FRI Timeline Freshman Spring Summer Sophomore Fall Computational










- Slides: 61


FRI Timeline Freshman Spring Summer Sophomore Fall Computational Materials Research Stream CH 367 C • Intro to python, linux, software, algorithms, and theory for molecular simulations • Begin Research Optional • Fellowship • Volunteer • Head start on Fall Research CH 369 K • Independent Research • Opportunity for publication Spring • Continue research • Peer Mentor • Join faculty labs, REUs, internships The application for the summer 2018 FRI fellowships has opened and is due March 25 th!

Molecular Dynamics

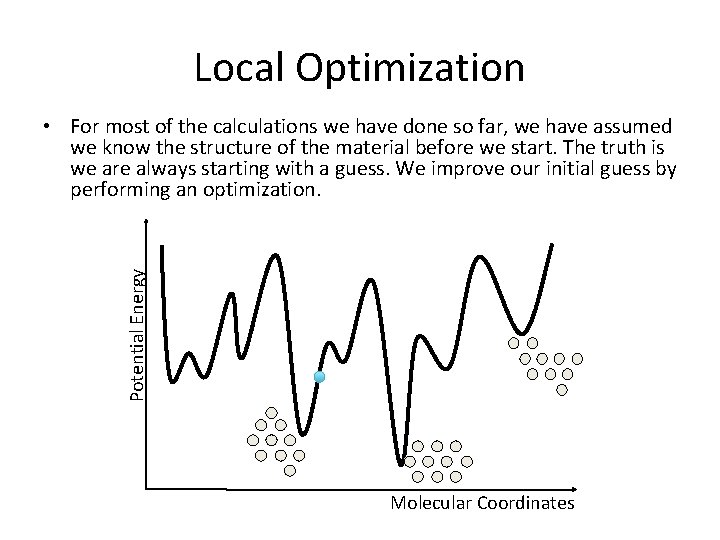
Local Optimization Potential Energy • For most of the calculations we have done so far, we have assumed we know the structure of the material before we start. The truth is we are always starting with a guess. We improve our initial guess by performing an optimization. Molecular Coordinates

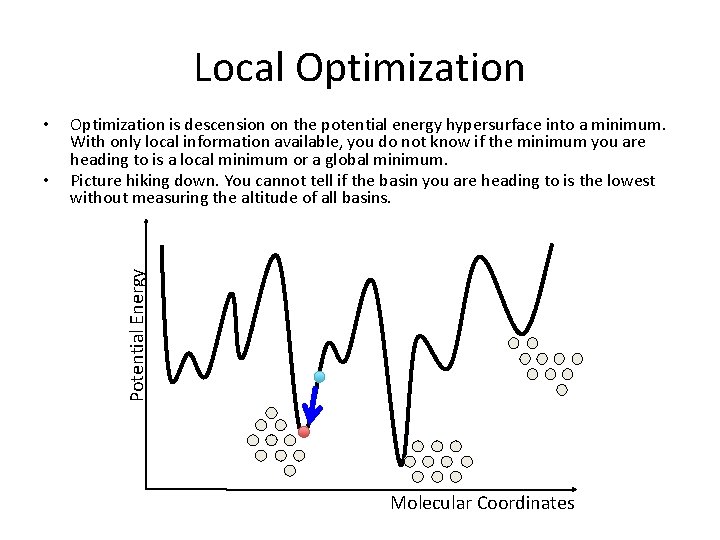
Local Optimization • Optimization is descension on the potential energy hypersurface into a minimum. With only local information available, you do not know if the minimum you are heading to is a local minimum or a global minimum. Picture hiking down. You cannot tell if the basin you are heading to is the lowest without measuring the altitude of all basins. Potential Energy • Molecular Coordinates

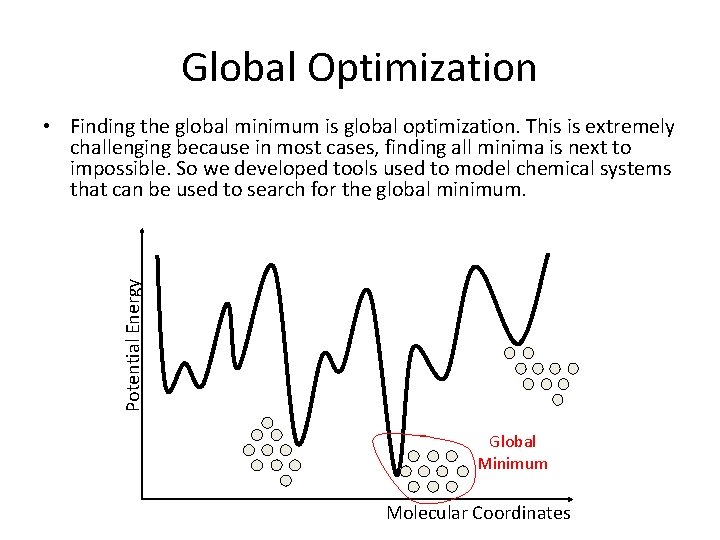
Global Optimization Potential Energy • Finding the global minimum is global optimization. This is extremely challenging because in most cases, finding all minima is next to impossible. So we developed tools used to model chemical systems that can be used to search for the global minimum. Global Minimum Molecular Coordinates

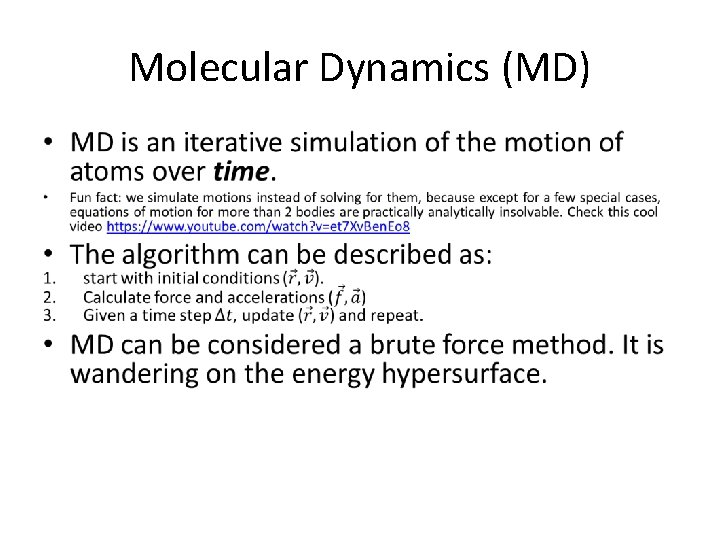
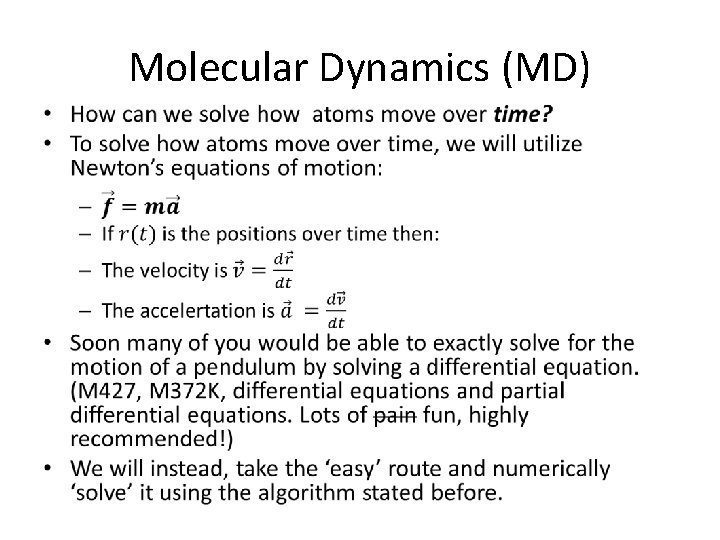
Molecular Dynamics (MD) •

