Formula Units Moles Representative Particles The form in

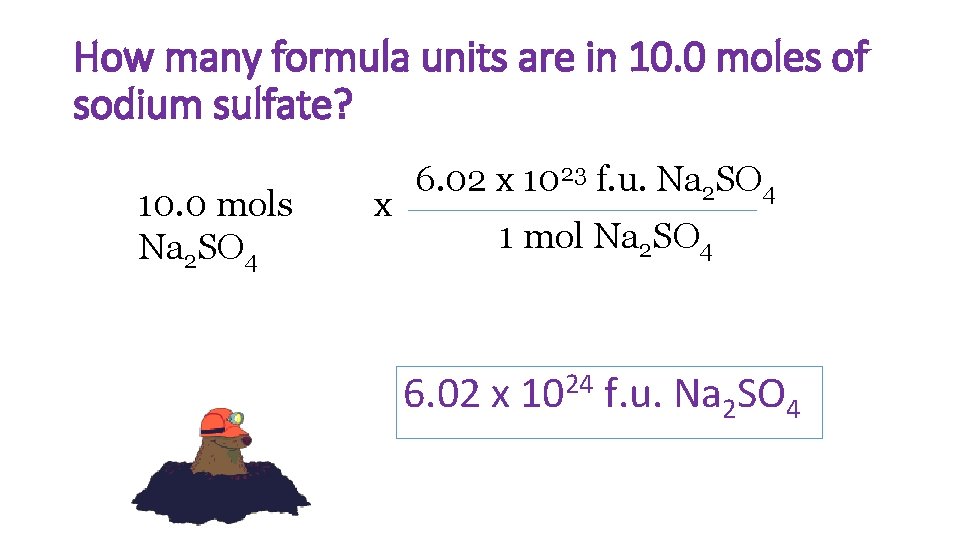
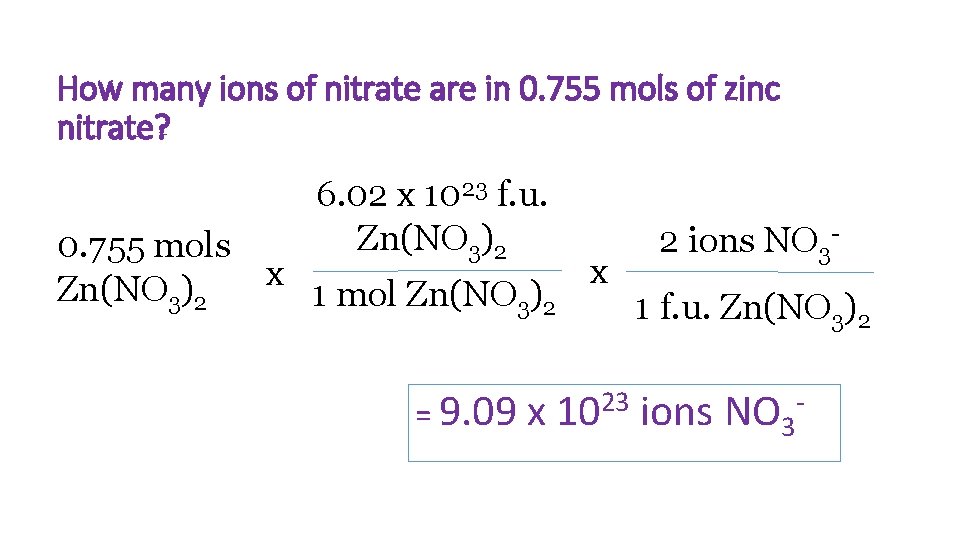


