Fractional Flow Reserve and Intravascular Ultrasound Relation Ship






- Slides: 27

Fractional Flow Reserve and Intravascular Ultrasound Relation. Ship THE F 1 RST TRIAL Ron Waksman, MD on Behalf of the F 1 RST Investigators

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship • Grant/Research Support • Consulting Fees/Honoraria Company • Volcano • Medtronic Vascular • Abbott Vascular • Boston Scientific • Biotronik • Medtronic • Abbott Vascular • Boston Scientific • Lilly Daiichi • Astra Zeneca

Background I • An intermediate coronary stenosis, defined as a luminal narrowing with a diameter stenosis between 40 -70% on angiography, is a source of controversy with regard to the appropriate criteria for undertaking revascularization. • It has been previously reported that postponing revascularization of intermediate severity lesions on angiography with FFR>0. 80 is safe and results in an excellent clinical outcome.

Background II • The PROSPECT trial followed 700 patients with acute coronary syndrome for 3 years, reported that the MACE was low with one of the correlated of non-culprit lesion related events was an MLA <4 mm 2 by IVUS • To date, few data are available regarding the relationship between the anatomical and morphological IVUS parameters and the functional FFR results.

Purpose and Aims • To evaluate the relationship between IVUS VH ® anatomical parameters and FFR value in patients with intermediate coronary stenoses. • To determine the IVUS VH ® anatomical criteria and cutoff value associated with significantly functional criteria of FFR< 0. 8.

Endpoints • Primary Correlation between MLA and FFR and to identity a cut-off value for MLA corresponding to and FFR of 0. 8 • Secondary ¡ Correlation between FFR and plaque burden and to identify a cut-off value for plaque burden corresponding to and FFR of 0. 8 ¡ Assess the relationship between FFR (<0. 80) and determined cut-off values of MLA with ¡ Presence of: • Thin cup fibroatheroma (TCFA) defined as Necrotic Core (NC) >10%, confluent over 3 consecutive frames with a plaque burden of > 40% and NC against the lumen surface > 30 degrees • Plaque burden ≥ 70%

Hypothesis We hypothesize that IVUS VH morphological criteria, such as minimal lumen area, plaque burden and plaque type by VH, can predict physiological ischemia by FFR.

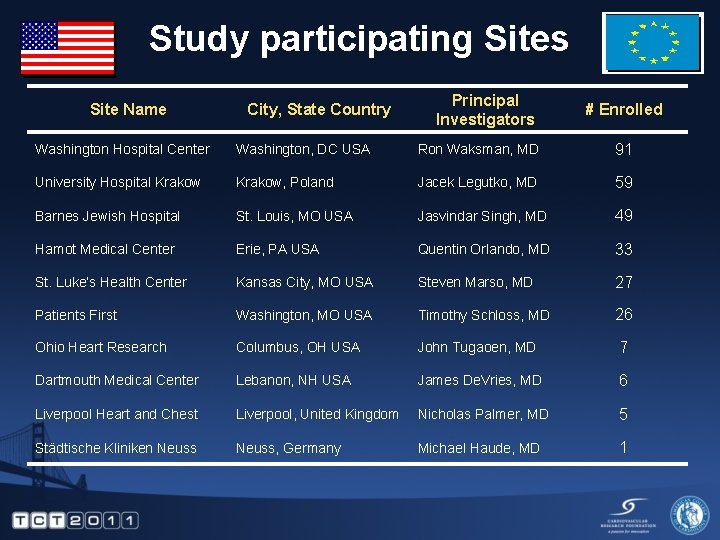
Study participating Sites Site Name City, State Country Principal Investigators # Enrolled Washington Hospital Center Washington, DC USA Ron Waksman, MD 91 University Hospital Krakow, Poland Jacek Legutko, MD 59 Barnes Jewish Hospital St. Louis, MO USA Jasvindar Singh, MD 49 Hamot Medical Center Erie, PA USA Quentin Orlando, MD 33 St. Luke’s Health Center Kansas City, MO USA Steven Marso, MD 27 Patients First Washington, MO USA Timothy Schloss, MD 26 Ohio Heart Research Columbus, OH USA John Tugaoen, MD 7 Dartmouth Medical Center Lebanon, NH USA James De. Vries, MD 6 Liverpool Heart and Chest Liverpool, United Kingdom Nicholas Palmer, MD 5 Städtische Kliniken Neuss, Germany Michael Haude, MD 1