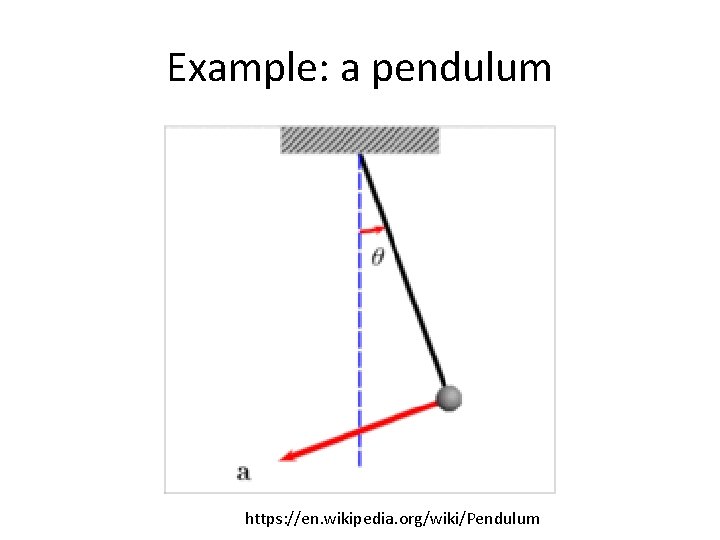
Example: a pendulum Assume you are holding a pendulum at the configuration below. What would happen if I let go in a world with no friction?

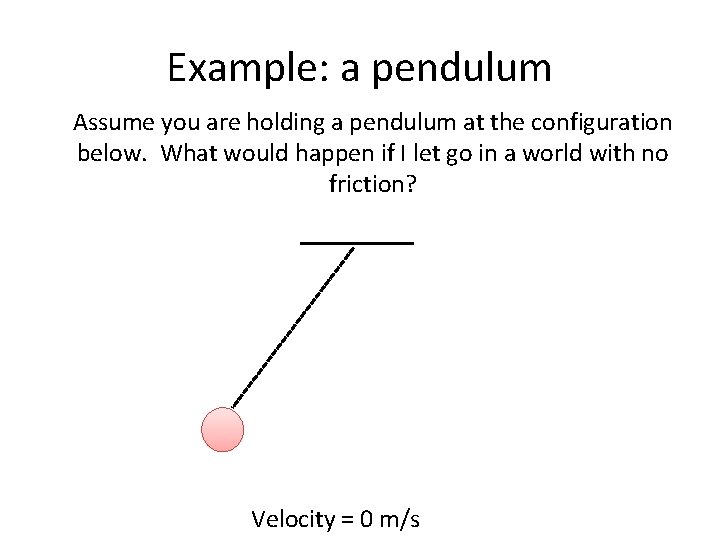
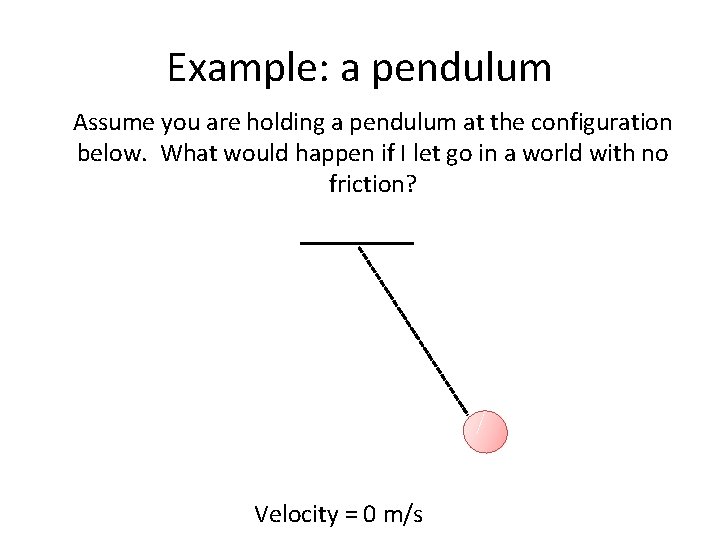
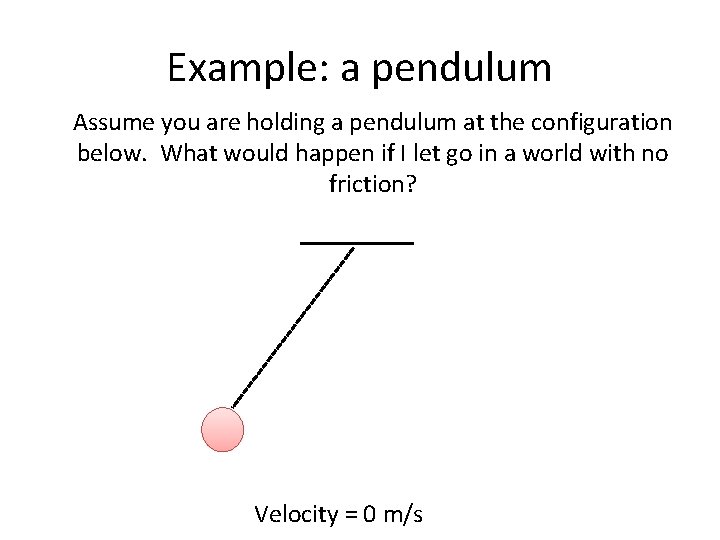
Example: a pendulum Assume you are holding a pendulum at the configuration below. What would happen if I let go in a world with no friction? Velocity = 0 m/s

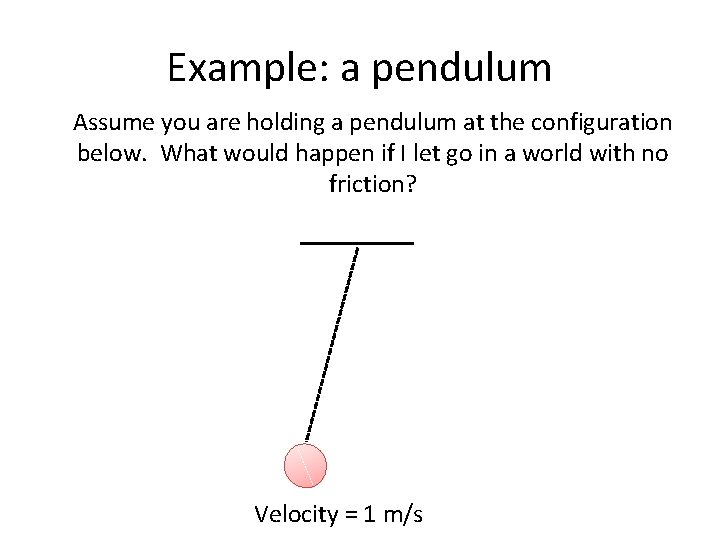
Example: a pendulum Assume you are holding a pendulum at the configuration below. What would happen if I let go in a world with no friction? Velocity = 1 m/s

