GINA The Role of LTRA Leukotriene Receptor Antagonist














- Slides: 30

GINA : The Role of LTRA (Leukotriene Receptor Antagonist) in Management of Asthma SUSANTHY DJ Medical Functional Staf Saiful Anwar Hospital, Pulmonology and Respiratory Medicine, Medical Faculty, Brawijaya University Malang 1 © Global Initiative for Asthma

Goals of asthma management The long-term goals of asthma management are 1. Symptom control: to achieve good control of symptoms and maintain normal activity levels 2. Risk reduction: to minimize future risk of exacerbations, fixed airflow limitation and medication sideeffects 2 GINA 2016 © Global Initiative for Asthma

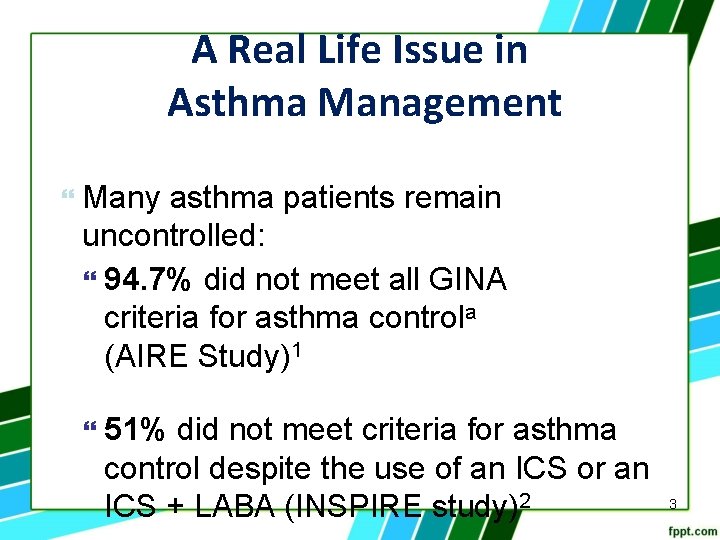
A Real Life Issue in Asthma Management Many asthma patients remain uncontrolled: 94. 7% did not meet all GINA criteria for asthma controla (AIRE Study)1 51% did not meet criteria for asthma control despite the use of an ICS or an ICS + LABA (INSPIRE study)2 3

Studi INSPIRE • Subjek: 3, 415 pasien asma dewasa ≥ 16 thn dari 11 negara yang diresepkan ICS atau ICS+LABA • Hasil: – – 74% masih menggunakan SABA setiap hari; 51% pasien memiliki asma yang tidak terkontrol; 21% pasien memiliki asma yang tidak terkontrol dengan baik; dan hanya 28% pasien yang diklasifikasikan memiliki asma yang terkontrol dengan baik. Partridge MR, et al. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulmonary Medicine 2006; 6: 13. 4

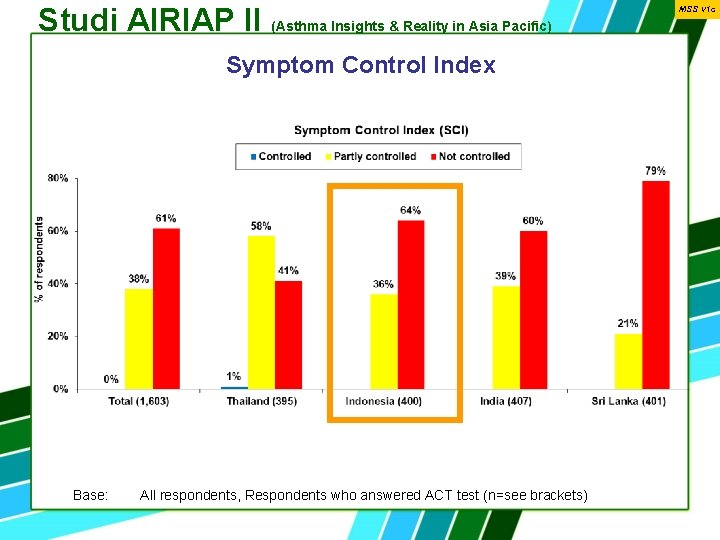
Studi AIRIAP II (Asthma Insights & Reality in Asia Pacific) Symptom Control Index Base: All respondents, Respondents who answered ACT test (n=see brackets) MSS v 1 c