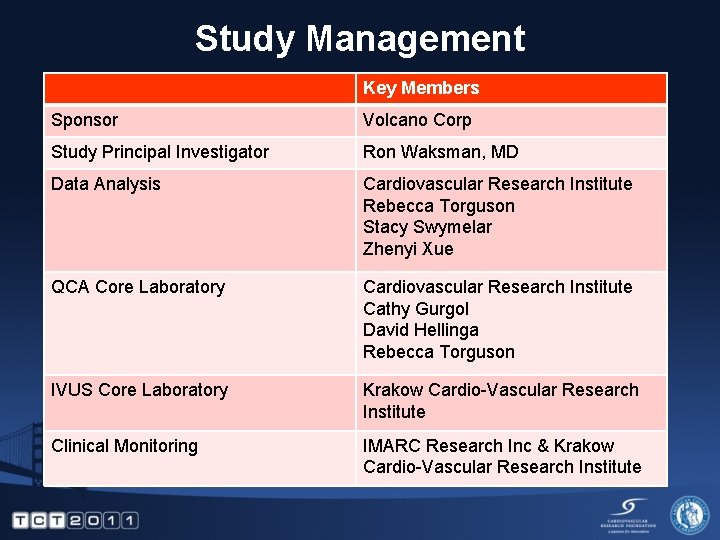
Study Management Key Members Sponsor Volcano Corp Study Principal Investigator Ron Waksman, MD Data Analysis Cardiovascular Research Institute Rebecca Torguson Stacy Swymelar Zhenyi Xue QCA Core Laboratory Cardiovascular Research Institute Cathy Gurgol David Hellinga Rebecca Torguson IVUS Core Laboratory Krakow Cardio-Vascular Research Institute Clinical Monitoring IMARC Research Inc & Krakow Cardio-Vascular Research Institute

Inclusion/Exclusion Criteria Inclusions • • • Exclusions Clinical indication for coronary angiography for stable or unstable angina • • STEMI within the past 24 hours Intermediate coronary lesion with stenosis 40 -80% in one or more native, major epicardial coronary artery • Left ventricular hypertrophy >1. 5 cm by echo • • History of bronchospasm or asthma • • Unprotected left main or ostial lesions • • • Lesions in arterial or saphenous vein grafts • More than one lesion in an epicardial vessel Lesions suitable for FFR and IVUS/VH Decompensated HF or hypotension requiring intubation, inotropes, intravenous diuretics or IABP ECG evidence of conduction defect (2º or 3º AVB) Severe calcification or tortuosity of the target vessel Thrombus Lesion in a vessel with <2. 5 mm reference diameter

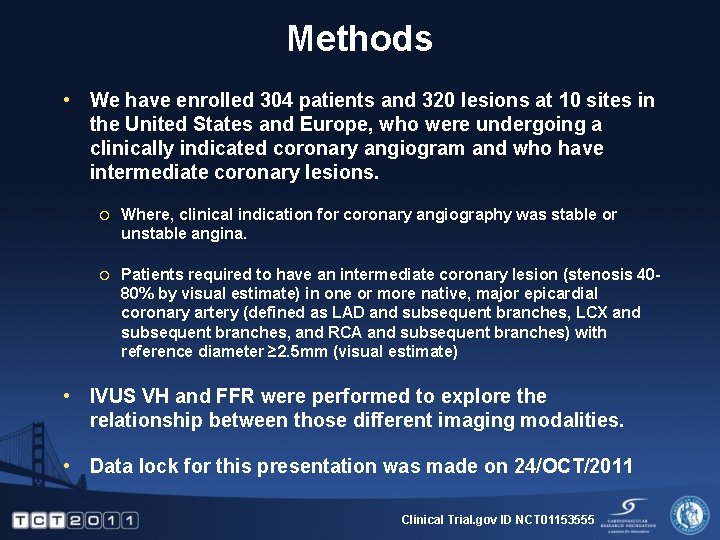
Methods • We have enrolled 304 patients and 320 lesions at 10 sites in the United States and Europe, who were undergoing a clinically indicated coronary angiogram and who have intermediate coronary lesions. ¡ Where, clinical indication for coronary angiography was stable or unstable angina. ¡ Patients required to have an intermediate coronary lesion (stenosis 4080% by visual estimate) in one or more native, major epicardial coronary artery (defined as LAD and subsequent branches, LCX and subsequent branches, and RCA and subsequent branches) with reference diameter ≥ 2. 5 mm (visual estimate) • IVUS VH and FFR were performed to explore the relationship between those different imaging modalities. • Data lock for this presentation was made on 24/OCT/2011 Clinical Trial. gov ID NCT 01153555