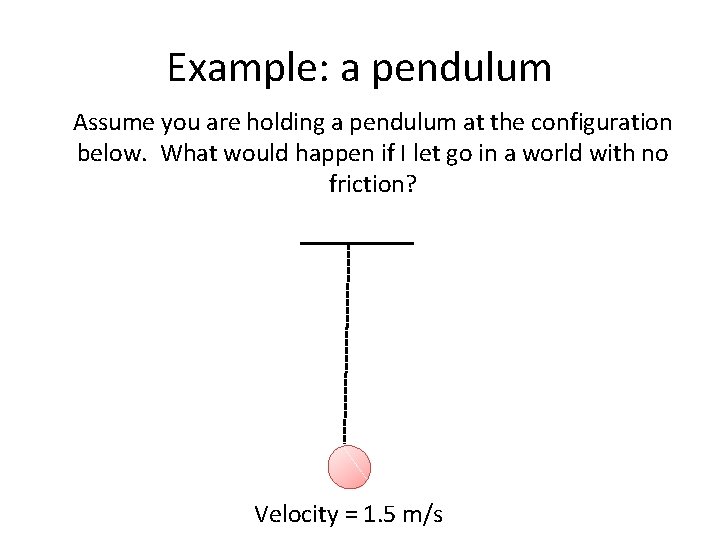
Example: a pendulum Assume you are holding a pendulum at the configuration below. What would happen if I let go in a world with no friction? Velocity = 1. 5 m/s

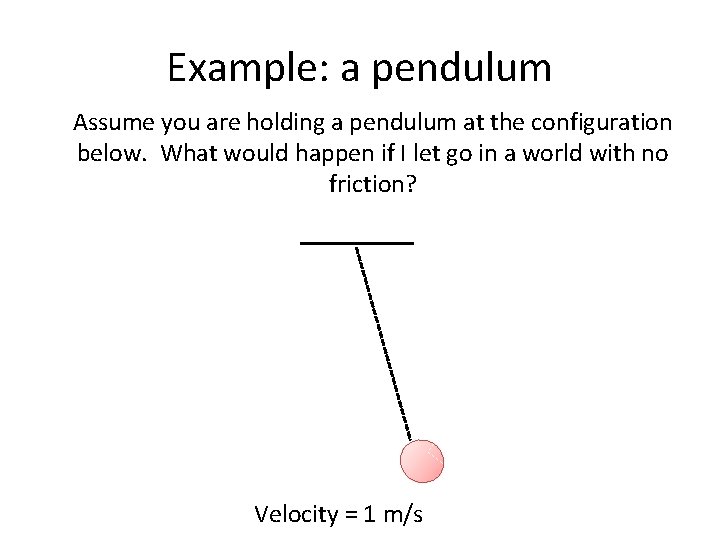
Example: a pendulum Assume you are holding a pendulum at the configuration below. What would happen if I let go in a world with no friction? Velocity = 1 m/s

Example: a pendulum Assume you are holding a pendulum at the configuration below. What would happen if I let go in a world with no friction? Velocity = 0 m/s

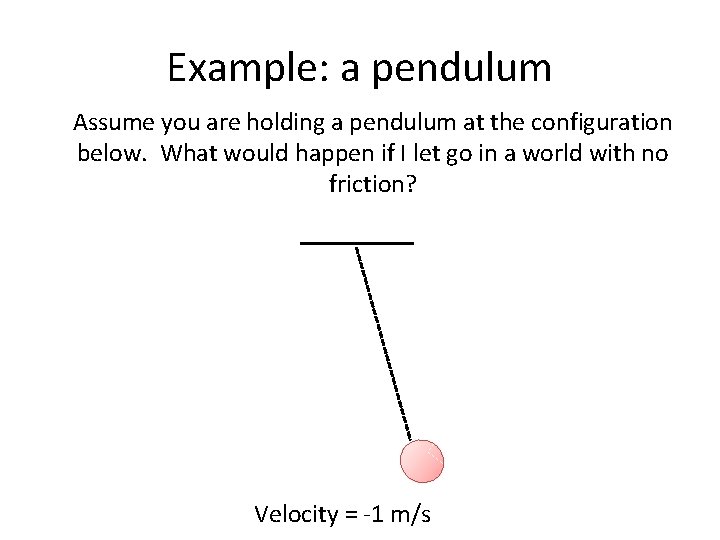
Example: a pendulum Assume you are holding a pendulum at the configuration below. What would happen if I let go in a world with no friction? Velocity = -1 m/s

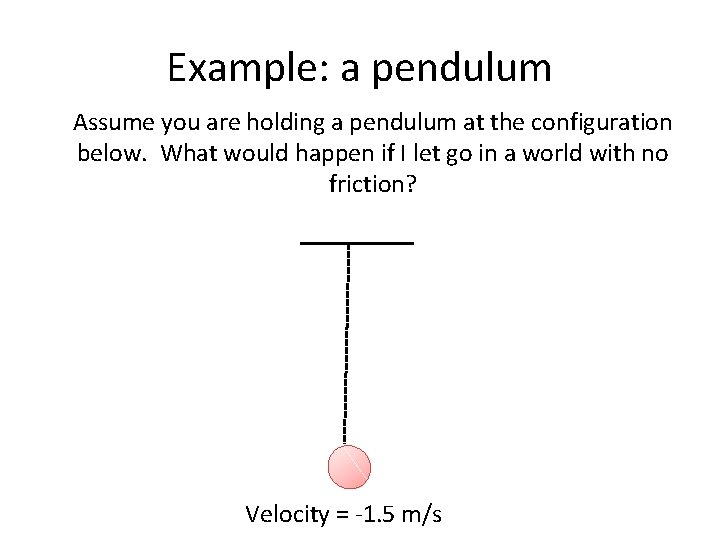
Example: a pendulum Assume you are holding a pendulum at the configuration below. What would happen if I let go in a world with no friction? Velocity = -1. 5 m/s

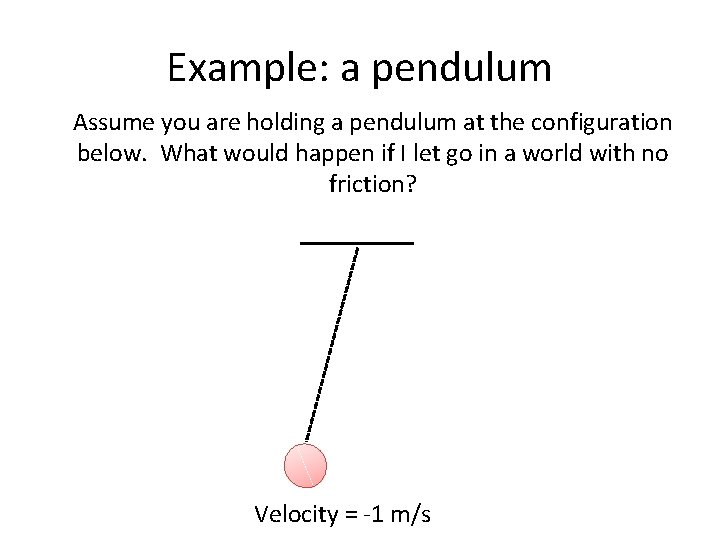
Example: a pendulum Assume you are holding a pendulum at the configuration below. What would happen if I let go in a world with no friction? Velocity = -1 m/s