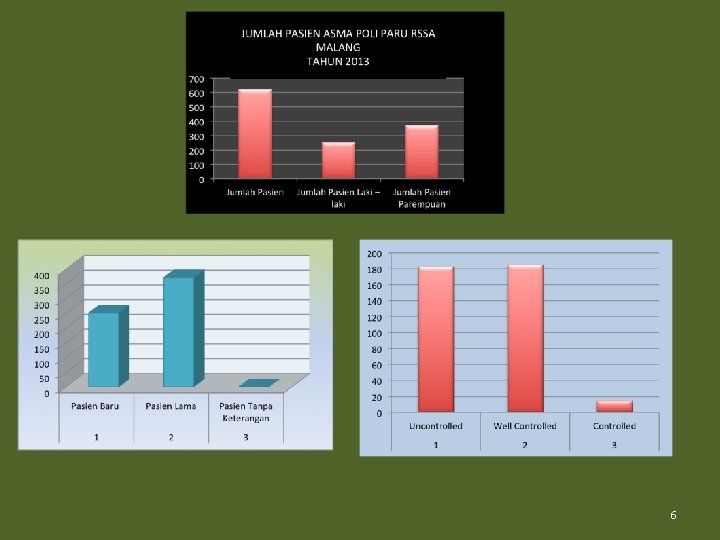
6

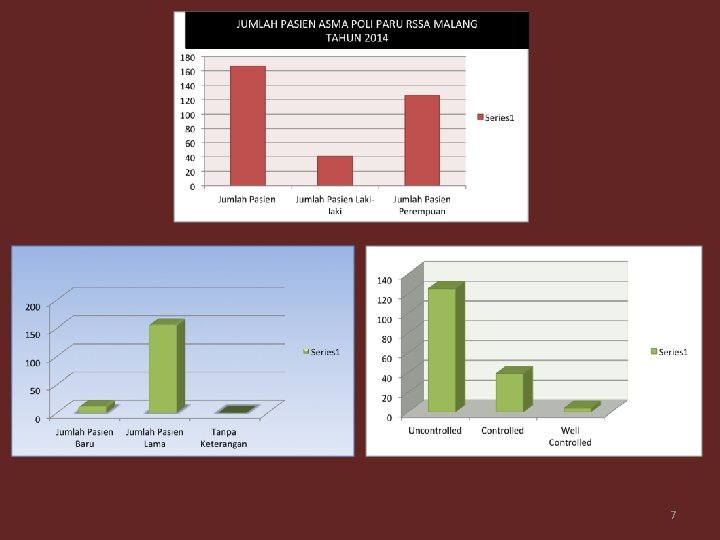
7



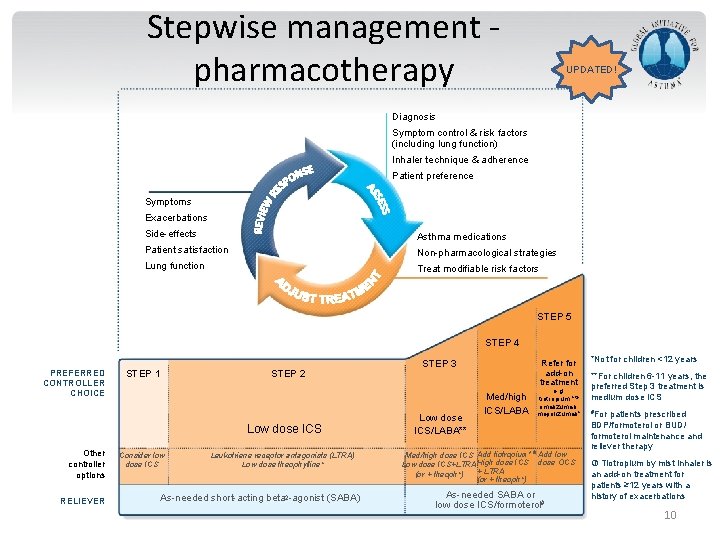
Stepwise management pharmacotherapy UPDATED! Diagnosis Symptom control & risk factors (including lung function) Inhaler technique & adherence Patient preference Symptoms Exacerbations Side-effects Asthma medications Patient satisfaction Non-pharmacological strategies Lung function Treat modifiable risk factors STEP 5 STEP 4 PREFERRED CONTROLLER CHOICE STEP 1 STEP 2 Low dose ICS Other controller options RELIEVER Consider low dose ICS Leukotriene receptor antagonists (LTRA) Low dose theophylline* As-needed short-acting beta 2 -agonist (SABA) GINA 2016, Box 3 -5 (2/8) (upper part) STEP 3 Low dose ICS/LABA** Refer for add-on treatment Med/high ICS/LABA e. g. tiotropium, * omalizumab, mepolizumab* Med/high dose ICS Add tiotropium* Add low Low dose ICS+LTRA High dose ICS dose OCS + LTRA (or + theoph*) As-needed SABA or low dose ICS/formoterol# *Not for children <12 years **For children 6 -11 years, the preferred Step 3 treatment is medium dose ICS #For patients prescribed BDP/formoterol or BUD/ formoterol maintenance and reliever therapy Tiotropium by mist inhaler is an add-on treatment for patients ≥ 12 years with a history of exacerbations 10