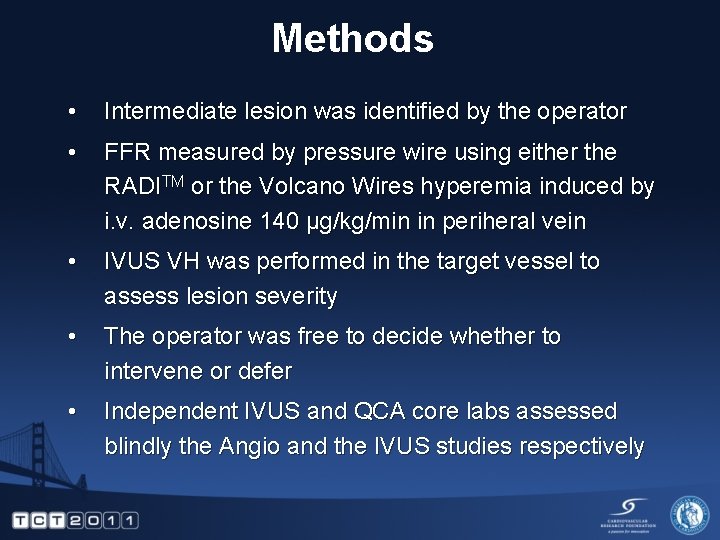
Methods • Intermediate lesion was identified by the operator • FFR measured by pressure wire using either the RADITM or the Volcano Wires hyperemia induced by i. v. adenosine 140 µg/kg/min in periheral vein • IVUS VH was performed in the target vessel to assess lesion severity • The operator was free to decide whether to intervene or defer • Independent IVUS and QCA core labs assessed blindly the Angio and the IVUS studies respectively

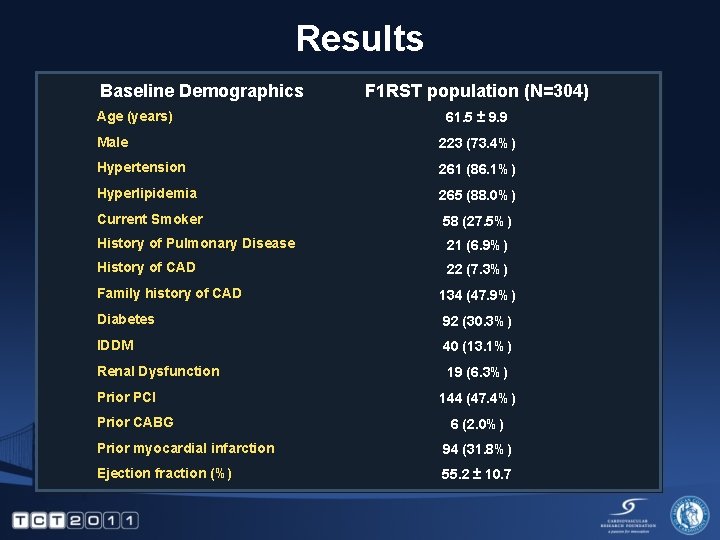
Results Baseline Demographics Age (years) F 1 RST population (N=304) 61. 5 ± 9. 9 Male 223 (73. 4%) Hypertension 261 (86. 1%) Hyperlipidemia 265 (88. 0%) Current Smoker 58 (27. 5%) History of Pulmonary Disease 21 (6. 9%) History of CAD 22 (7. 3%) Family history of CAD 134 (47. 9%) Diabetes 92 (30. 3%) IDDM 40 (13. 1%) Renal Dysfunction 19 (6. 3%) Prior PCI Prior CABG 144 (47. 4%) 6 (2. 0%) Prior myocardial infarction 94 (31. 8%) Ejection fraction (%) 55. 2 ± 10. 7

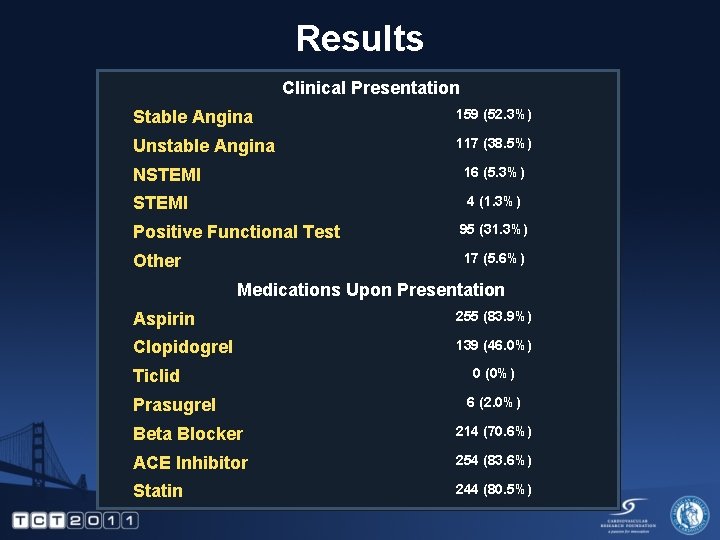
Results Clinical Presentation Stable Angina 159 (52. 3%) Unstable Angina 117 (38. 5%) NSTEMI 16 (5. 3%) STEMI 4 (1. 3%) Positive Functional Test 95 (31. 3%) Other 17 (5. 6%) Medications Upon Presentation Aspirin 255 (83. 9%) Clopidogrel 139 (46. 0%) Ticlid Prasugrel 0 (0%) 6 (2. 0%) Beta Blocker 214 (70. 6%) ACE Inhibitor 254 (83. 6%) Statin 244 (80. 5%)