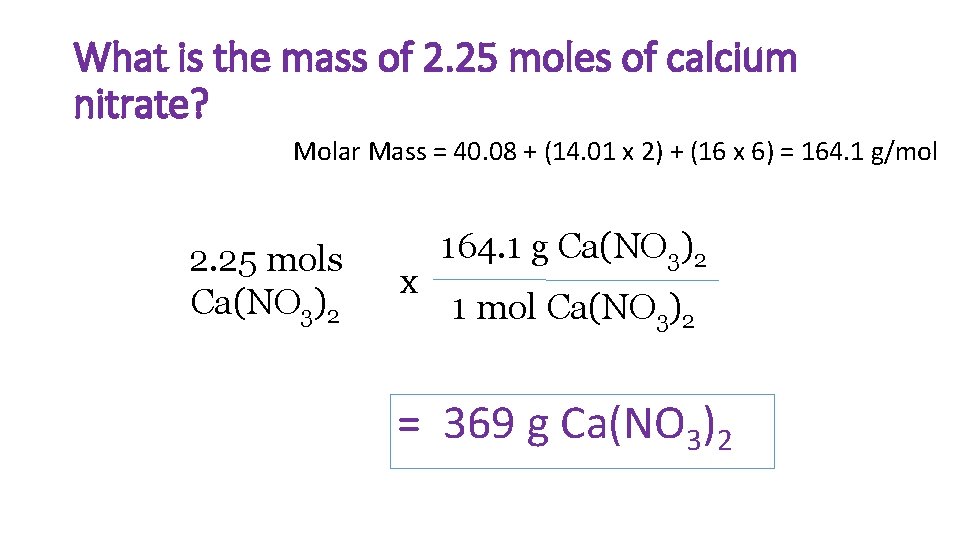

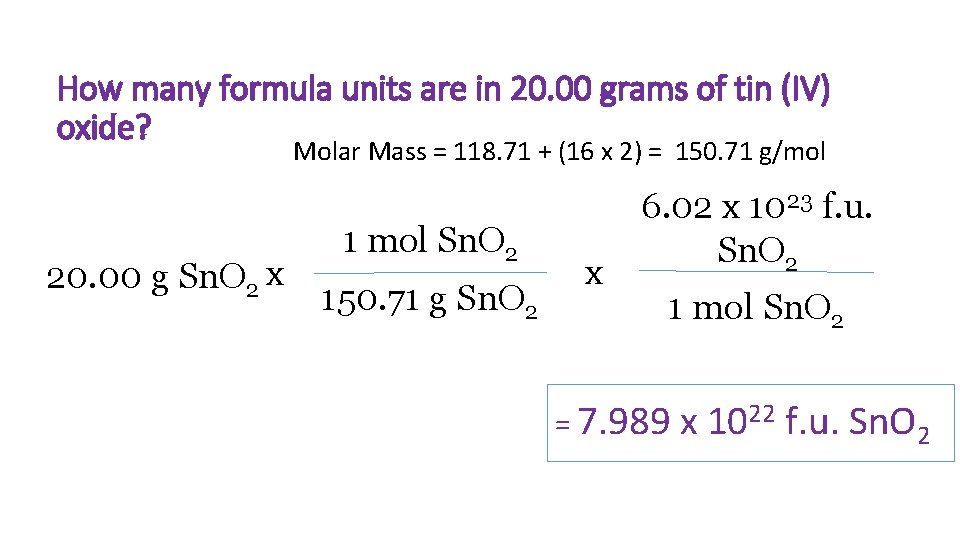
- Slides: 20

Formula Units & Moles

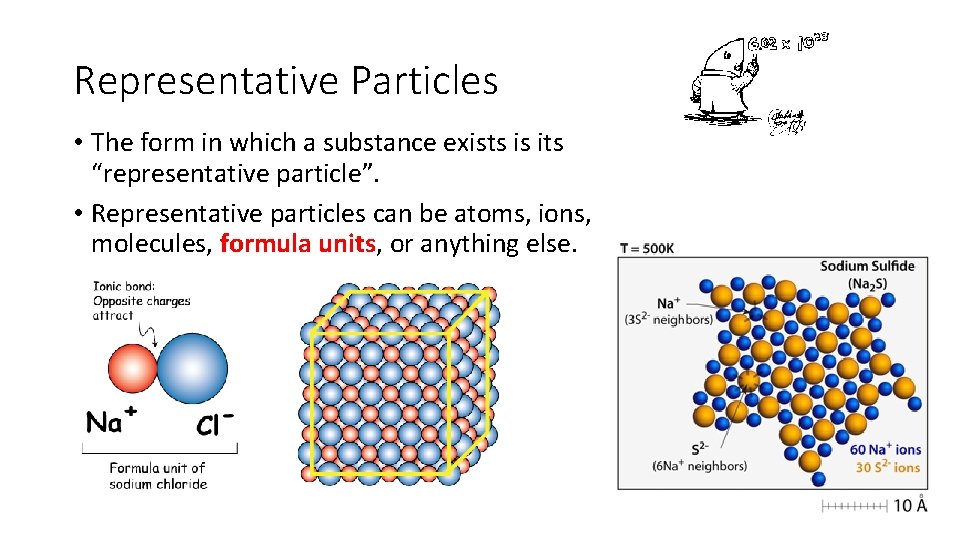
Representative Particles • The form in which a substance exists is its “representative particle”. • Representative particles can be atoms, ions, molecules, formula units, or anything else.

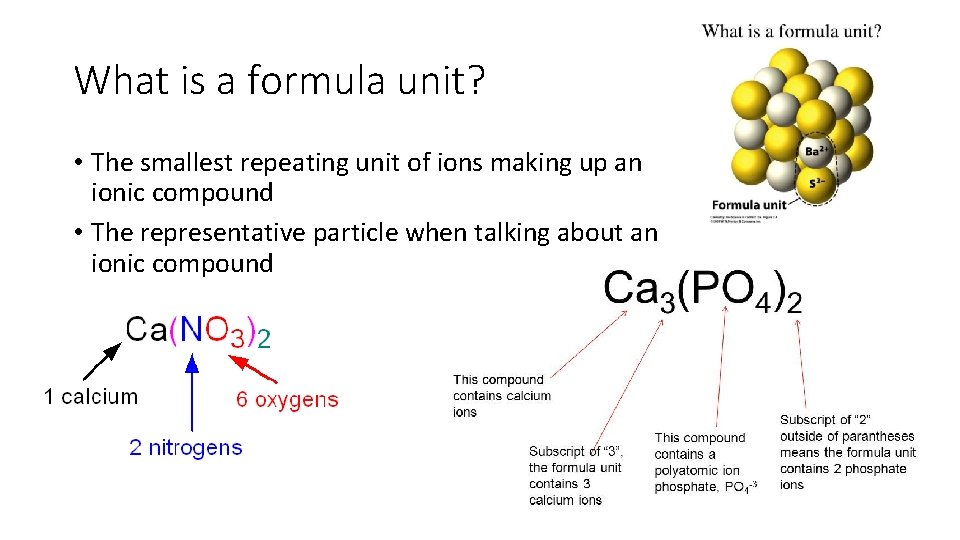
What is a formula unit? • The smallest repeating unit of ions making up an ionic compound • The representative particle when talking about an ionic compound

Avogadro’s Number and Formula Units • What do we already know? • 1 mole of an element = 6. 02 x 1023 atoms • What we will use this unit? • 1 mole of an ionic compound = 6. 02 x 1023 formula units • Representative Particles: unit needs to be applicable to what is being calculated.

