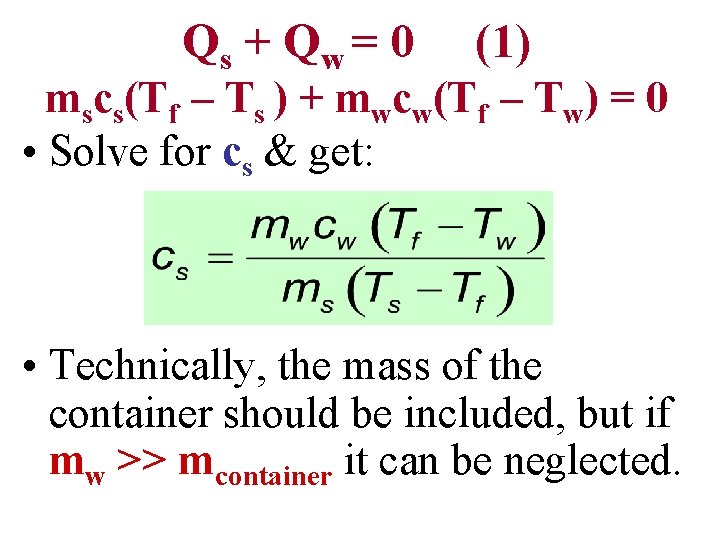

- Slides: 39

Section 4. 4: Heat Capacity & Specific Heat

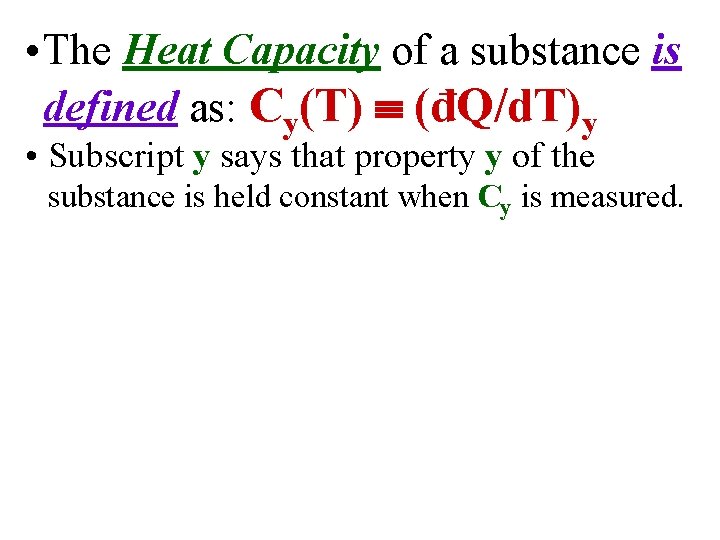
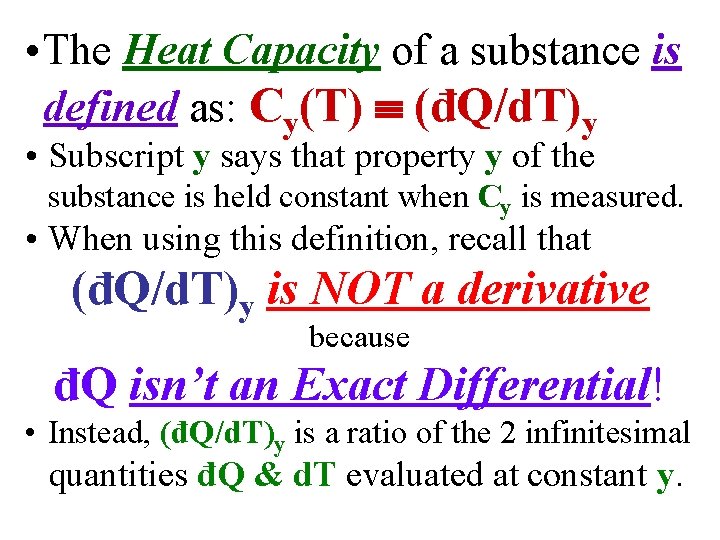
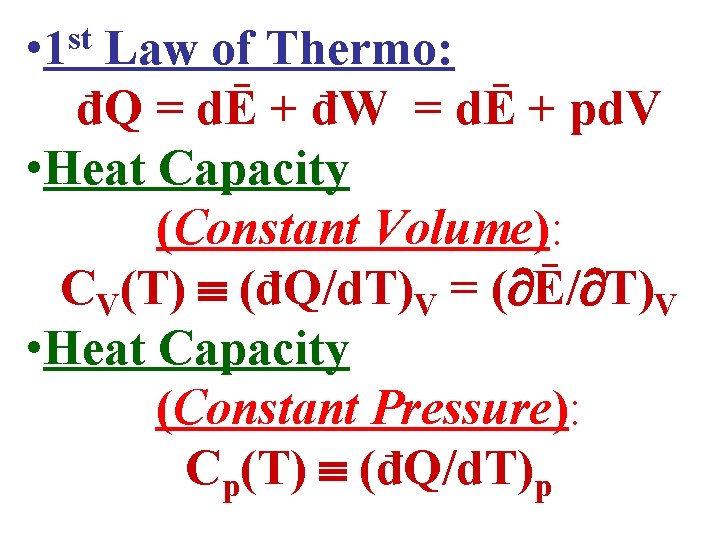
• The Heat Capacity of a substance is defined as: Cy(T) (đQ/d. T)y • Subscript y says that property y of the substance is held constant when Cy is measured.

• The Heat Capacity of a substance is defined as: Cy(T) (đQ/d. T)y • Subscript y says that property y of the substance is held constant when Cy is measured. • When using this definition, recall that (đQ/d. T)y is NOT a derivative because đQ isn’t an Exact Differential! • Instead, (đQ/d. T)y is a ratio of the 2 infinitesimal quantities đQ & d. T evaluated at constant y.