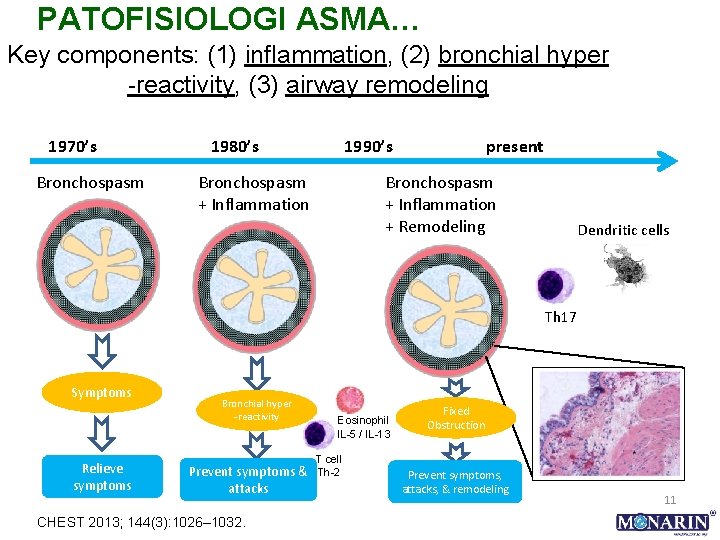
PATOFISIOLOGI ASMA… Key components: (1) inflammation, (2) bronchial hyper -reactivity, (3) airway remodeling 1970’s Bronchospasm 1980’s 1990’s Bronchospasm + Inflammation present Bronchospasm + Inflammation + Remodeling Dendritic cells Th 17 Symptoms Relieve symptoms Bronchial hyper -reactivity Prevent symptoms & attacks CHEST 2013; 144(3): 1026– 1032. Eosinophil IL-5 / IL-13 T cell Th-2 Fixed Obstruction Prevent symptoms, attacks, & remodeling 11

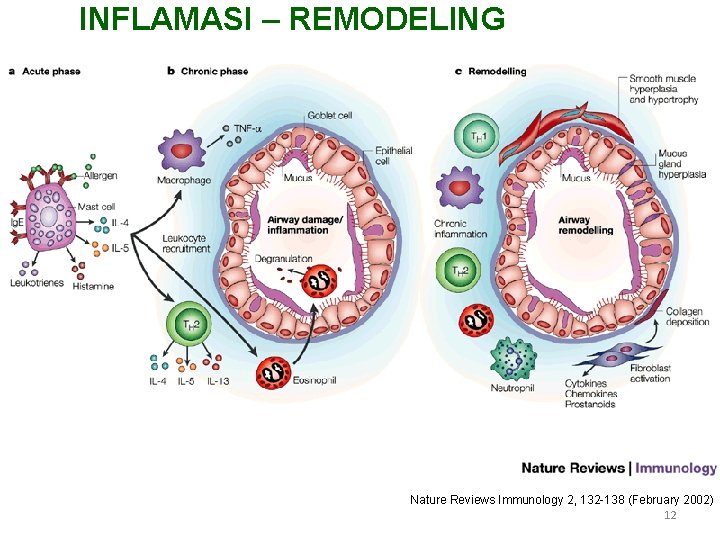
INFLAMASI – REMODELING Nature Reviews Immunology 2, 132 -138 (February 2002) 12