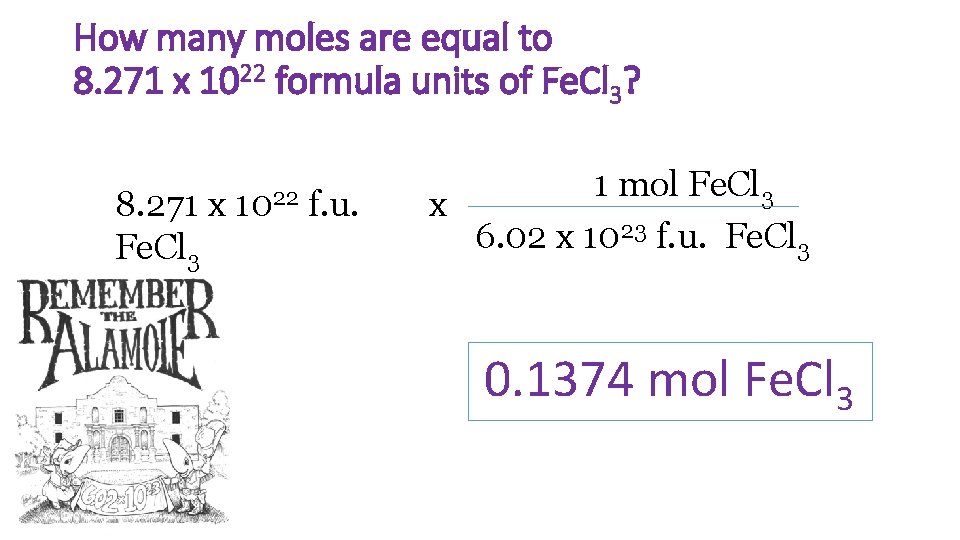
How many moles are equal to 8. 271 x 1022 formula units of Fe. Cl 3? 8. 271 x Fe. Cl 3 1022 f. u. 1 mol Fe. Cl 3 x 6. 02 x 1023 f. u. Fe. Cl 3 0. 1374 mol Fe. Cl 3
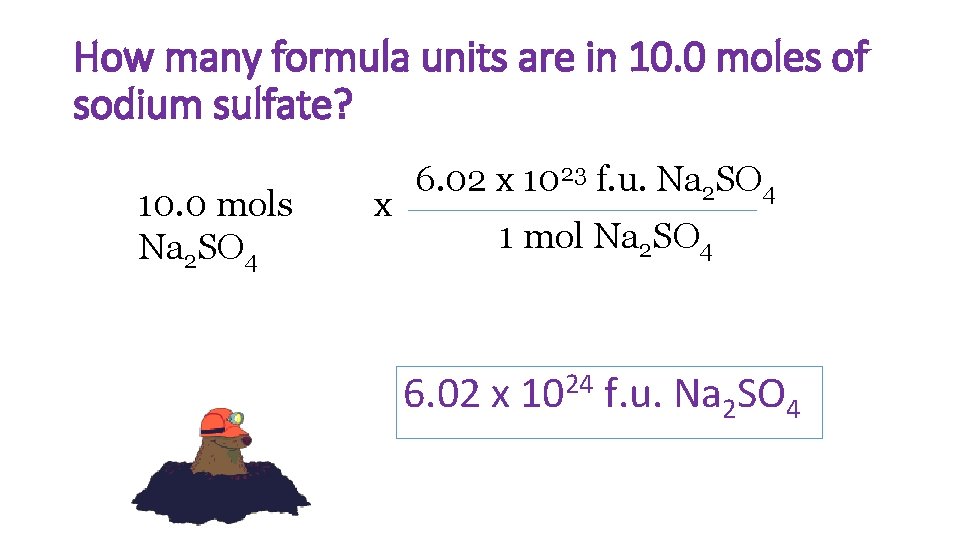
How many formula units are in 10. 0 moles of sodium sulfate? 10. 0 mols Na 2 SO 4 x 6. 02 x 1023 f. u. Na 2 SO 4 1 mol Na 2 SO 4 6. 02 x 1024 f. u. Na 2 SO 4
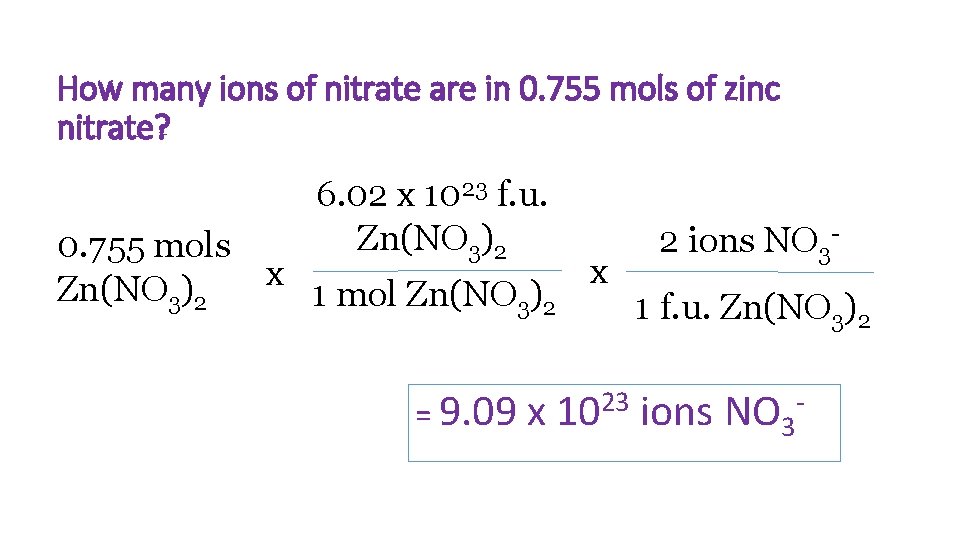
How many ions of nitrate are in 0. 755 mols of zinc nitrate? 6. 02 x 1023 f. u. Zn(NO 3)2 2 ions NO 3 - 0. 755 mols x x Zn(NO 3)2 1 mol Zn(NO 3)2 1 f. u. Zn(NO 3)2 = 9. 09 x 23 10 ions NO 3 -