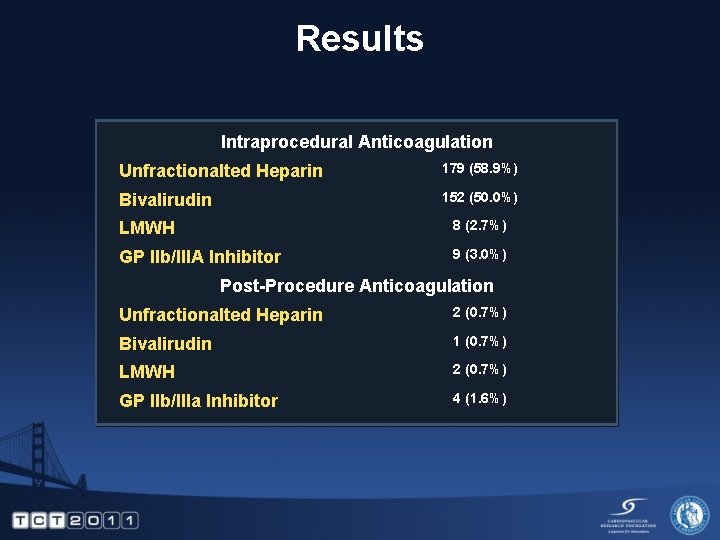
Results Intraprocedural Anticoagulation Unfractionalted Heparin 179 (58. 9%) Bivalirudin 152 (50. 0%) LMWH 8 (2. 7%) GP IIb/IIIA Inhibitor 9 (3. 0%) Post-Procedure Anticoagulation Unfractionalted Heparin 2 (0. 7%) Bivalirudin 1 (0. 7%) LMWH 2 (0. 7%) GP IIb/IIIa Inhibitor 4 (1. 6%)

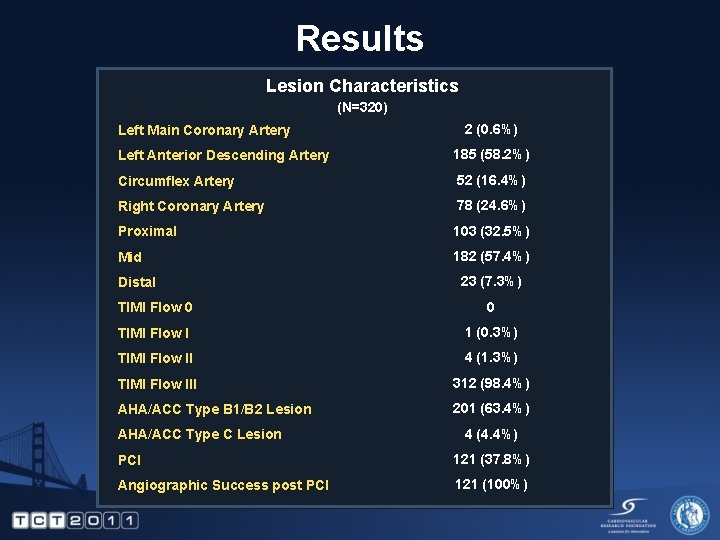
Results Lesion Characteristics (N=320) Left Main Coronary Artery 2 (0. 6%) Left Anterior Descending Artery 185 (58. 2%) Circumflex Artery 52 (16. 4%) Right Coronary Artery 78 (24. 6%) Proximal 103 (32. 5%) Mid 182 (57. 4%) Distal 23 (7. 3%) TIMI Flow 0 0 TIMI Flow I 1 (0. 3%) TIMI Flow II 4 (1. 3%) TIMI Flow III 312 (98. 4%) AHA/ACC Type B 1/B 2 Lesion 201 (63. 4%) AHA/ACC Type C Lesion 4 (4. 4%) PCI 121 (37. 8%) Angiographic Success post PCI 121 (100%)