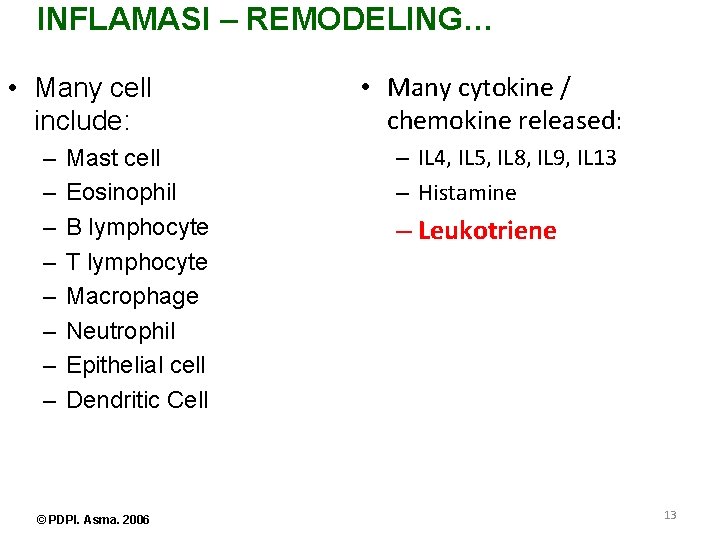
INFLAMASI – REMODELING… • Many cell include: – – – – Mast cell Eosinophil B lymphocyte T lymphocyte Macrophage Neutrophil Epithelial cell Dendritic Cell © PDPI. Asma. 2006 • Many cytokine / chemokine released: – IL 4, IL 5, IL 8, IL 9, IL 13 – Histamine – Leukotriene 13

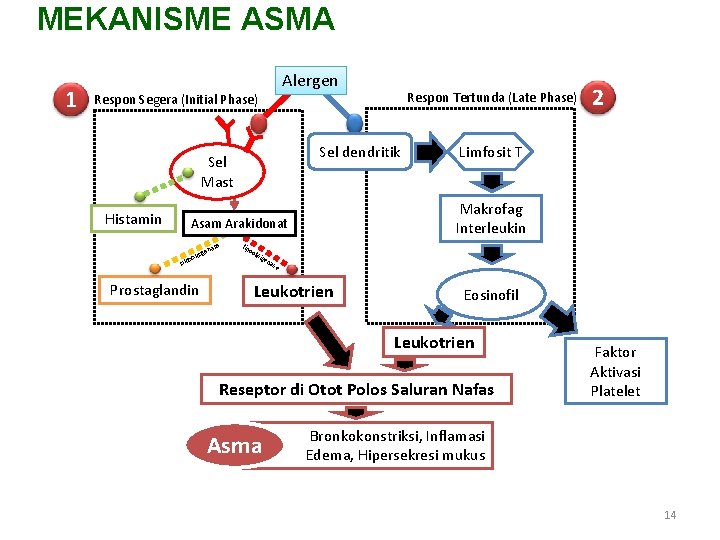
MEKANISME ASMA 1 Alergen Respon Segera (Initial Phase) Sel dendritik Sel Mast Histamin se ena s Prostaglandin lip 2 Limfosit T Makrofag Interleukin Asam Arakidonat ig oks iklo Respon Tertunda (Late Phase) oo ksi ge na se Leukotrien Eosinofil Leukotrien Reseptor di Otot Polos Saluran Nafas Asma Faktor Aktivasi Platelet Bronkokonstriksi, Inflamasi Edema, Hipersekresi mukus 14

15

ALASAN KONTROL ASMA BURUK § The wrong diagnosis § Incorrect choice of inhaler, poor technique § Smoking - Relative steroid resistance among smokers asthma § Co-morbid Rhinitis § Patients’ beliefs and adherence § Individual variation in response to treatment Haughney J, Price D, Kaplan A, et al. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med 2008; 102: 1681 -93