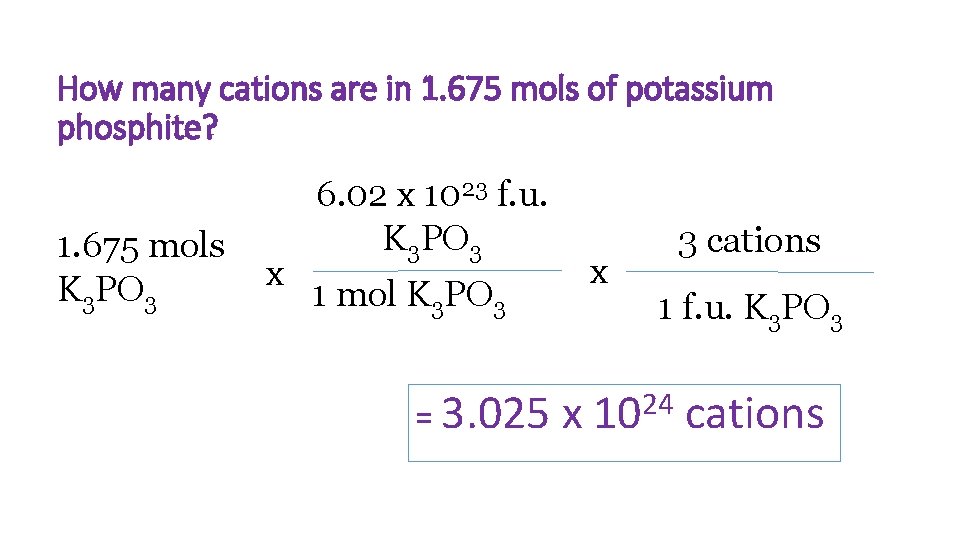
How many cations are in 1. 675 mols of potassium phosphite? 1. 675 mols K 3 PO 3 x 6. 02 x 1023 f. u. K 3 PO 3 x 1 mol K 3 PO 3 = 3. 025 x 3 cations 1 f. u. K 3 PO 3 24 10 cations