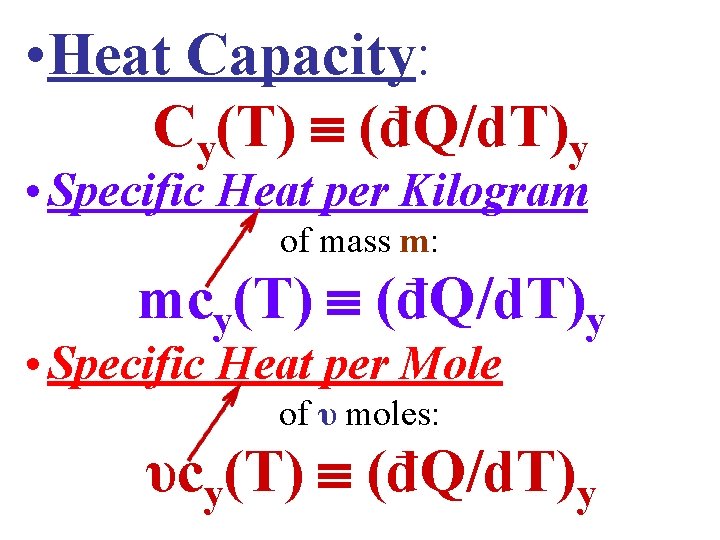
• Heat Capacity: Cy(T) (đQ/d. T)y • Specific Heat per Kilogram of mass m: mcy(T) (đQ/d. T)y • Specific Heat per Mole of υ moles: υcy(T) (đQ/d. T)y

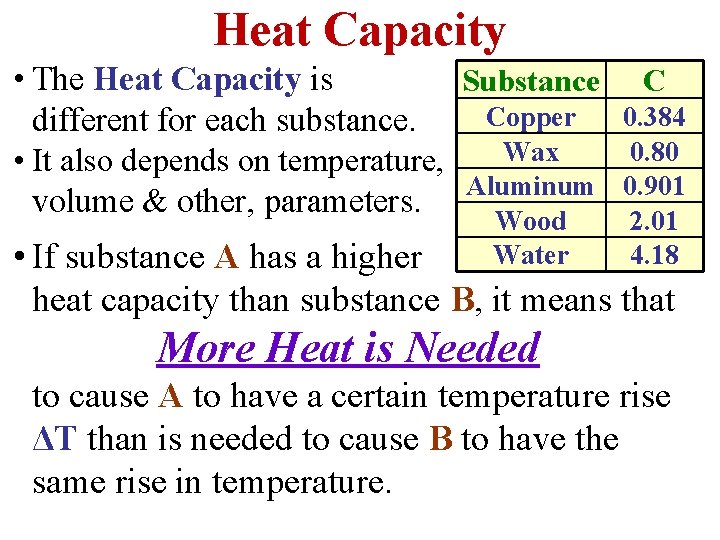
Heat Capacity • The Heat Capacity is Substance C Copper 0. 384 different for each substance. Wax 0. 80 • It also depends on temperature, Aluminum 0. 901 volume & other, parameters. Wood Water 2. 01 4. 18 • If substance A has a higher heat capacity than substance B, it means that More Heat is Needed to cause A to have a certain temperature rise ΔT than is needed to cause B to have the same rise in temperature.