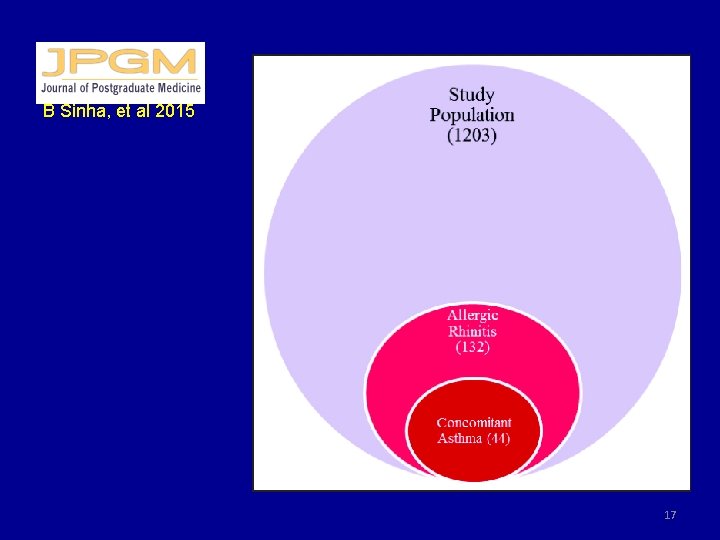
B Sinha, et al 2015 17

Perubahan Target Terapi: LEUKOTRIEN • Diproduksi oleh leukosit dan memiliki struktur tiga rantai ganda (triena) • Mediator pro-inflamasi, hasil dari aktivasi sel-sel imun pada membran sel akibat adanya alergen • Bila berikatan pada reseptor Cys. LT di otot polos saluran nafas kontraksi otot polos, menarik eosinofil, meningkatkan sekresi mukus, meningkatkan permeabilitas vaskuler proliferasi otot polos perubahan model saluran nafas (bronkokonstriksi, hipersekresi) • Sintesis & pelepasan leukotrien tidak dihambat oleh kortikosteroid perlu anti-leukotrien / antagonis reseptor leukotrien (LTRA). Vora AC. Montelukast – place in therapy. Supplement to Journal of The Association of Physicians of India 2014(62): 46 -50. 18 Benninger MS & Waters H. Montelukast: Pharmacology, safety, tolerability and efficacy. Therapeutics 2009(1): 1253 -61.

MONTELUKAST • Montelukast: LTRA paling poten dan spesifik • Sebagai pengontrol pada alergi, asma, maupun asma akibat olahraga (exercise-induced) • Berikatan dengan kuat dan selektif pada reseptor Cys. LT 1 untuk menghambat pengikatan leukotrien LTD 4 antagonis leukotrien anti-inflamasi • Aman & ditoleransi dengan baik tidak ada perbedaan bermakna dengan plasebo Vora AC. Montelukast – place in therapy. Supplement to Journal of The Association of Physicians of India 2014(62): 46 -50. Benninger MS & Waters H. Montelukast: Pharmacology, safety, tolerability and efficacy. Therapeutics 2009(1): 1253 -61. 19

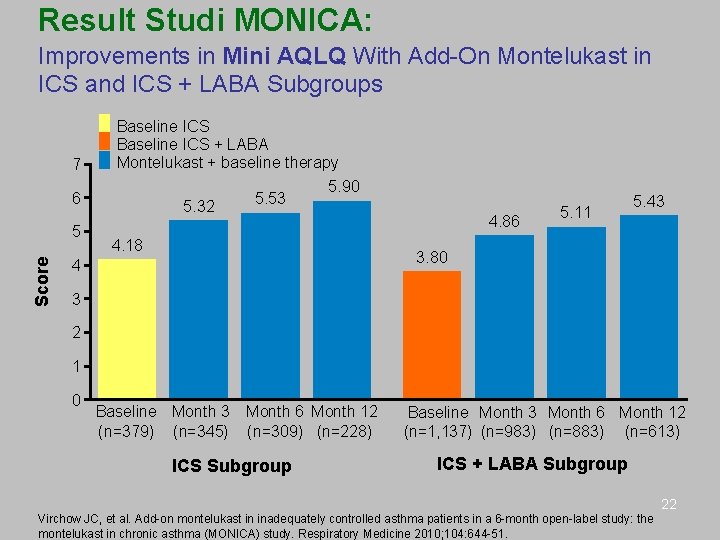
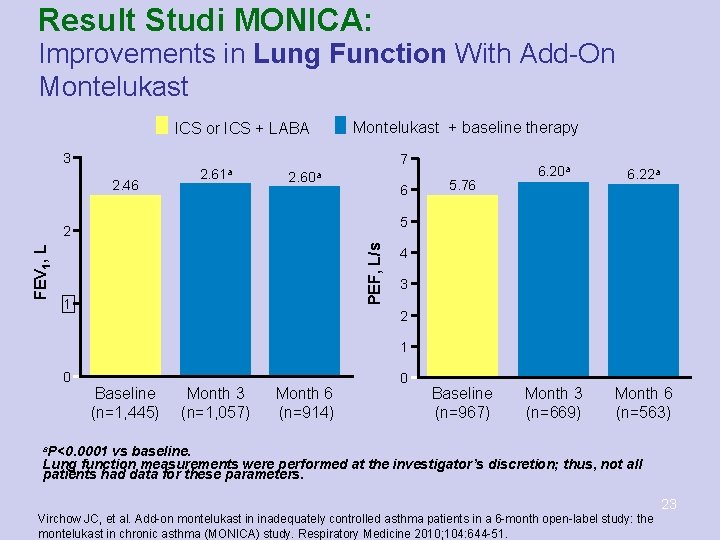
MONTELUKAST – Studi MONICA • • Subjek: ≥ 18 thn dengan asma ringan-sedang diberikan MON 10 mg 1 x 1 sebagai add-on ICS atau ICS+LABA selama 6 bulan Hasil: Terjadi perbaikan klinis ketika montelukast ditambahkan pada terapi ICS atau ICS+LABA. Perbaikan tersebut mencakup kontrol asma, kualitas hidup, fungsi paru, serta status asma, dengan profil keamanan yang baik. Virchow JC, et al. Add-on montelukast in inadequately controlled asthma patients in a 6 -month open-label study: the 20 montelukast in chronic asthma (MONICA) study. Respiratory Medicine 2010; 104: 644 -51.