Example: a pendulum Assume you are holding a pendulum at the configuration below. What would happen if I let go in a world with no friction? Velocity = 0 m/s

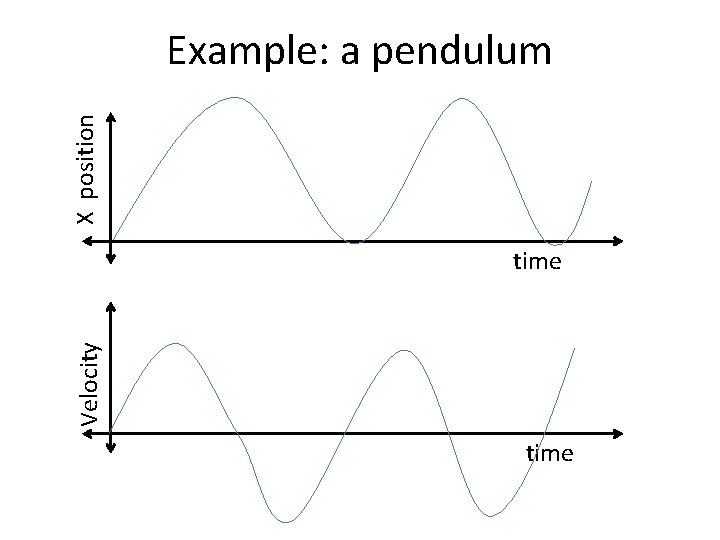
X position Example: a pendulum Velocity time

Molecular Dynamics (MD) •

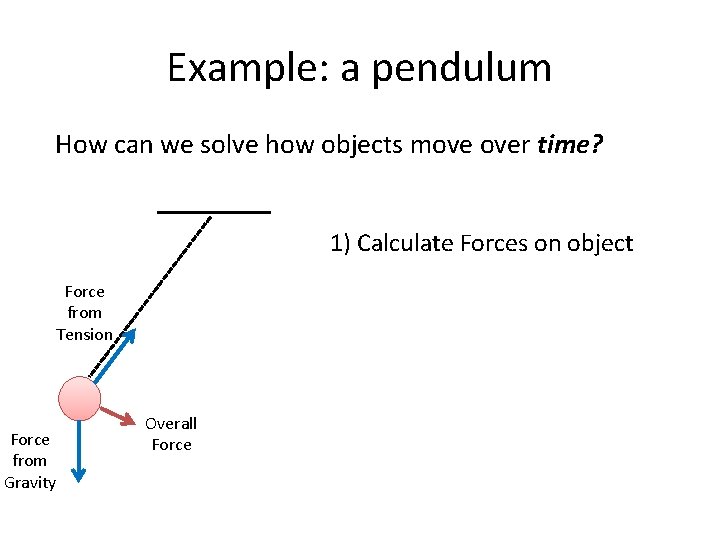
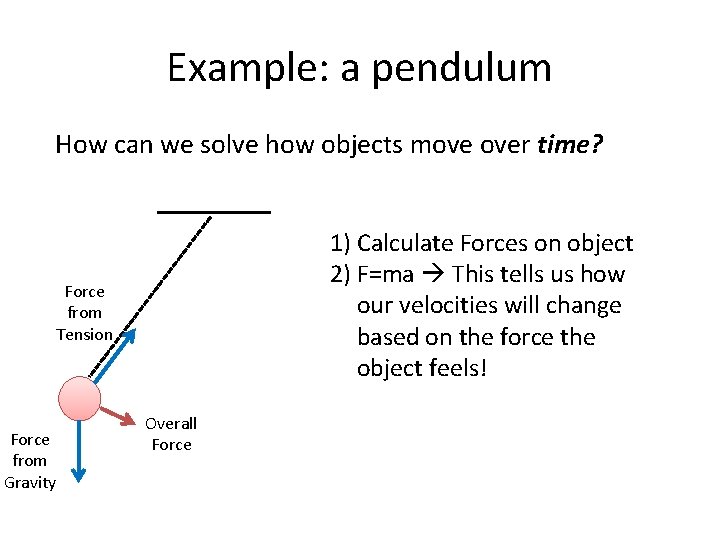
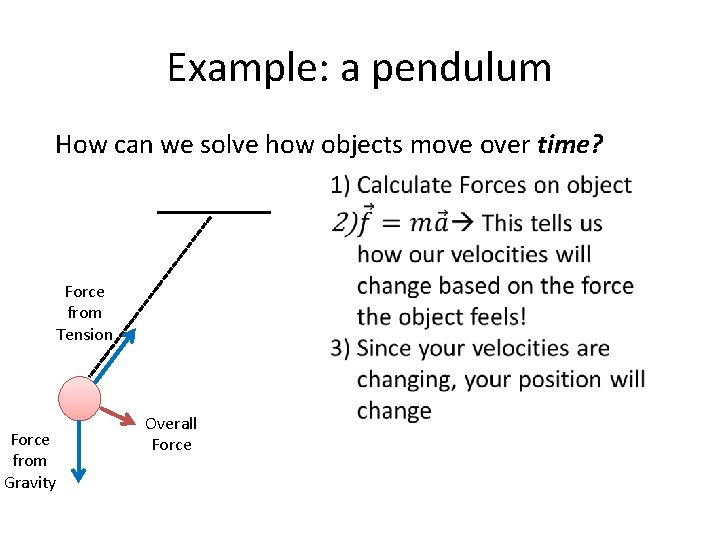
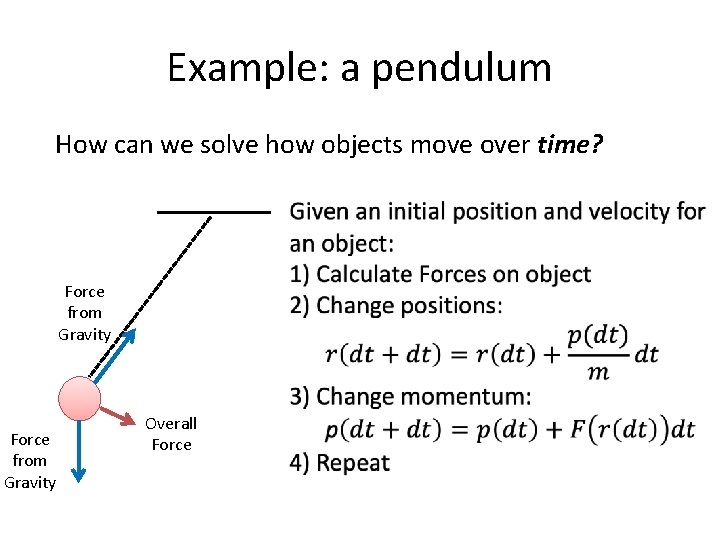
Example: a pendulum How can we solve how objects move over time? 1) Calculate Forces on object Force from Tension Force from Gravity Overall Force

Example: a pendulum How can we solve how objects move over time? 1) Calculate Forces on object 2) F=ma This tells us how our velocities will change based on the force the object feels! Force from Tension Force from Gravity Overall Force

Example: a pendulum How can we solve how objects move over time? Force from Tension Force from Gravity Overall Force

Example: a pendulum https: //en. wikipedia. org/wiki/Pendulum

Example: a pendulum How can we solve how objects move over time? Force from Gravity Overall Force