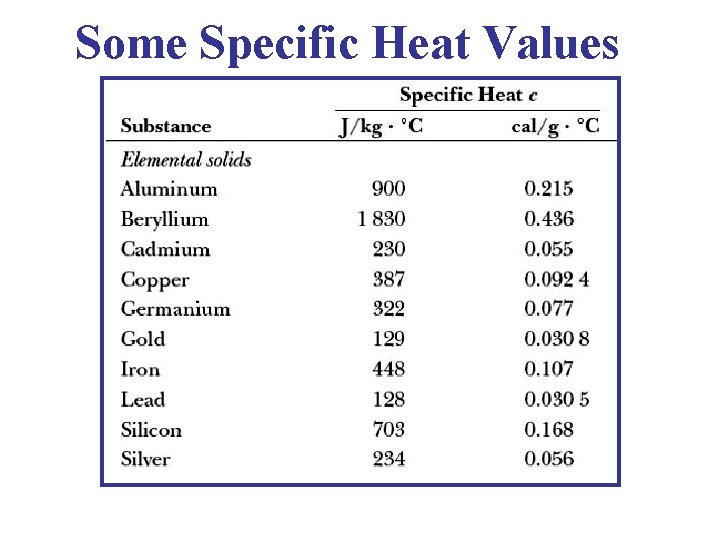
Some Specific Heat Values

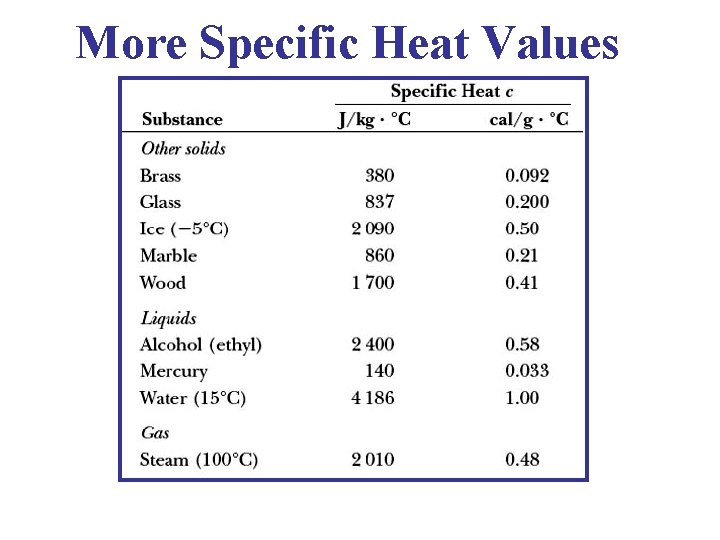
More Specific Heat Values

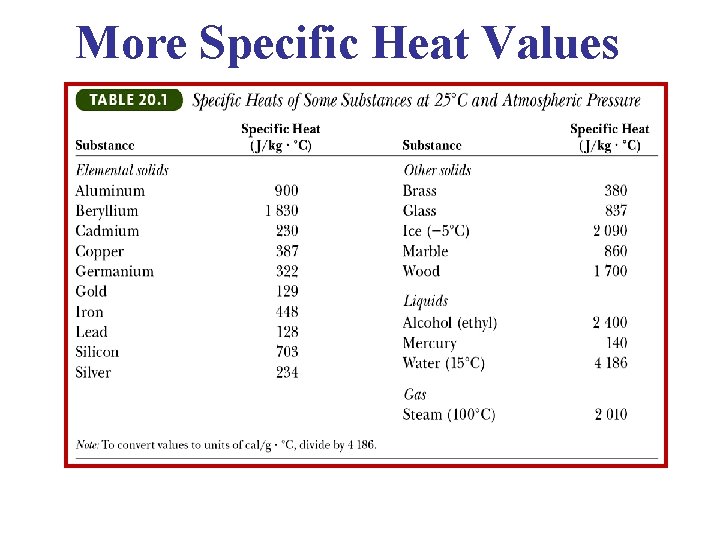
More Specific Heat Values

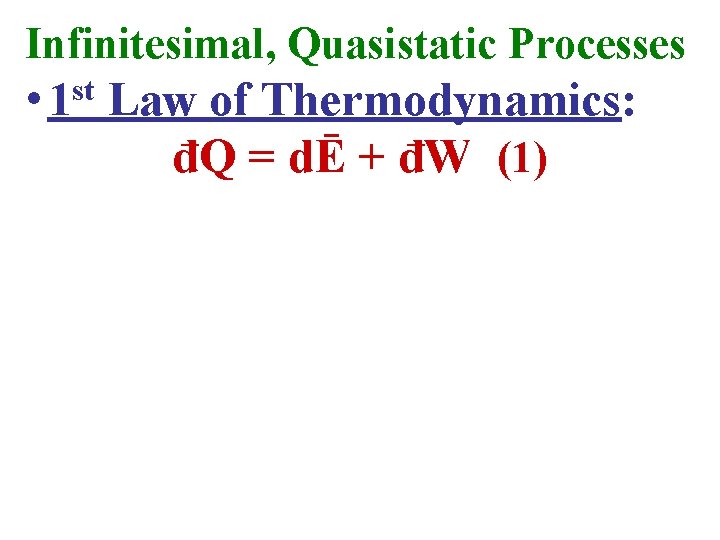
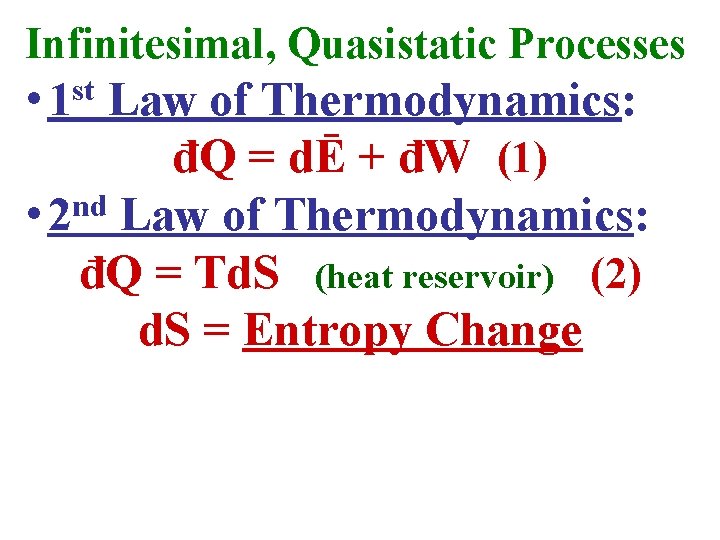
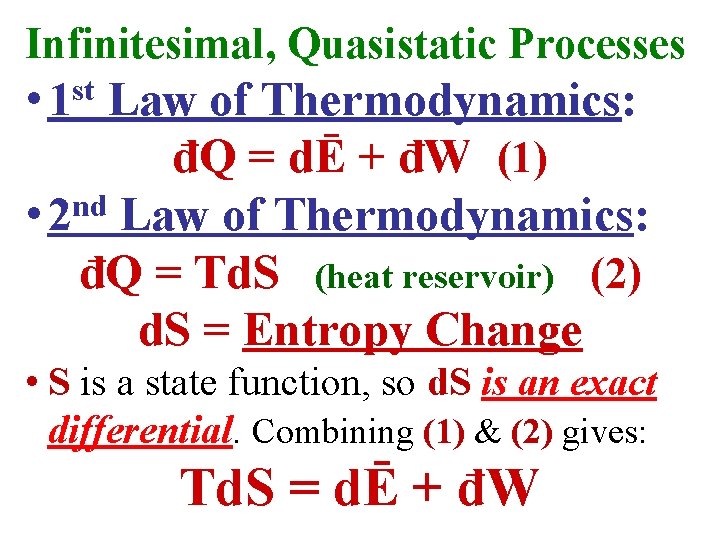
Infinitesimal, Quasistatic Processes • 1 st Law of Thermodynamics: đQ = dĒ + đW (1)

Infinitesimal, Quasistatic Processes • 1 st Law of Thermodynamics: đQ = dĒ + đW (1) nd • 2 Law of Thermodynamics: đQ = Td. S (heat reservoir) (2) d. S = Entropy Change

Infinitesimal, Quasistatic Processes • 1 st Law of Thermodynamics: đQ = dĒ + đW (1) nd • 2 Law of Thermodynamics: đQ = Td. S (heat reservoir) (2) d. S = Entropy Change • S is a state function, so d. S is an exact differential. Combining (1) & (2) gives: Td. S = dĒ + đW