Result Studi MONICA: Patients in Each ACT Category, % Improvements in ACT Scores With Add-On Montelukast in ICS and ICS + LABA Subgroups ACT Scores 25 (Completely controlled) 20– 24 (Well controlled) 100 2. 1 19. 5 9. 4 15. 2 23. 5 75 29. 8 47. 5 50 25 0 54. 3 48. 3 Baseline (n=388) 39. 3 9. 6 15. 1 45. 2 45. 8 53. 3 27. 6 63. 1 19. 2 14. 1 10. 8 Month 3 (n=357) Month 6 (n=318) ICS Subgroup • 25. 0 16– 19 (Poorly controlled) <16 (Uncontrolled) 0. 9 6. 0 11. 9 13. 4 27. 9 23. 8 6. 5 Month 12 (n=230) Baseline Month 3 (n=1, 163) (n=1, 035) 23. 1 21. 3 20. 4 16. 7 Month 6 (n=911) Month 12 (n=622) ICS + LABA Subgroup ACT scores: LS mean ACT score improved from baseline to Month 12 of add-on montelukast by 6. 6 in the ICS subgroup and 5. 4 in the ICS + LABA subgroup. ICS or ICS + LABA at baseline; add-on montelukast at Months 3, 6, and 12 Slide 21 Virchow JC, et al. Add-on montelukast in inadequately controlled asthma patients in a 6 -month open-label study: the montelukast in chronic asthma (MONICA) study. Respiratory Medicine 2010; 104: 644 -51.

Result Studi MONICA: Improvements in Mini AQLQ With Add-On Montelukast in ICS and ICS + LABA Subgroups 7 Baseline ICS + LABA Montelukast + baseline therapy 6 Score 5 5. 32 5. 53 5. 90 4. 86 4. 18 5. 11 5. 43 3. 80 4 3 2 1 0 Baseline Month 3 (n=379) (n=345) Month 6 Month 12 (n=309) (n=228) ICS Subgroup Baseline Month 3 Month 6 Month 12 (n=1, 137) (n=983) (n=883) (n=613) ICS + LABA Subgroup 22 Virchow JC, et al. Add-on montelukast in inadequately controlled asthma patients in a 6 -month open-label study: the montelukast in chronic asthma (MONICA) study. Respiratory Medicine 2010; 104: 644 -51.

Result Studi MONICA: Improvements in Lung Function With Add-On Montelukast ICS or ICS + LABA Montelukast + baseline therapy 3 2. 46 2. 61 a 7 2. 60 a 6 6. 22 a Month 3 (n=669) Month 6 (n=563) 5 PEF, L/s 2 FEV 1, L 5. 76 6. 20 a 1 4 3 2 1 0 Baseline (n=1, 445) Month 3 (n=1, 057) Month 6 (n=914) 0 Baseline (n=967) a. P<0. 0001 vs baseline. Lung function measurements were performed at the investigator’s discretion; thus, not all patients had data for these parameters. 23 Virchow JC, et al. Add-on montelukast in inadequately controlled asthma patients in a 6 -month open-label study: the montelukast in chronic asthma (MONICA) study. Respiratory Medicine 2010; 104: 644 -51.