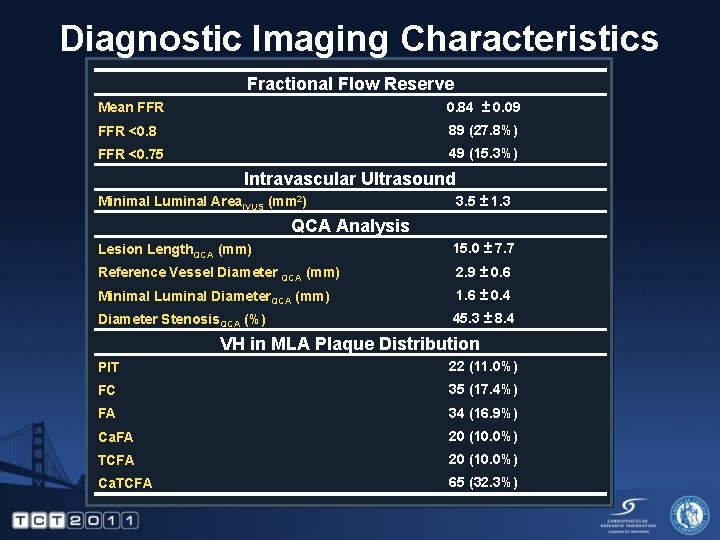
Diagnostic Imaging Characteristics Fractional Flow Reserve Mean FFR 0. 84 ± 0. 09 FFR <0. 8 89 (27. 8%) FFR <0. 75 49 (15. 3%) Intravascular Ultrasound Minimal Luminal Area. IVUS (mm 2) 3. 5 ± 1. 3 QCA Analysis Lesion Length. QCA (mm) 15. 0 ± 7. 7 Reference Vessel Diameter QCA (mm) 2. 9 ± 0. 6 Minimal Luminal Diameter. QCA (mm) 1. 6 ± 0. 4 Diameter Stenosis. QCA (%) 45. 3 ± 8. 4 VH in MLA Plaque Distribution PIT 22 (11. 0%) FC 35 (17. 4%) FA 34 (16. 9%) Ca. FA 20 (10. 0%) TCFA 20 (10. 0%) Ca. TCFA 65 (32. 3%)

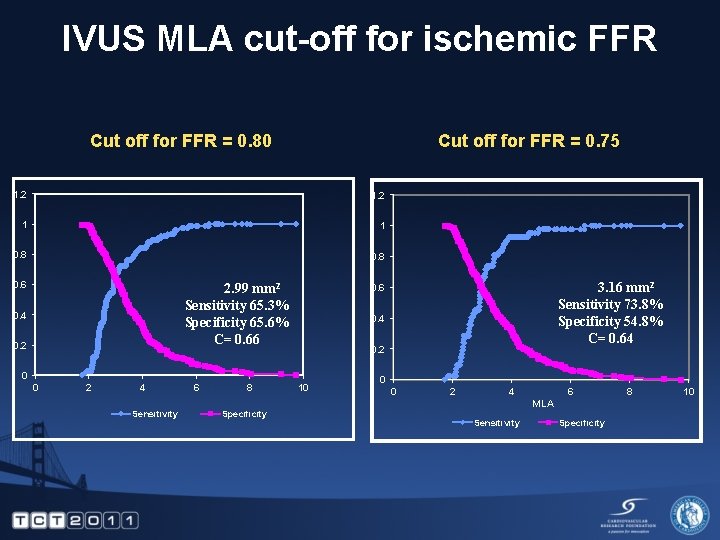
IVUS MLA cut-off for ischemic FFR Cut off for FFR = 0. 75 Cut off for FFR = 0. 80 1. 2 1 1 0. 8 0. 6 2. 99 mm 2 Sensitivity 65. 3% Specificity 65. 6% C= 0. 66 0. 4 0. 2 0 0 2 4 Sensitivity 6 8 Specificity 3. 16 mm 2 Sensitivity 73. 8% Specificity 54. 8% C= 0. 64 0. 6 10 0 0 2 4 6 MLA Sensitivity Specificity 8 10

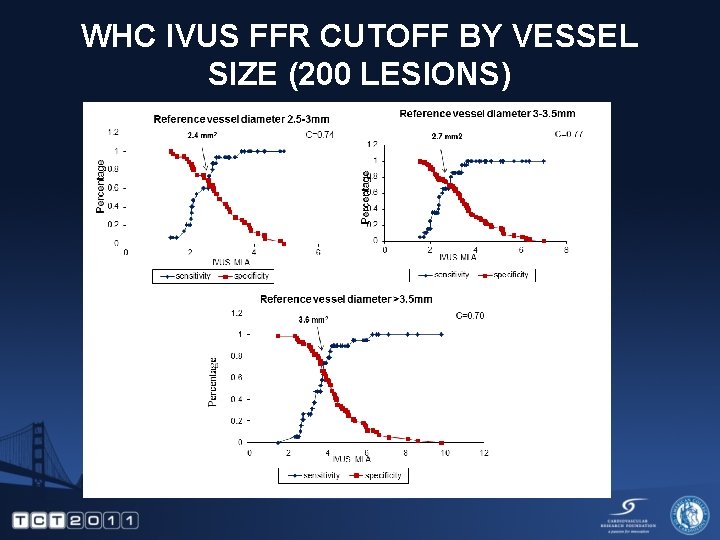
WHC IVUS FFR CUTOFF BY VESSEL SIZE (200 LESIONS)