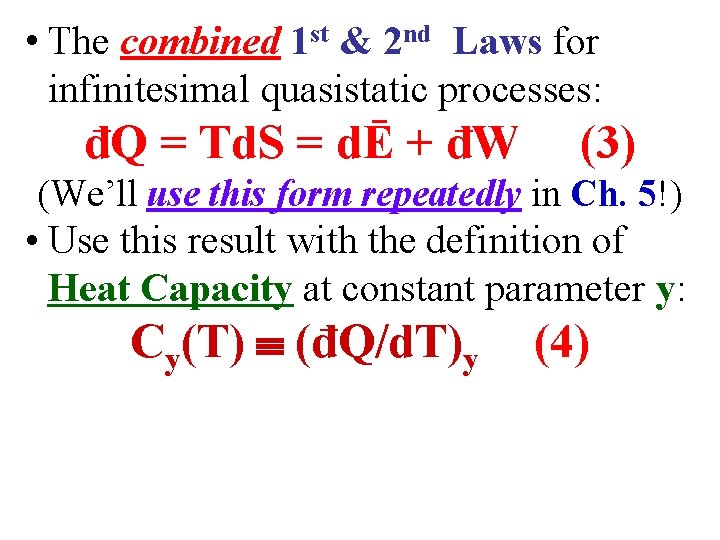
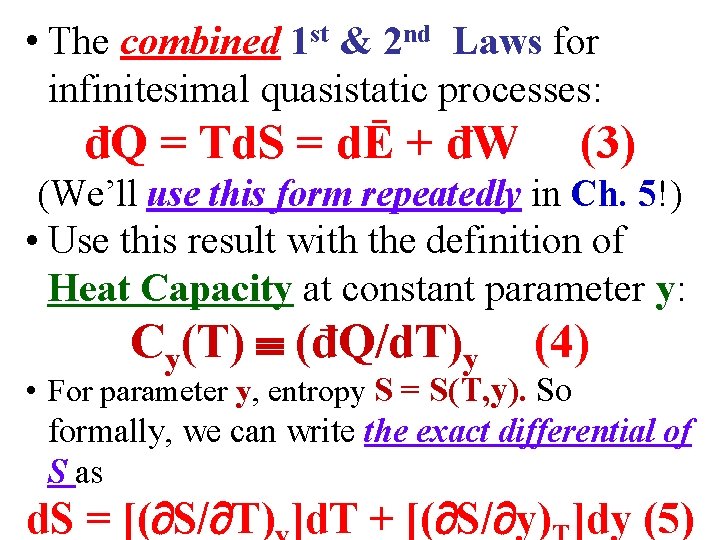
• The combined 1 st & 2 nd Laws for infinitesimal quasistatic processes: đQ = Td. S = dĒ + đW (3) (We’ll use this form repeatedly in Ch. 5!)

• The combined 1 st & 2 nd Laws for infinitesimal quasistatic processes: đQ = Td. S = dĒ + đW (3) (We’ll use this form repeatedly in Ch. 5!) • Use this result with the definition of Heat Capacity at constant parameter y: Cy(T) (đQ/d. T)y (4)

• The combined 1 st & 2 nd Laws for infinitesimal quasistatic processes: đQ = Td. S = dĒ + đW (3) (We’ll use this form repeatedly in Ch. 5!) • Use this result with the definition of Heat Capacity at constant parameter y: Cy(T) (đQ/d. T)y (4) • For parameter y, entropy S = S(T, y). So formally, we can write the exact differential of S as d. S = [( S/ T) ]d. T + [( S/ y) ]dy (5)

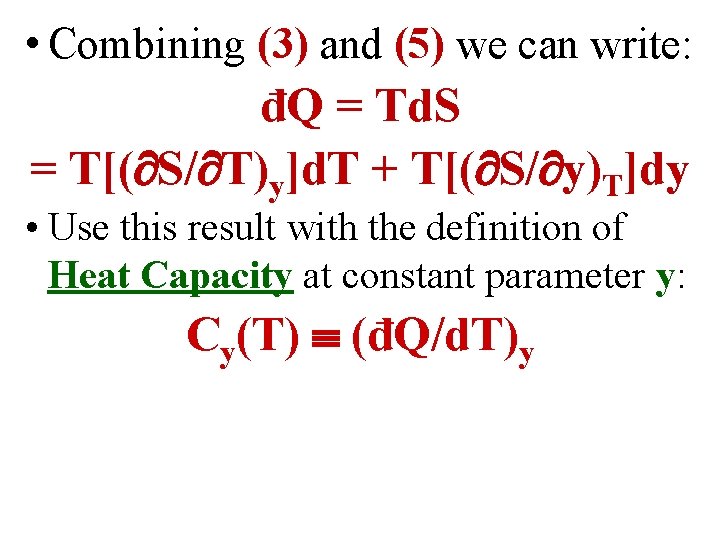
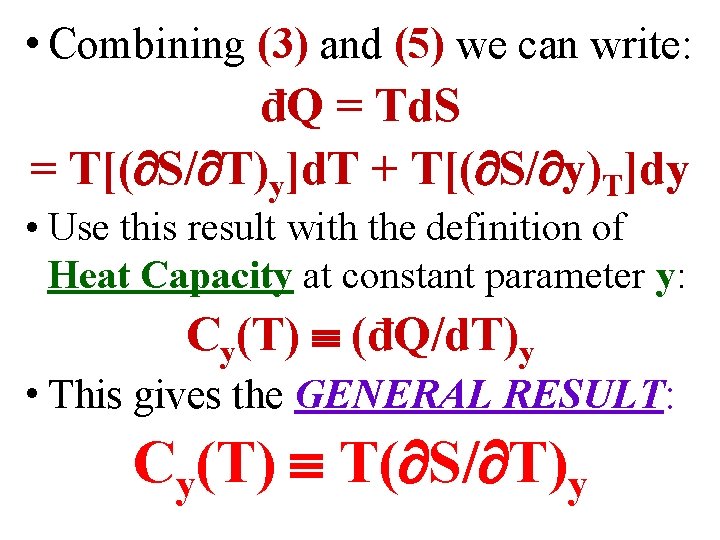
• Combining (3) and (5) we can write: đQ = Td. S = T[( S/ T)y]d. T + T[( S/ y)T]dy

• Combining (3) and (5) we can write: đQ = Td. S = T[( S/ T)y]d. T + T[( S/ y)T]dy • Use this result with the definition of Heat Capacity at constant parameter y: Cy(T) (đQ/d. T)y

• Combining (3) and (5) we can write: đQ = Td. S = T[( S/ T)y]d. T + T[( S/ y)T]dy • Use this result with the definition of Heat Capacity at constant parameter y: Cy(T) (đQ/d. T)y • This gives the GENERAL RESULT: Cy(T) T( S/ T)y