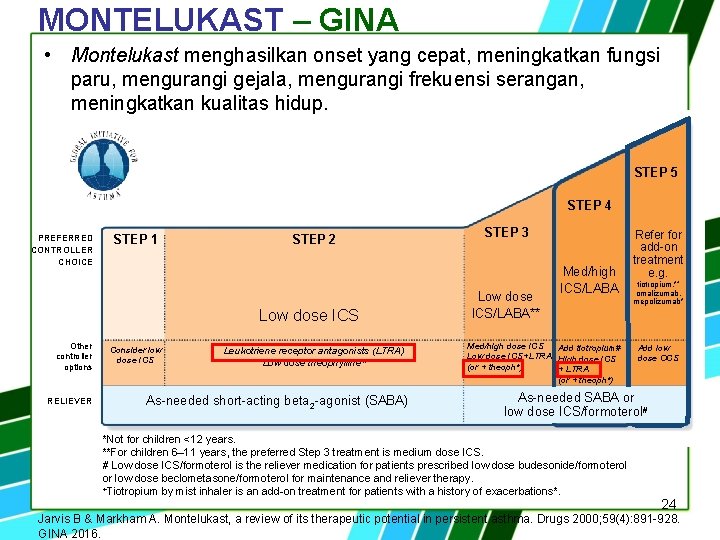
MONTELUKAST – GINA • Montelukast menghasilkan onset yang cepat, meningkatkan fungsi paru, mengurangi gejala, mengurangi frekuensi serangan, meningkatkan kualitas hidup. STEP 5 STEP 4 PREFERRED CONTROLLER CHOICE STEP 1 STEP 2 Low dose ICS Other controller options RELIEVER Consider low dose ICS Leukotriene receptor antagonists (LTRA) Low dose theophylline* As-needed short-acting beta 2 -agonist (SABA) STEP 3 Low dose ICS/LABA** Med/high ICS/LABA Med/high dose ICS Add tiotropium# Low dose ICS+LTRA High dose ICS (or + theoph*) + LTRA (or + theoph*) Refer for add-on treatment e. g. tiotropium, *+ omalizumab, mepolizumab* Add low dose OCS As-needed SABA or low dose ICS/formoterol# *Not for children <12 years. **For children 6– 11 years, the preferred Step 3 treatment is medium dose ICS. # Low dose ICS/formoterol is the reliever medication for patients prescribed low dose budesonide/formoterol or low dose beclometasone/formoterol for maintenance and reliever therapy. +Tiotropium by mist inhaler is an add-on treatment for patients with a history of exacerbations*. 24 Jarvis B & Markham A. Montelukast, a review of its therapeutic potential in persistent asthma. Drugs 2000; 59(4): 891 -928. GINA 2016.

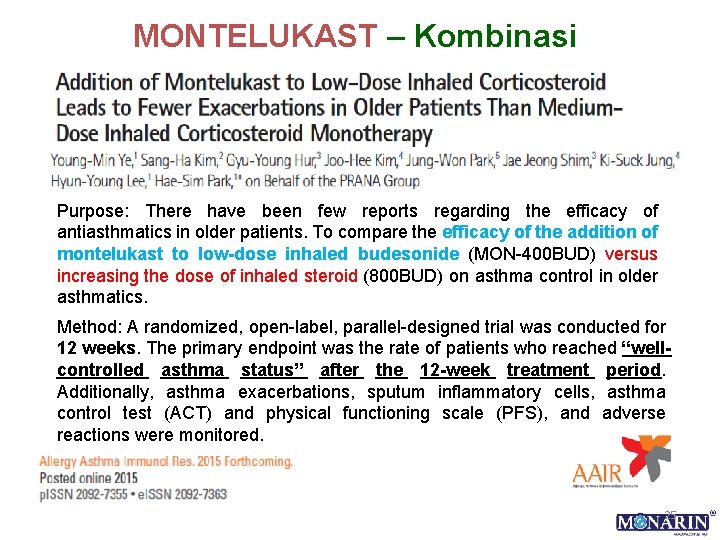
MONTELUKAST – Kombinasi Purpose: There have been few reports regarding the efficacy of antiasthmatics in older patients. To compare the efficacy of the addition of montelukast to low-dose inhaled budesonide (MON-400 BUD) versus increasing the dose of inhaled steroid (800 BUD) on asthma control in older asthmatics. Method: A randomized, open-label, parallel-designed trial was conducted for 12 weeks. The primary endpoint was the rate of patients who reached “wellcontrolled asthma status” after the 12 -week treatment period. Additionally, asthma exacerbations, sputum inflammatory cells, asthma control test (ACT) and physical functioning scale (PFS), and adverse reactions were monitored. 25