Mole-Mass Relationships

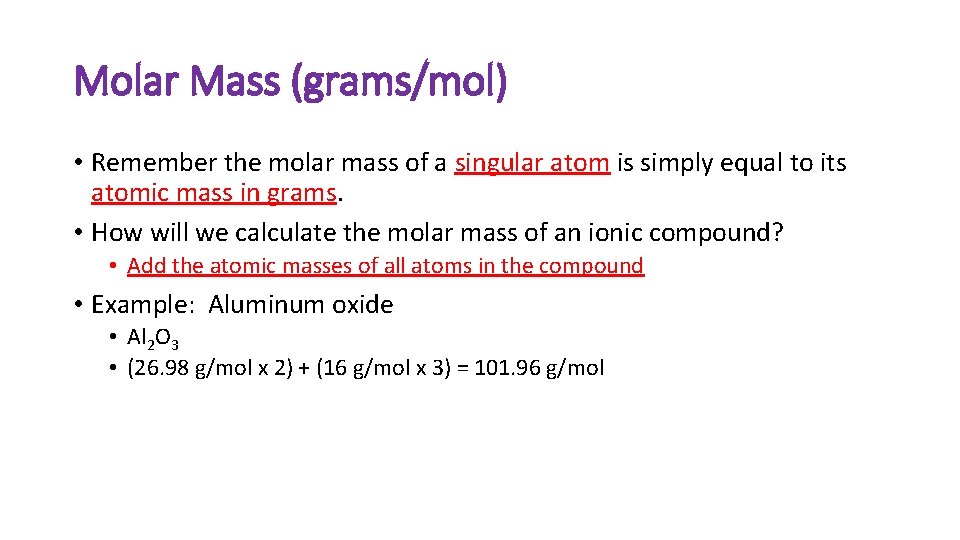
Molar Mass (grams/mol) • Remember the molar mass of a singular atom is simply equal to its atomic mass in grams. • How will we calculate the molar mass of an ionic compound? • Add the atomic masses of all atoms in the compound • Example: Aluminum oxide • Al 2 O 3 • (26. 98 g/mol x 2) + (16 g/mol x 3) = 101. 96 g/mol

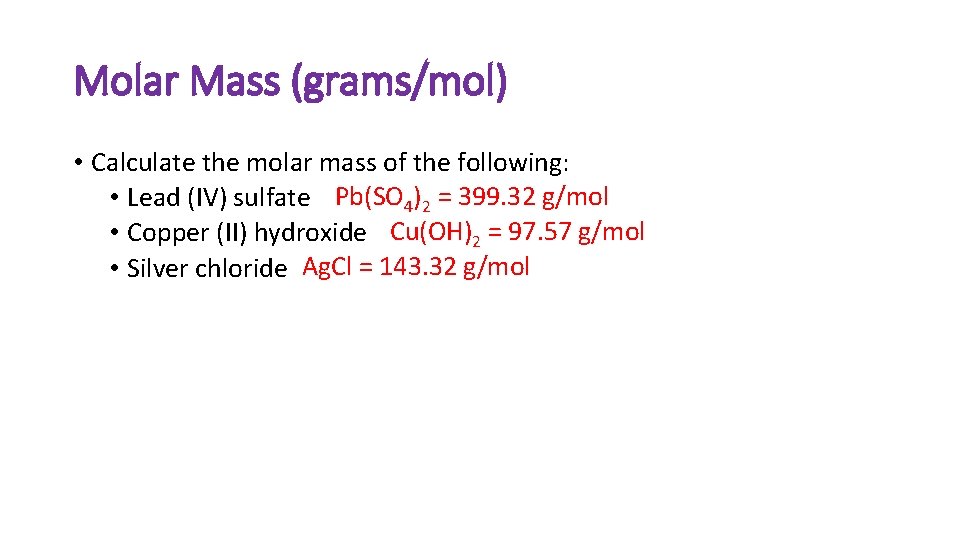
Molar Mass (grams/mol) • Calculate the molar mass of the following: • Lead (IV) sulfate Pb(SO 4)2 = 399. 32 g/mol • Copper (II) hydroxide Cu(OH)2 = 97. 57 g/mol • Silver chloride Ag. Cl = 143. 32 g/mol