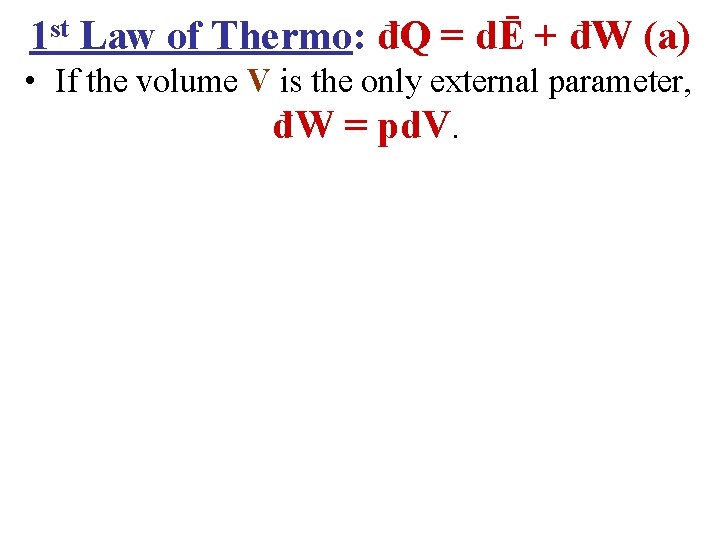
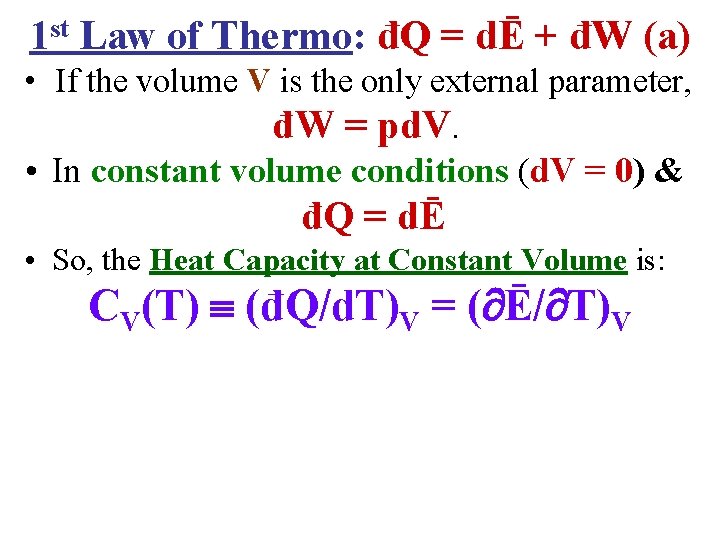
1 st Law of Thermo: đQ = dĒ + đW (a) • If the volume V is the only external parameter, đW = pd. V.

1 st Law of Thermo: đQ = dĒ + đW (a) • If the volume V is the only external parameter, đW = pd. V. • In constant volume conditions (d. V = 0) & đQ = dĒ • So, the Heat Capacity at Constant Volume is: CV(T) (đQ/d. T)V = ( Ē/ T)V

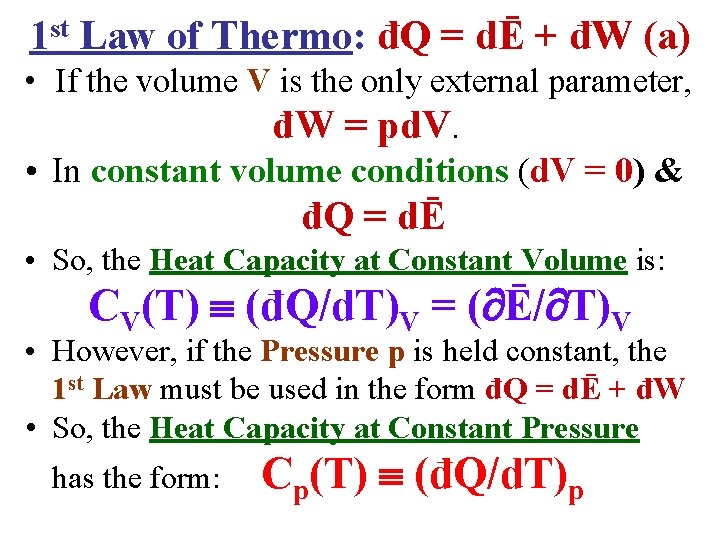
1 st Law of Thermo: đQ = dĒ + đW (a) • If the volume V is the only external parameter, đW = pd. V. • In constant volume conditions (d. V = 0) & đQ = dĒ • So, the Heat Capacity at Constant Volume is: CV(T) (đQ/d. T)V = ( Ē/ T)V • However, if the Pressure p is held constant, the 1 st Law must be used in the form đQ = dĒ + đW • So, the Heat Capacity at Constant Pressure has the form: Cp(T) (đQ/d. T)p

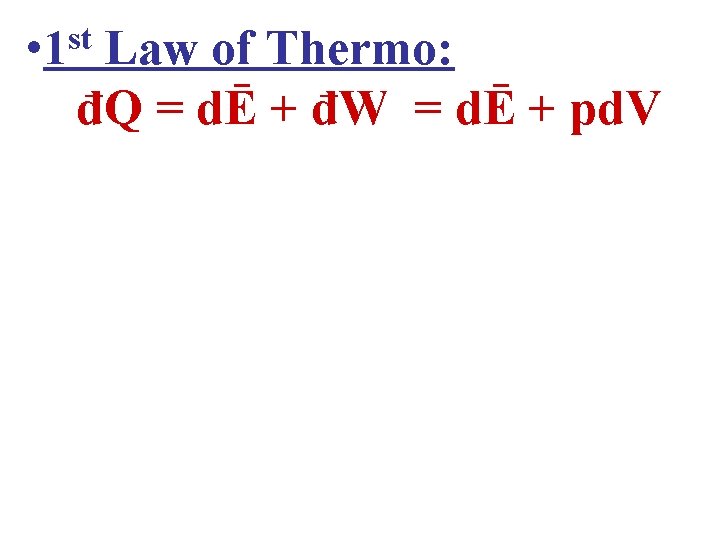
• 1 st Law of Thermo: đQ = dĒ + đW = dĒ + pd. V