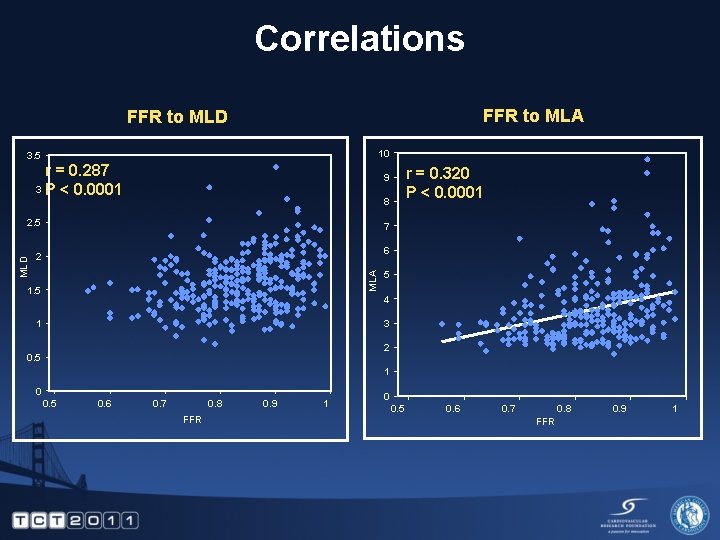
Correlations FFR to MLA FFR to MLD 10 3. 5 r = 0. 287 3 P < 0. 0001 8 2. 5 7 6 2 MLA MLD r = 0. 320 P < 0. 0001 9 1. 5 5 4 3 1 2 0. 5 1 0 0. 5 0. 6 0. 7 0. 8 FFR 0. 9 1

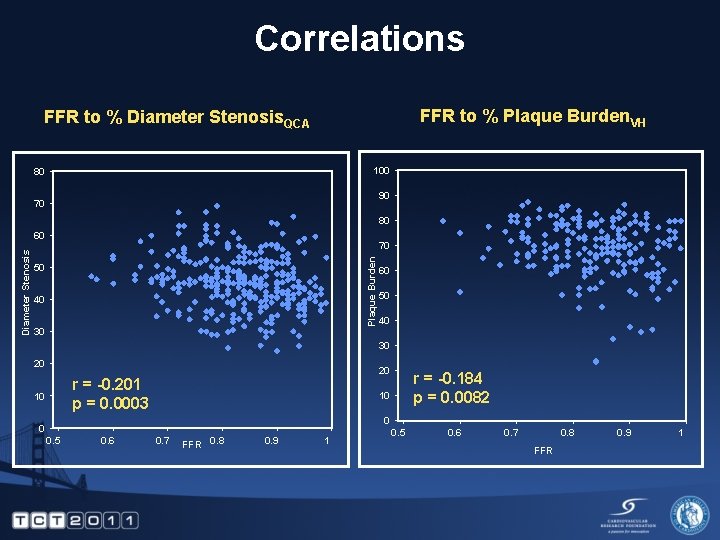
Correlations FFR to % Plaque Burden. VH FFR to % Diameter Stenosis. QCA 100 80 90 70 80 70 Plaque Burden Diameter Stenosis 60 50 40 30 20 20 r = -0. 201 p = 0. 0003 10 r = -0. 184 p = 0. 0082 10 0 0 0. 5 0. 6 0. 7 FFR 0. 8 0. 9 1 0. 5 0. 6 0. 7 0. 8 FFR 0. 9 1

Correlations of FFR to MLA by RVD < 3. 0 mm RVD 3. 0 to 3. 5 mm 8 7 6 12 r = 0. 234 p = 0. 0036 10 8 MLA 5 MLA 4 3 r = 0. 352 p = 0. 0024 6 4 2 2 1 0 0. 6 0. 7 FFR 0. 8 0. 9 0 1 0. 5 0. 6 RVD > 3. 5 mm 10 9 8 7 r = 0. 339 p = 0. 0261 6 MLA 0. 5 5 4 3 2 1 0 0. 5 0. 7 FFR 0. 9 0. 7 FFR 0. 8 0. 9 1