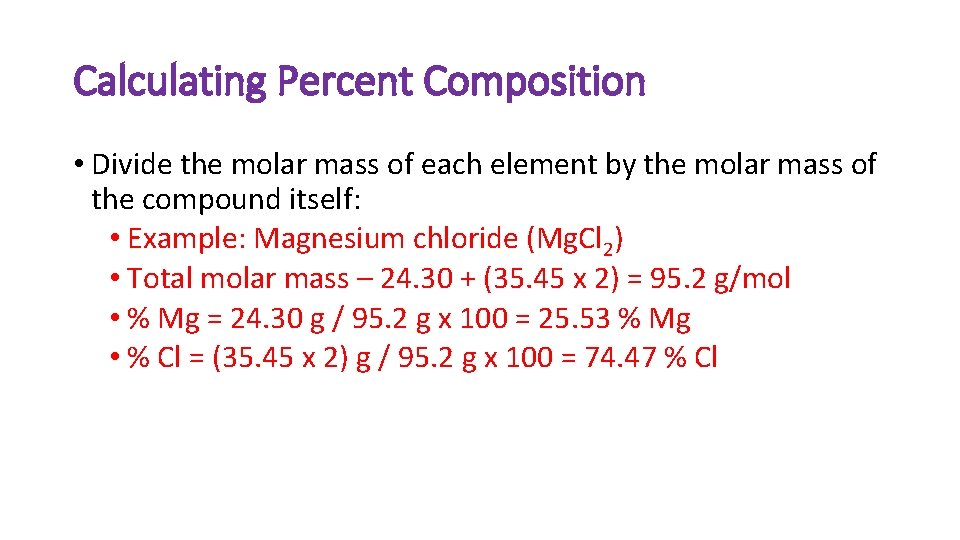
Calculating Percent Composition • Divide the molar mass of each element by the molar mass of the compound itself: • Example: Magnesium chloride (Mg. Cl 2) • Total molar mass – 24. 30 + (35. 45 x 2) = 95. 2 g/mol • % Mg = 24. 30 g / 95. 2 g x 100 = 25. 53 % Mg • % Cl = (35. 45 x 2) g / 95. 2 g x 100 = 74. 47 % Cl

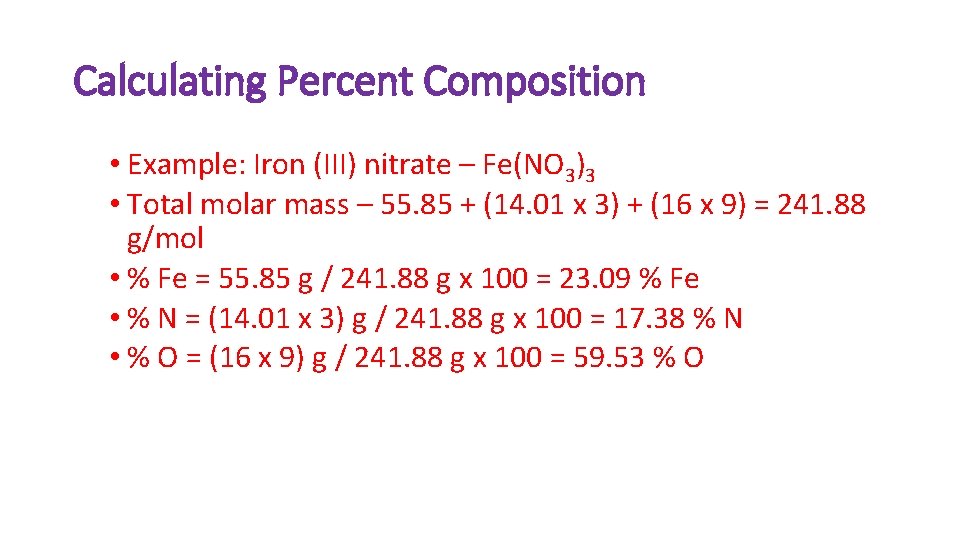
Calculating Percent Composition • Example: Iron (III) nitrate – Fe(NO 3)3 • Total molar mass – 55. 85 + (14. 01 x 3) + (16 x 9) = 241. 88 g/mol • % Fe = 55. 85 g / 241. 88 g x 100 = 23. 09 % Fe • % N = (14. 01 x 3) g / 241. 88 g x 100 = 17. 38 % N • % O = (16 x 9) g / 241. 88 g x 100 = 59. 53 % O