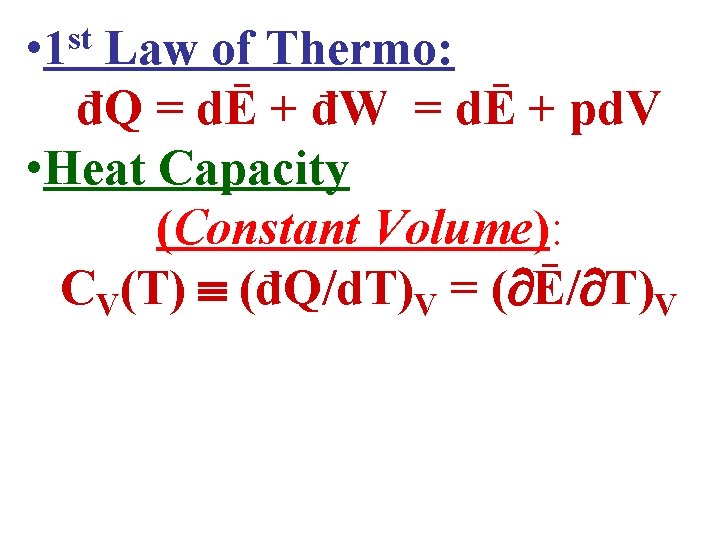
• 1 st Law of Thermo: đQ = dĒ + đW = dĒ + pd. V • Heat Capacity (Constant Volume): CV(T) (đQ/d. T)V = ( Ē/ T)V

• 1 st Law of Thermo: đQ = dĒ + đW = dĒ + pd. V • Heat Capacity (Constant Volume): CV(T) (đQ/d. T)V = ( Ē/ T)V • Heat Capacity (Constant Pressure): Cp(T) (đQ/d. T)p

• Clearly, in general, Cp ≠ CV. • Further, in general, Cp > CV. • However, the measured Cp & CV are very similar for solids & liquids, but very different for gases, so be sure you know which one you’re using if you look one up in a table!

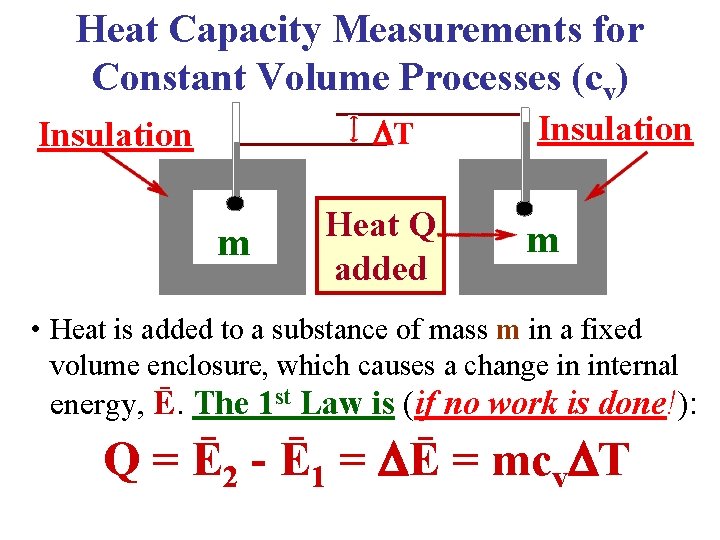
Heat Capacity Measurements for Constant Volume Processes (cv) T Insulation m Heat Q added Insulation m • Heat is added to a substance of mass m in a fixed volume enclosure, which causes a change in internal energy, Ē. The 1 st Law is (if no work is done!): Q = Ē 2 - Ē 1 = Ē = mcv T

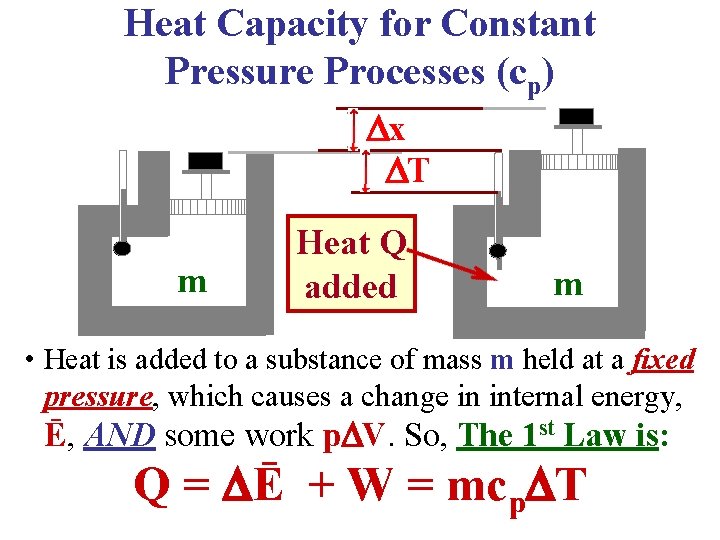
Heat Capacity for Constant Pressure Processes (cp) x T m Heat Q added m • Heat is added to a substance of mass m held at a fixed pressure, which causes a change in internal energy, Ē, AND some work p V. So, The 1 st Law is: Q = Ē + W = mcp T

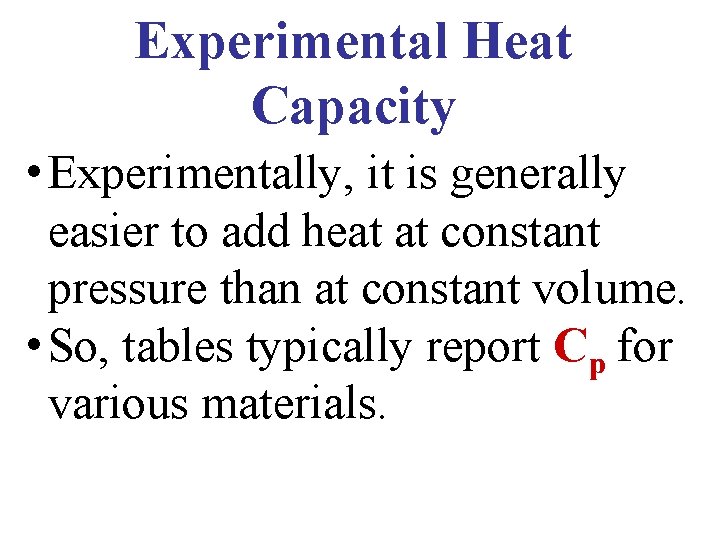
Experimental Heat Capacity • Experimentally, it is generally easier to add heat at constant pressure than at constant volume. • So, tables typically report Cp for various materials.