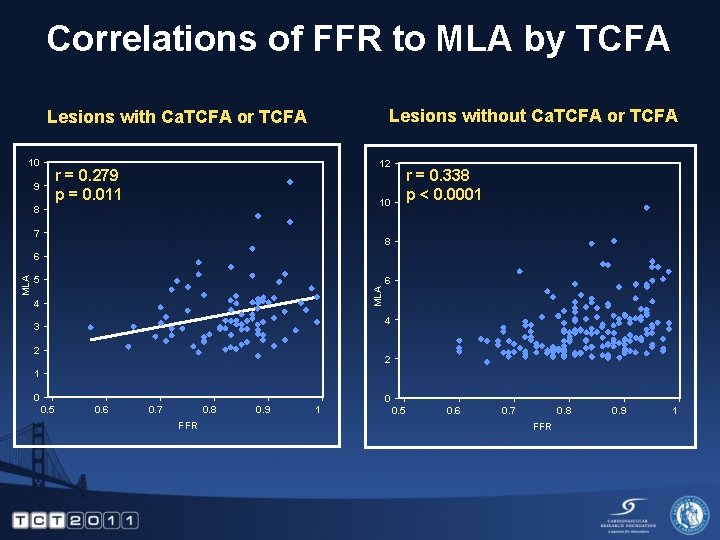
Correlations of FFR to MLA by TCFA Lesions without Ca. TCFA or TCFA Lesions with Ca. TCFA or TCFA 10 12 r = 0. 279 p = 0. 011 9 10 8 7 r = 0. 338 p < 0. 0001 8 5 MLA 6 4 3 2 2 1 0 0. 5 0. 6 0. 7 0. 8 FFR 0. 9 1

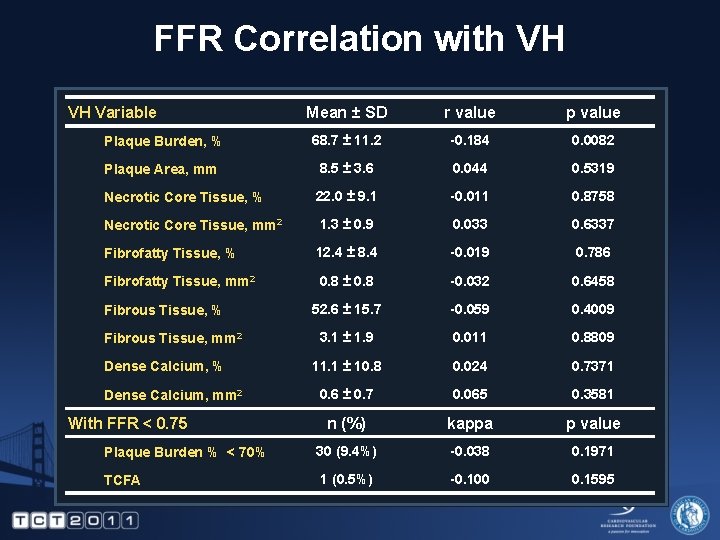
FFR Correlation with VH VH Variable Mean ± SD r value p value Plaque Burden, % 68. 7 ± 11. 2 -0. 184 0. 0082 Plaque Area, mm 8. 5 ± 3. 6 0. 044 0. 5319 Necrotic Core Tissue, % 22. 0 ± 9. 1 -0. 011 0. 8758 Necrotic Core Tissue, mm 2 1. 3 ± 0. 9 0. 033 0. 6337 Fibrofatty Tissue, % 12. 4 ± 8. 4 -0. 019 0. 786 Fibrofatty Tissue, mm 2 0. 8 ± 0. 8 -0. 032 0. 6458 52. 6 ± 15. 7 -0. 059 0. 4009 3. 1 ± 1. 9 0. 011 0. 8809 11. 1 ± 10. 8 0. 024 0. 7371 0. 6 ± 0. 7 0. 065 0. 3581 n (%) kappa p value Plaque Burden % < 70% 30 (9. 4%) -0. 038 0. 1971 TCFA 1 (0. 5%) -0. 100 0. 1595 Fibrous Tissue, % Fibrous Tissue, mm 2 Dense Calcium, % Dense Calcium, mm 2 With FFR < 0. 75

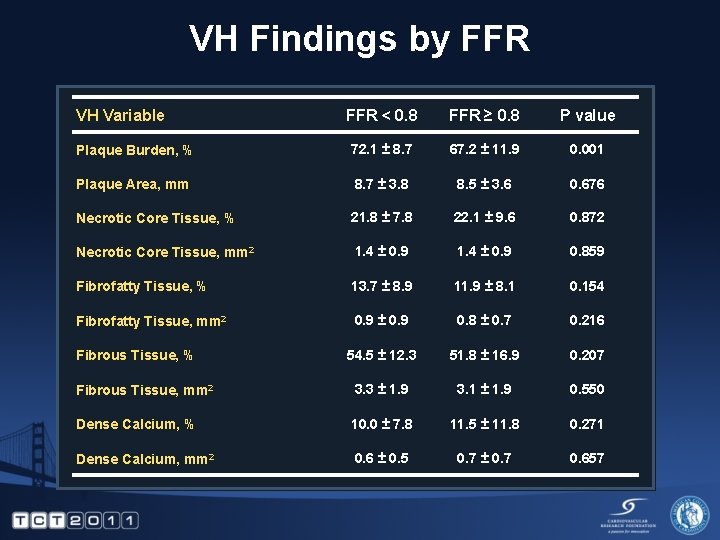
VH Findings by FFR VH Variable FFR < 0. 8 FFR ≥ 0. 8 P value Plaque Burden, % 72. 1 ± 8. 7 67. 2 ± 11. 9 0. 001 Plaque Area, mm 8. 7 ± 3. 8 8. 5 ± 3. 6 0. 676 Necrotic Core Tissue, % 21. 8 ± 7. 8 22. 1 ± 9. 6 0. 872 Necrotic Core Tissue, mm 2 1. 4 ± 0. 9 0. 859 Fibrofatty Tissue, % 13. 7 ± 8. 9 11. 9 ± 8. 1 0. 154 Fibrofatty Tissue, mm 2 0. 9 ± 0. 9 0. 8 ± 0. 7 0. 216 54. 5 ± 12. 3 51. 8 ± 16. 9 0. 207 Fibrous Tissue, mm 2 3. 3 ± 1. 9 3. 1 ± 1. 9 0. 550 Dense Calcium, % 10. 0 ± 7. 8 11. 5 ± 11. 8 0. 271 Dense Calcium, mm 2 0. 6 ± 0. 5 0. 7 ± 0. 7 0. 657 Fibrous Tissue, %