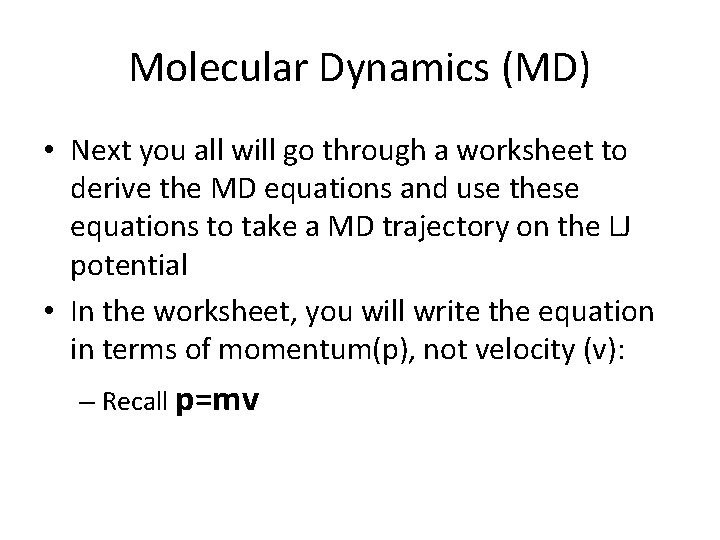
Molecular Dynamics (MD) • Next you all will go through a worksheet to derive the MD equations and use these equations to take a MD trajectory on the LJ potential • In the worksheet, you will write the equation in terms of momentum(p), not velocity (v): – Recall p=mv

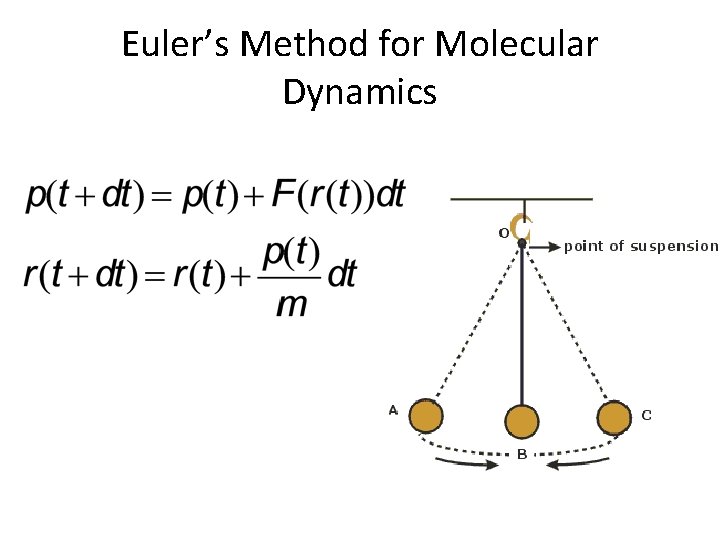
Euler’s Method for Molecular Dynamics

Euler’s Method for Molecular Dynamics

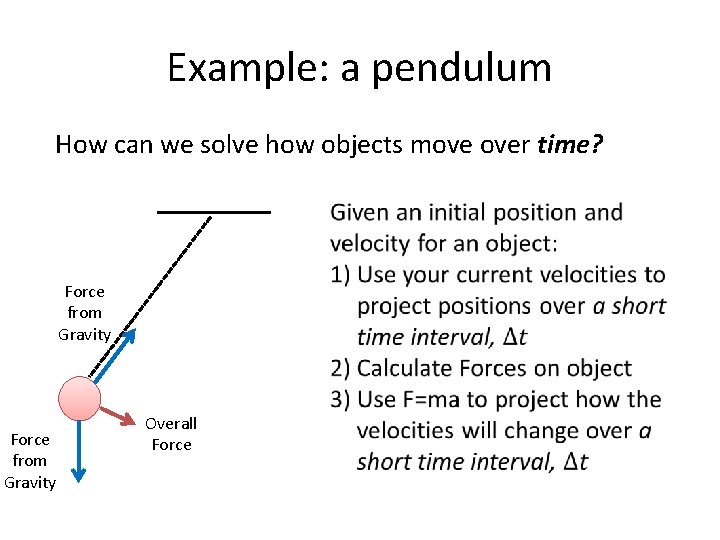
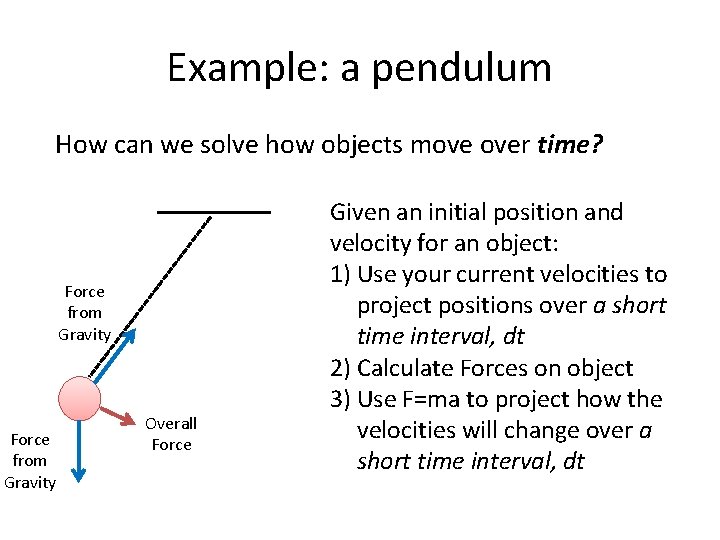
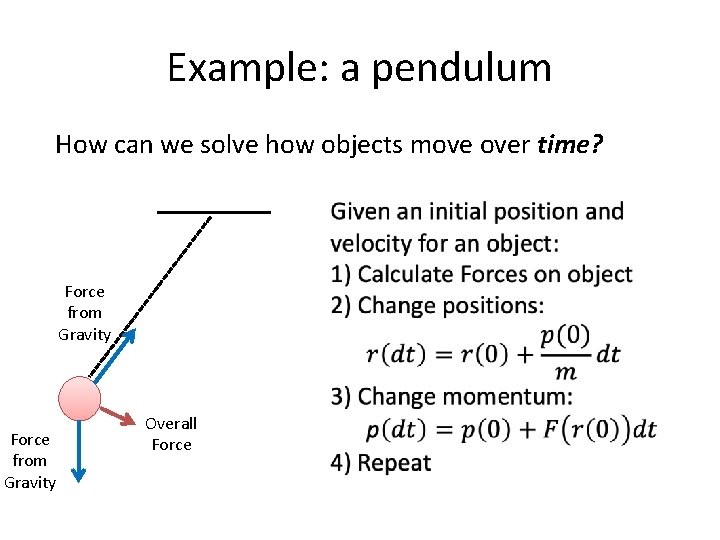
Example: a pendulum How can we solve how objects move over time? Force from Gravity Overall Force Given an initial position and velocity for an object: 1) Use your current velocities to project positions over a short time interval, dt 2) Calculate Forces on object 3) Use F=ma to project how the velocities will change over a short time interval, dt

Example: a pendulum How can we solve how objects move over time? Force from Gravity Overall Force

Example: a pendulum How can we solve how objects move over time? Force from Gravity Overall Force