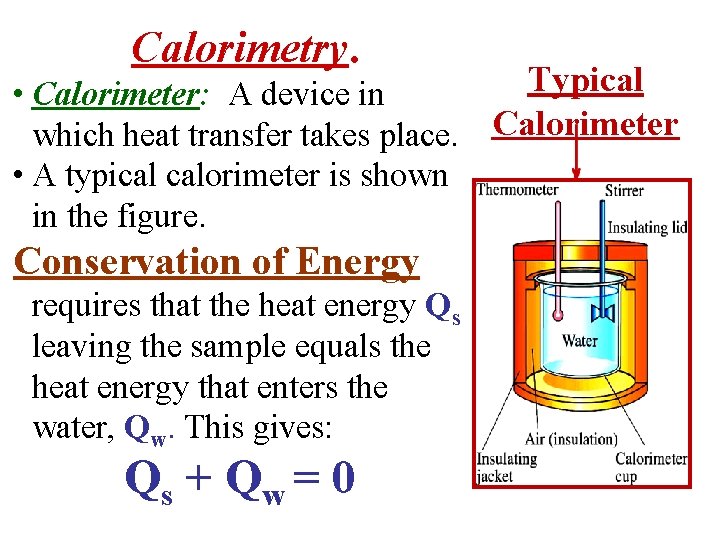
Calorimetry Example: Similar to Reif, pages 141 -142 • A technique to Measure Specific Heat is to heat a sample of material, add it to water, & record the final temperature. • This technique is called Calorimetry. Calorimeter A device in which heat transfer takes place.

Calorimetry. Typical • Calorimeter: A device in which heat transfer takes place. Calorimeter • A typical calorimeter is shown in the figure. Conservation of Energy requires that the heat energy Qs leaving the sample equals the heat energy that enters the water, Qw. This gives: Qs + Q w = 0

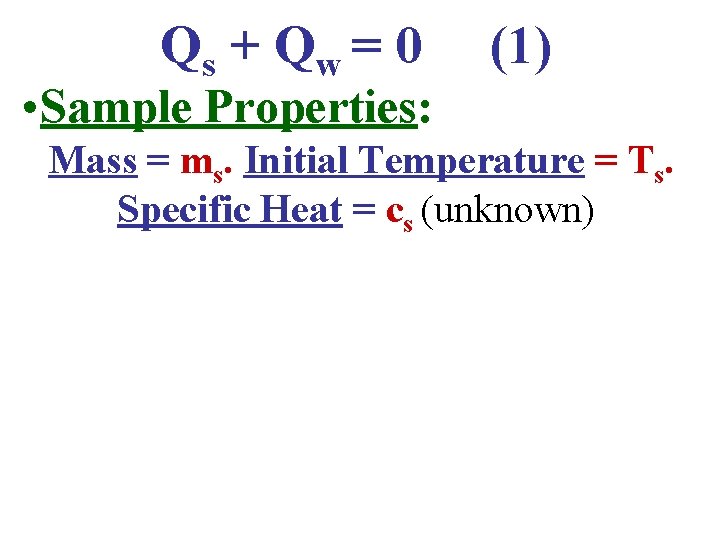
Qs + Q w = 0 (1) • Sample Properties: Mass = ms. Initial Temperature = Ts. Specific Heat = cs (unknown)

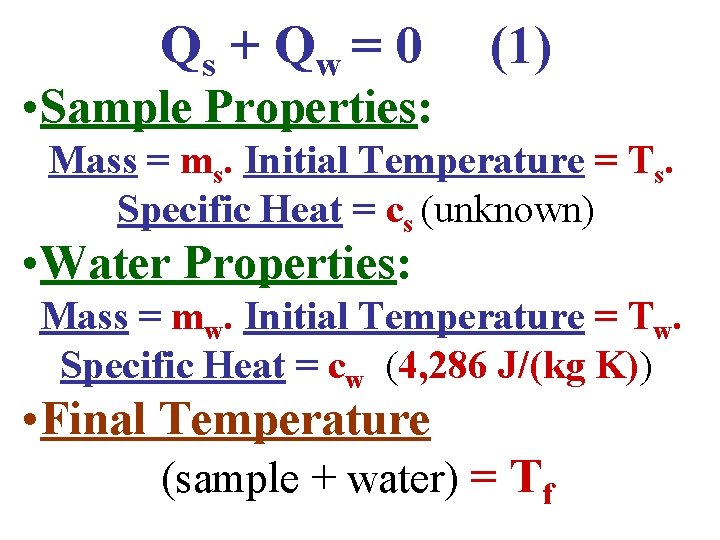
Qs + Q w = 0 (1) • Sample Properties: Mass = ms. Initial Temperature = Ts. Specific Heat = cs (unknown) • Water Properties: Mass = mw. Initial Temperature = Tw. Specific Heat = cw (4, 286 J/(kg K))

Qs + Q w = 0 (1) • Sample Properties: Mass = ms. Initial Temperature = Ts. Specific Heat = cs (unknown) • Water Properties: Mass = mw. Initial Temperature = Tw. Specific Heat = cw (4, 286 J/(kg K)) • Final Temperature (sample + water) = Tf

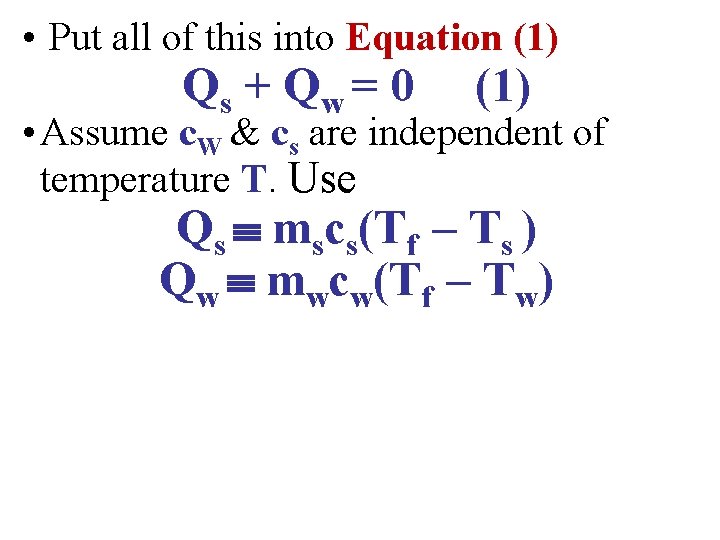
• Put all of this into Equation (1) Qs + Q w = 0 (1)