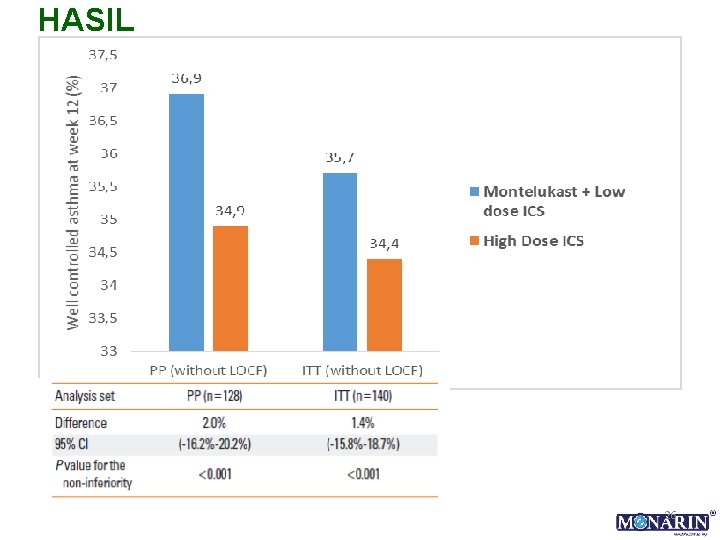
HASIL 26

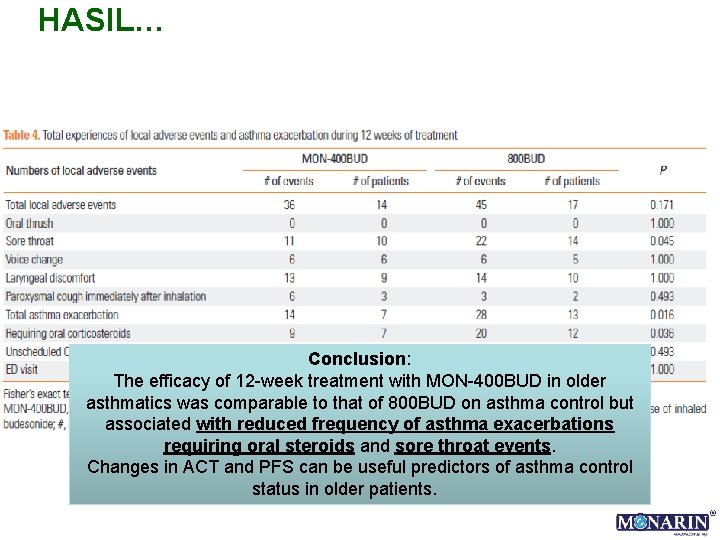
HASIL… Conclusion: The efficacy of 12 -week treatment with MON-400 BUD in older asthmatics was comparable to that of 800 BUD on asthma control but associated with reduced frequency of asthma exacerbations requiring oral steroids and sore throat events. Changes in ACT and PFS can be useful predictors of asthma control status in older patients.

MONTELUKAST – EIB Pemberian kombinasi SABA dan Leukotrien Antagonis sebelum berolahraga sangat direkomendasikan pada pasien yang memiliki resiko EIB 28

SUMMARY • Studi INSPIRE & AIRIAP menunjukkan bahwa terapi terkini belum mengontrol asma dengan optimal. • Reaksi asma melibatkan peranan mediator pro-inflamasi leukotrien yang tidak bisa dihambat dengan terapi kortikosteroid, sehingga dibutuhkan antagonis reseptor leukotrien (LTRA), seperti montelukast. • LTRA direkomendasikan dalam guideline GINA. • Dari uji klinis, montelukast efektif digunakan sebagai monoterapi maupun kombinasi, dengan profil keamanan setara dengan plasebo pada dewasa maupun pediatri. • MONARIN mengandung LTRA montelukast sebagai antiinflamasi pada kasus asma dengan/tanpa rinitis. 29

TERIMA KASIH 30
 Calcitonin gene-related peptide receptor antagonist
Calcitonin gene-related peptide receptor antagonist Azure web role worker role example
Azure web role worker role example Rollendistanz krappmann beispiel
Rollendistanz krappmann beispiel Role conflict occurs when fulfilling the role expectations
Role conflict occurs when fulfilling the role expectations Clasificacion de severidad del asma
Clasificacion de severidad del asma Montelukast gina
Montelukast gina What is gina
What is gina Gina donahue
Gina donahue Photo gina lollobrigida aujourd'hui
Photo gina lollobrigida aujourd'hui Geografijos viktorina
Geografijos viktorina Gina wilson rational functions
Gina wilson rational functions Gina lee-olukoya
Gina lee-olukoya Rctq
Rctq Gina intervention today
Gina intervention today Gina abbiate
Gina abbiate Global initiative for asthma
Global initiative for asthma Gina 2006
Gina 2006 Gina scala
Gina scala Gina fox has started her own company
Gina fox has started her own company Do you like to sing
Do you like to sing Gina ferreira
Gina ferreira Gina burkhardt
Gina burkhardt Gina fisk
Gina fisk Gina pocket
Gina pocket Gina
Gina Gina sells 216 cakes
Gina sells 216 cakes Hongo de gina
Hongo de gina Gina graham
Gina graham Gina
Gina In data analytics lifecycle gina stands for
In data analytics lifecycle gina stands for Mild persistent asthma
Mild persistent asthma