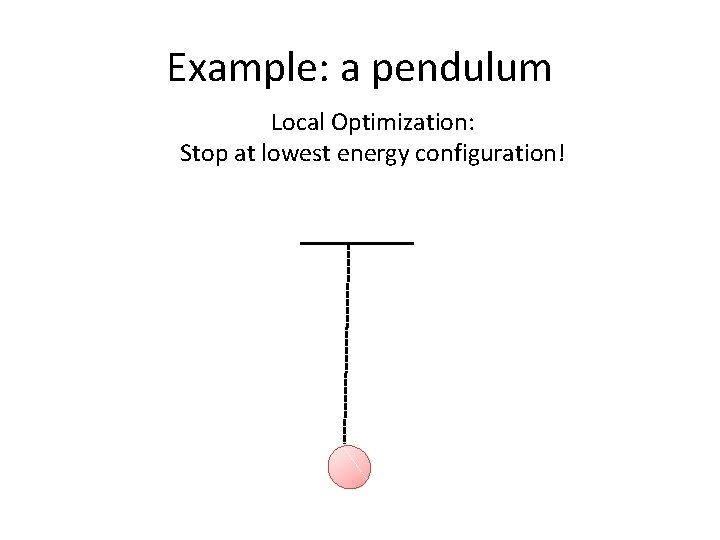
Difference to local optimization • If we were to minimize the potential energy of the pendulum, what would happen?

Example: a pendulum Local Optimization

Example: a pendulum Local Optimization

Example: a pendulum Local Optimization: Stop at lowest energy configuration!

Difference to local optimization •

Ensembles • When performing MD simulations we have options for simulating different environments. • First let’s review some important concepts: – What is temperature?

Ensembles • When performing MD simulations we have options for simulating different environments. • First let’s review some important concepts: – What is temperature? • A measure of the average kinetic energy of the system

Ensembles • When performing MD simulations we have options for simulating different environments. • First let’s review some important concepts: – What is temperature? • A measure of the average kinetic energy of the system – What is the law of conservation of energy?

Ensembles • When performing MD simulations we have options for simulating different environments. • First let’s review some important concepts: – What is temperature? • A measure of the average kinetic energy of the system – What is the law of conservation of energy? • Total energy remains fixed

Ensembles • Let’s assume I close the door, seal any holes in the wall so that the number of particles is fixed, turn off thermostat, and start a small fire. • What will happen to the total energy in this room and the temperature?

Ensembles • Let’s assume a close the door, seal any holes in the wall so that the number of particles is fixed, turn off thermostat, and start a small fire. • What will happen to the total energy in this room and the temperature? – Total energy is fixed – Temperature would increase • Why would the temperature increase?

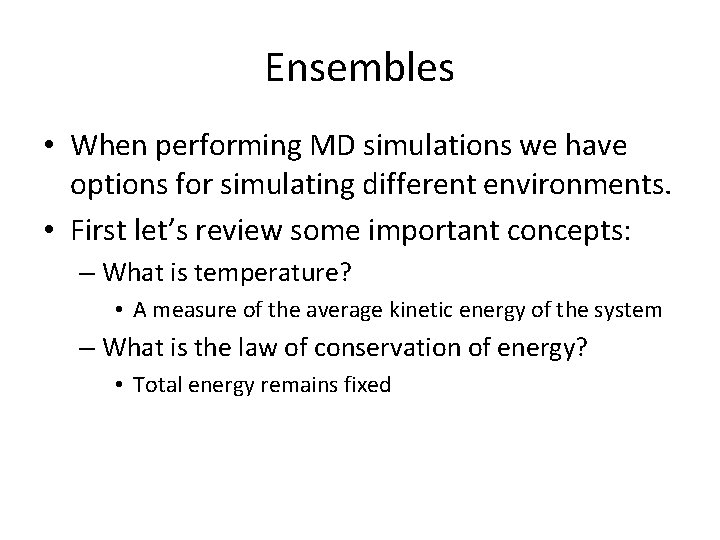
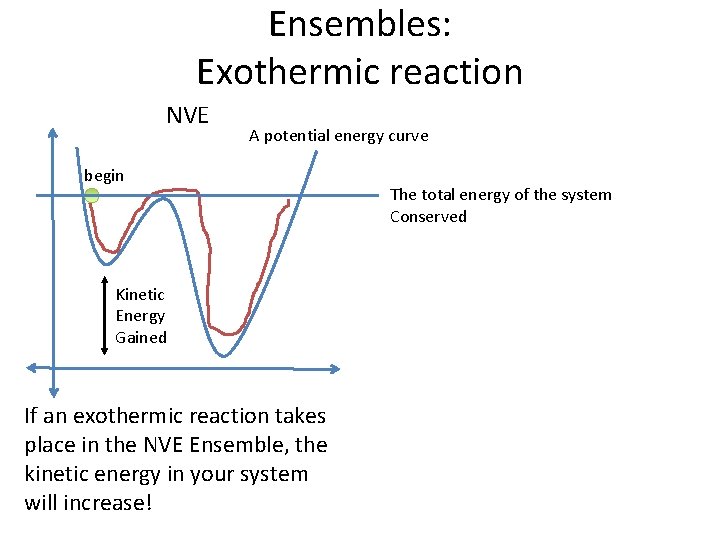
Ensembles: Exothermic reaction NVE A potential energy curve begin Kinetic Energy Gained If an exothermic reaction takes place in the NVE Ensemble, the kinetic energy in your system will increase! The total energy of the system Conserved

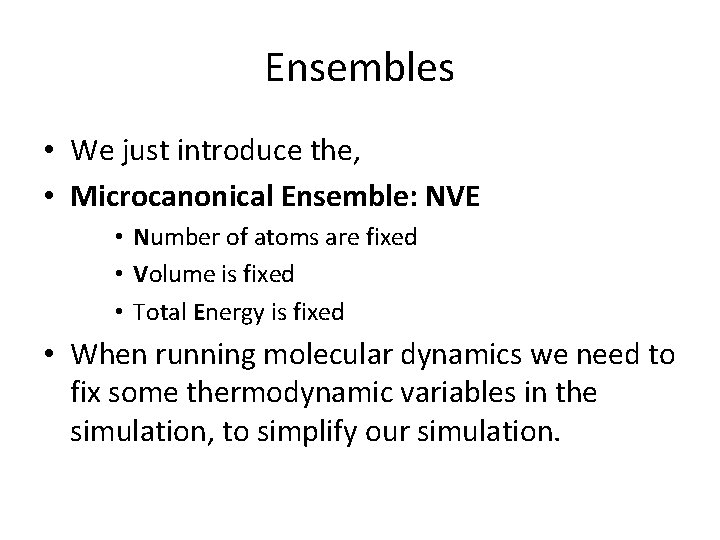
Ensembles • We just introduce the, • Microcanonical Ensemble: NVE • Number of atoms are fixed • Volume is fixed • Total Energy is fixed • When running molecular dynamics we need to fix some thermodynamic variables in the simulation, to simplify our simulation.

Ensembles • Let’s assume I close the door, seal any holes in the wall so that the number of particles is fixed, turn on thermostat, and start a small fire. • What will happen to the total energy in this room and the temperature?