• Put all of this into Equation (1) Qs + Q w = 0 (1) • Assume c. W & cs are independent of temperature T. Use Qs mscs(Tf – Ts ) Qw mwcw(Tf – Tw)

• Put all of this into Equation (1) Qs + Q w = 0 (1) • Assume c. W & cs are independent of temperature T. Use Qs mscs(Tf – Ts ) Qw mwcw(Tf – Tw) • This gives: mscs(Tf – Ts ) + mwcw(Tf – Tw) = 0
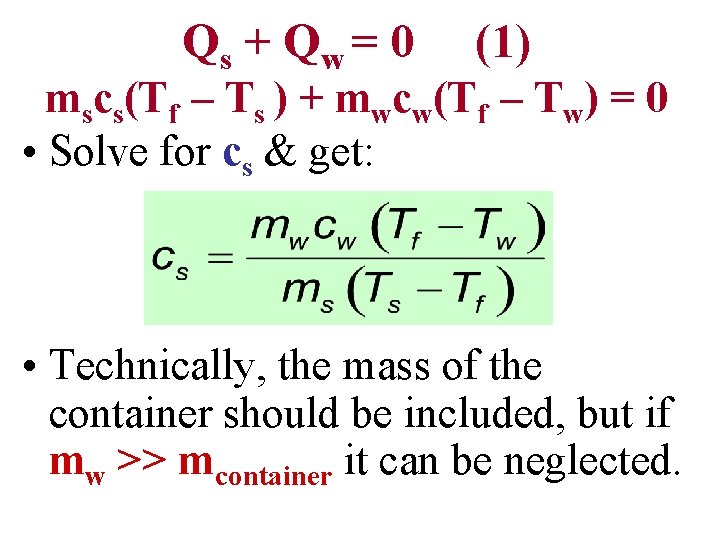
Qs + Q w = 0 (1) mscs(Tf – Ts ) + mwcw(Tf – Tw) = 0 • Solve for cs & get: • Technically, the mass of the container should be included, but if mw >> mcontainer it can be neglected.

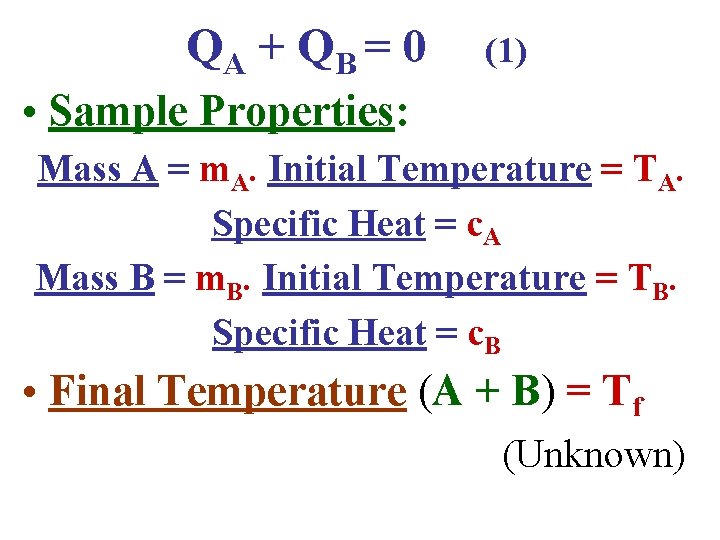
• Consider now a slightly different problem. 2 substances A & B, initially at different temperatures TA & TB. • The specific heats c. A & c. B are known. • The final temperature Tf is unknown. All steps are the same as the previous example until near the end! QA + Q B = 0 (1)

QA + Q B = 0 (1) • Sample Properties: Mass A = m. A. Initial Temperature = TA. Specific Heat = c. A Mass B = m. B. Initial Temperature = TB. Specific Heat = c. B • Final Temperature (A + B) = Tf (Unknown)

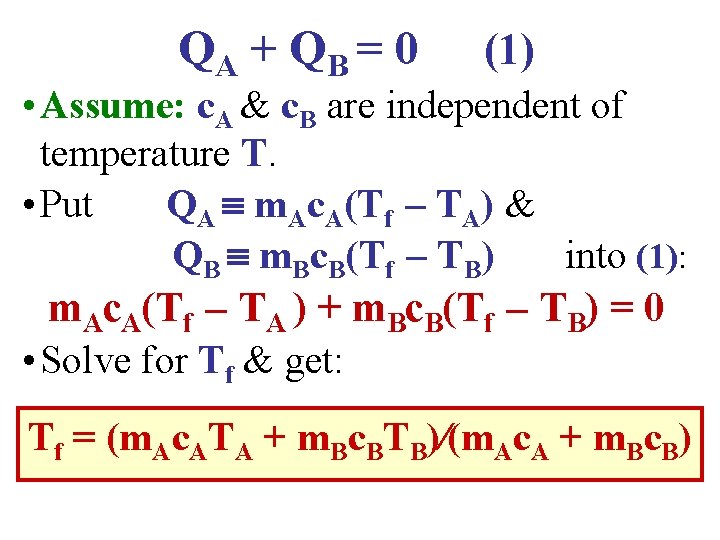
QA + Q B = 0 (1) • Assume: c. A & c. B are independent of temperature T. • Put QA m. Ac. A(Tf – TA) & QB m. Bc. B(Tf – TB) into (1): m. Ac. A(Tf – TA ) + m. Bc. B(Tf – TB) = 0 • Solve for Tf & get: Tf = (m. Ac. ATA + m. Bc. BTB)∕(m. Ac. A + m. Bc. B)