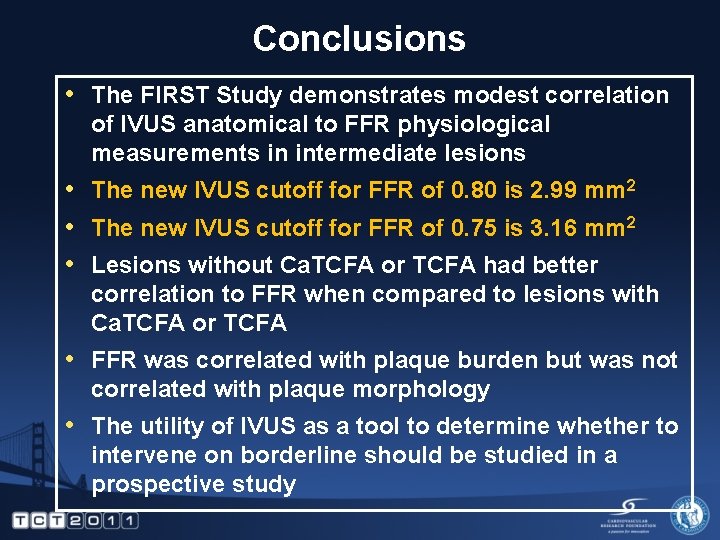
Conclusions • The FIRST Study demonstrates modest correlation of IVUS anatomical to FFR physiological measurements in intermediate lesions • The new IVUS cutoff for FFR of 0. 80 is 2. 99 mm 2 • The new IVUS cutoff for FFR of 0. 75 is 3. 16 mm 2 • Lesions without Ca. TCFA or TCFA had better correlation to FFR when compared to lesions with Ca. TCFA or TCFA • FFR was correlated with plaque burden but was not correlated with plaque morphology • The utility of IVUS as a tool to determine whether to intervene on borderline should be studied in a prospective study

Limitations • This trial does not assess long term outcome with respect to the IVUS/FFR findings. • Once this IVUS/FFR correlation has been established it would require to conduct an IVUS guided lesion assessment study to determine the need for intervention and the impact of this strategy on long term clinical outcome.
 Intravascular ultrasound
Intravascular ultrasound Ivus
Ivus Ferriman–gallwey score
Ferriman–gallwey score Difference between capital reserve and reserve capital
Difference between capital reserve and reserve capital Difference between capital reserve and reserve capital
Difference between capital reserve and reserve capital Fractional reserve banking
Fractional reserve banking Fractional reserve banking example
Fractional reserve banking example Fractional reserve banking example
Fractional reserve banking example Fractional reserve banking example
Fractional reserve banking example Fractional reserve banking system
Fractional reserve banking system Contractionary money policy
Contractionary money policy Fractional reserve theory
Fractional reserve theory Contingency reserve vs management reserve
Contingency reserve vs management reserve Laws of flotation
Laws of flotation Reserve buoyancy of ship
Reserve buoyancy of ship Reserve buoyancy of ship
Reserve buoyancy of ship Compared to an empty ship would a ship loaded
Compared to an empty ship would a ship loaded Ship ship routines and construction
Ship ship routines and construction Espaço intravascular
Espaço intravascular Intravascular hemolytic anemia
Intravascular hemolytic anemia Liquido intravascular
Liquido intravascular Disseminated intravascular coagulation pathophysiology
Disseminated intravascular coagulation pathophysiology Disforese
Disforese Liquido intravascular
Liquido intravascular Disseminated intravascular coagulation
Disseminated intravascular coagulation Espacio extracelular e intracelular
Espacio extracelular e intracelular Esquema de liquidos corporales
Esquema de liquidos corporales Distillation vs steam distillation
Distillation vs steam distillation