Ensembles • Let’s assume I close the door, seal any holes in the wall so that the number of particles is fixed, turn on thermostat, and start a small fire. • What will happen to the total energy in this room and the temperature? – Total energy is not fixed – Temperature would remain fixed

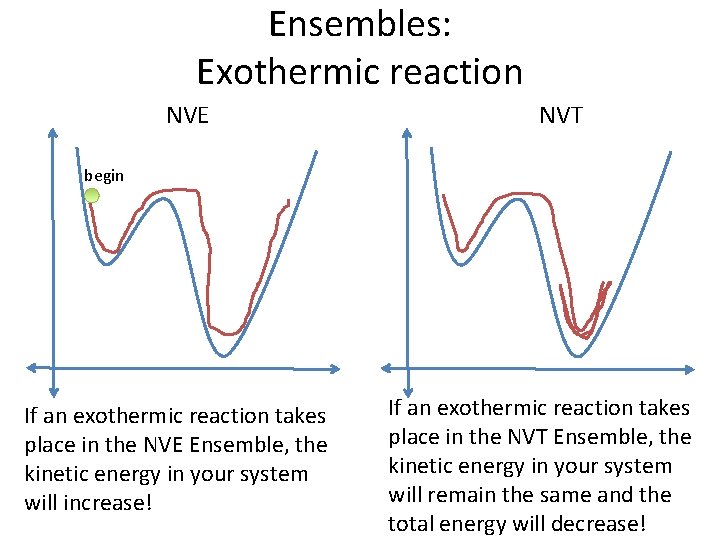
Ensembles: Exothermic reaction NVE NVT begin If an exothermic reaction takes place in the NVE Ensemble, the kinetic energy in your system will increase! If an exothermic reaction takes place in the NVT Ensemble, the kinetic energy in your system will remain the same and the total energy will decrease!

Ensembles • We just introduce the: • Canonical Ensemble: NVT • Number of atoms are fixed • Volume is fixed • Temperature is fixed

Ensembles • You used the NVE ensemble on the worksheet. • In lab 5, we will use the NVE ensemble to look for an optimal value for h, (i. e. Δt or timestep) • We will use the NVT ensemble to control the temperature • Next, we will introduce one way of fixing the temperature

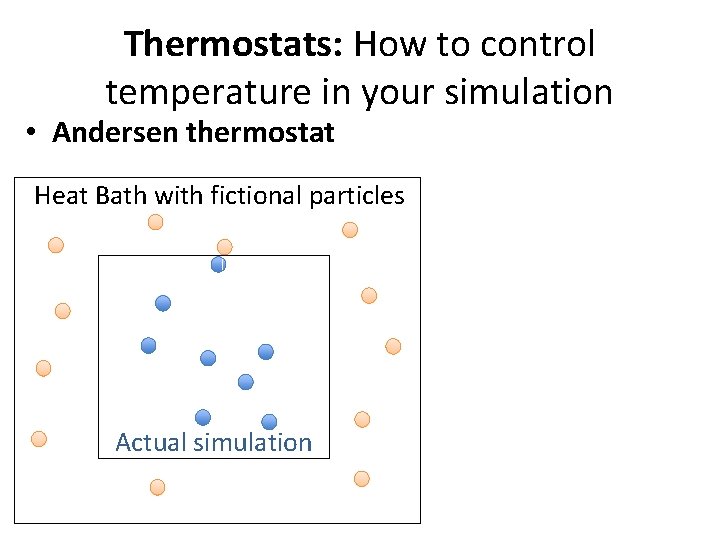
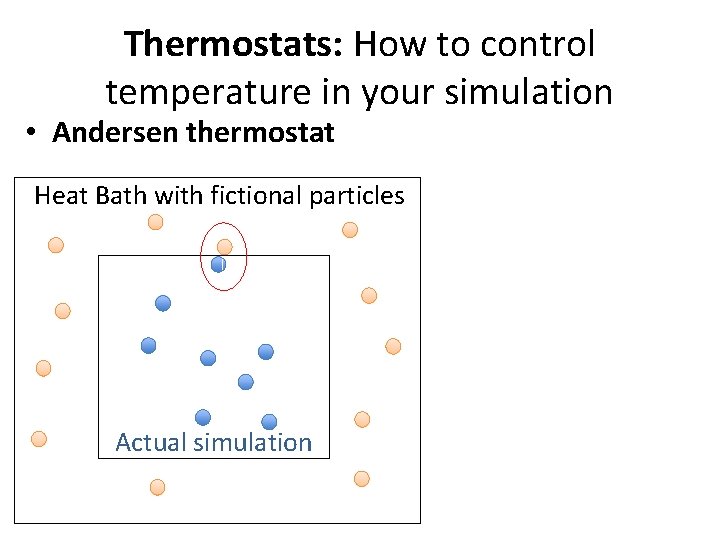
Thermostats: How to control temperature in your simulation • Andersen thermostat Heat Bath with fictional particles Actual simulation

Thermostats: How to control temperature in your simulation • Andersen thermostat Heat Bath with fictional particles Actual simulation

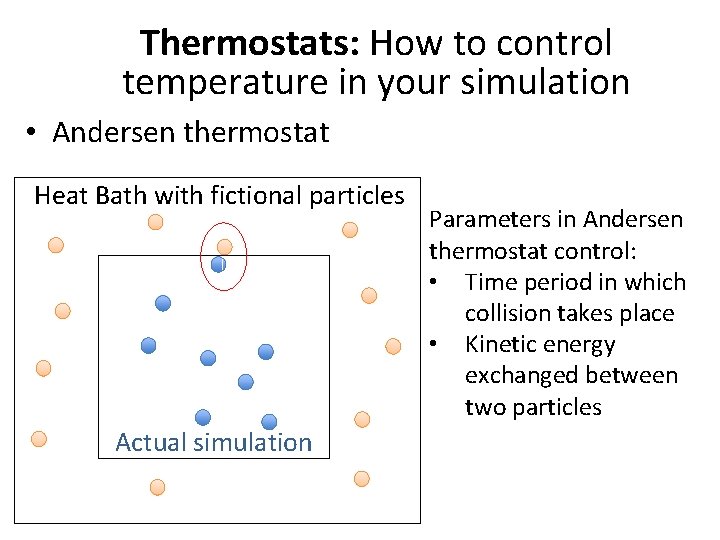
Thermostats: How to control temperature in your simulation • Andersen thermostat Heat Bath with fictional particles Actual simulation Parameters in Andersen thermostat control: • Time period in which collision takes place • Kinetic energy exchanged between two particles

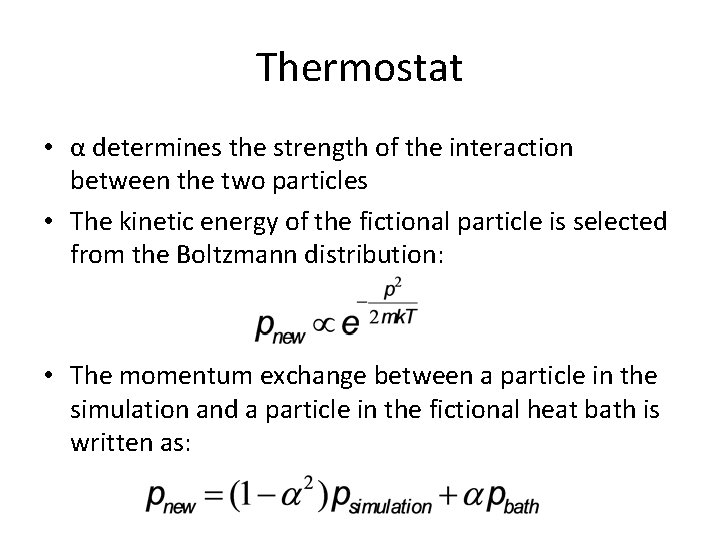
Thermostat • α determines the strength of the interaction between the two particles • The kinetic energy of the fictional particle is selected from the Boltzmann distribution: • The momentum exchange between a particle in the simulation and a particle in the fictional heat bath is written as:

Anderson Thermostat Summary • For every MD Step: – Select if kinetic energy will take place based on Pcol or the probability of collision occurring • If temperature changes: – Select a temperature for your bath from Boltzmann distribution at temperature, T – Perform a kinetic energy between the bath and your simulation • Else: – Temperature remains the same

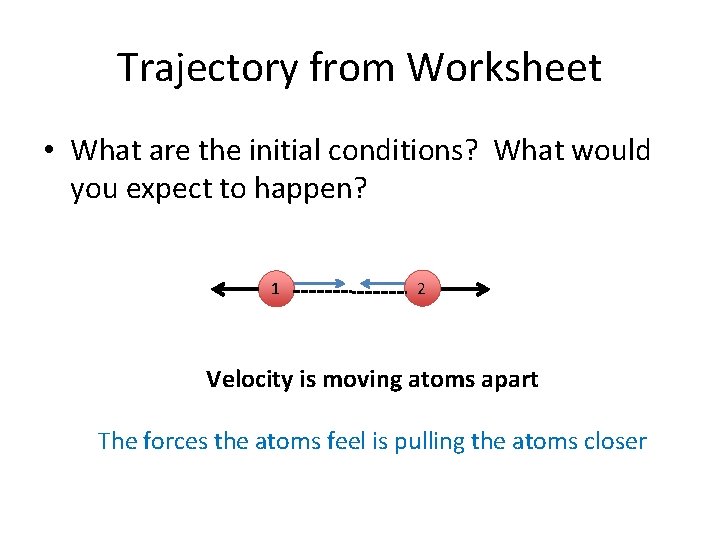
Trajectory from Worksheet • What are the initial conditions? What would you expect to happen?

Trajectory from Worksheet • What are the initial conditions? What would you expect to happen? 1 2 Velocity is moving atoms apart The forces the atoms feel is pulling the atoms closer

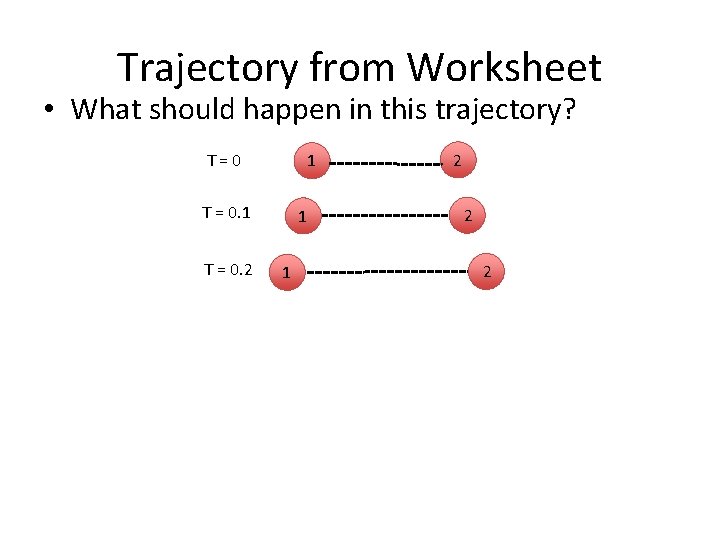
Trajectory from Worksheet • What should happen in this trajectory? T=0 1 T = 0. 2 1 1 2 2 2

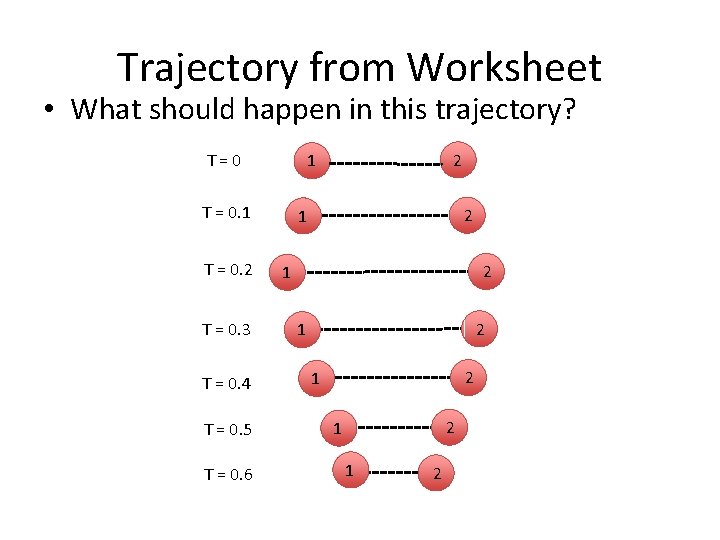
Trajectory from Worksheet • What should happen in this trajectory? T=0 T = 0. 1 T = 0. 2 T = 0. 3 T = 0. 4 T = 0. 5 T = 0. 6 2 1 2 1 2 1 1 2

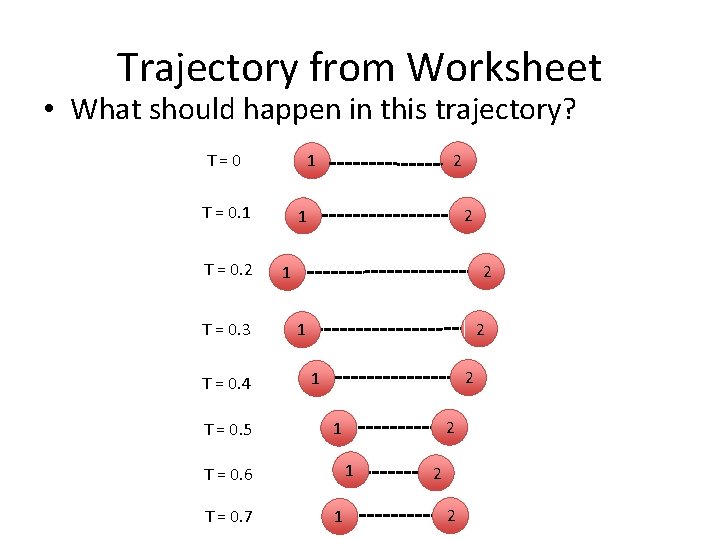
Trajectory from Worksheet • What should happen in this trajectory? T=0 T = 0. 1 T = 0. 2 T = 0. 3 T = 0. 4 T = 0. 5 2 1 2 1 2 1 1 T = 0. 6 T = 0. 7 2 1 1 2 2

Trajectory from Worksheet • What should happen to the total energy in the example trajectory? What did happen to the total energy in the worksheet? Why?

Trajectory from Worksheet • What happen to the total energy in the example trajectory you calculated in the worksheet? Why? – The total energy did not remain constant and it never will in MD due to numerical simulations! – The time step needs to small enough so that the total energy remains closer to where it starts • You will explore how to find an ideal timestep in Lab 5

Trajectory from Worksheet • What happen to the total energy in the example trajectory you calculated in the worksheet? Why? – Euler’s method is a first order numerical integrator (only uses first derivative) – A better method called the Velocity-Verlet Algorithm is commonly used and improves on Euler’s method by using approximate second derivatives!