Mole – Mass Conversion factor 1 mol = molar mass (g/mol)
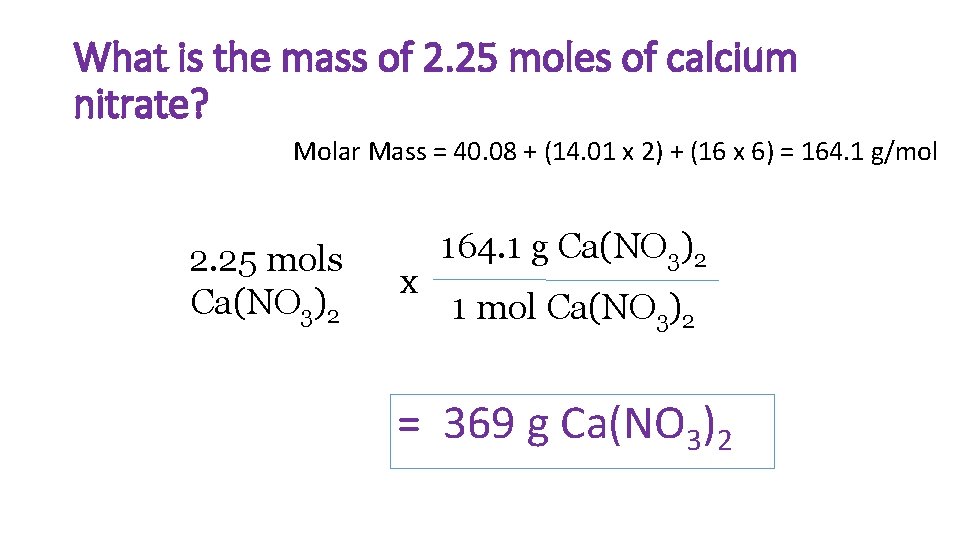
What is the mass of 2. 25 moles of calcium nitrate? Molar Mass = 40. 08 + (14. 01 x 2) + (16 x 6) = 164. 1 g/mol 2. 25 mols Ca(NO 3)2 x 164. 1 g Ca(NO 3)2 1 mol Ca(NO 3)2 = 369 g Ca(NO 3)2

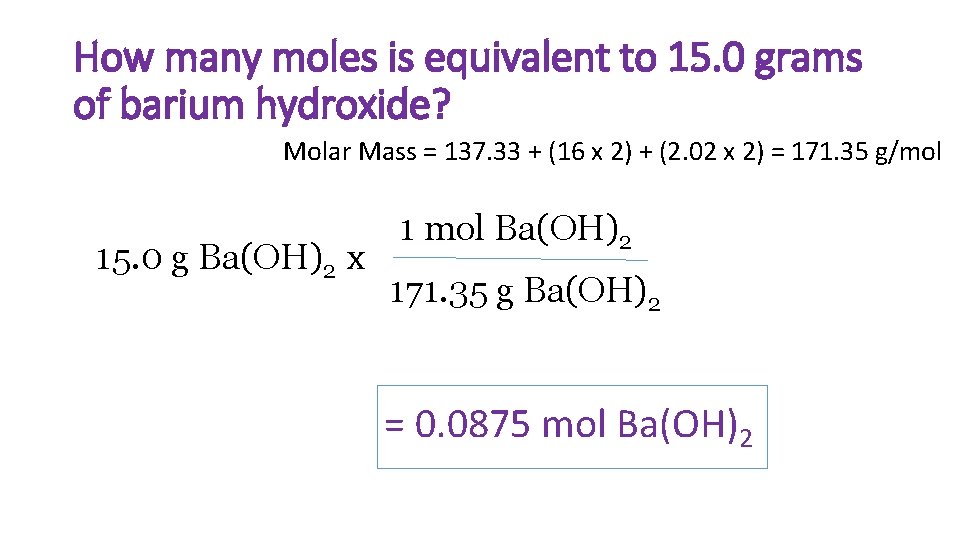
How many moles is equivalent to 15. 0 grams of barium hydroxide? Molar Mass = 137. 33 + (16 x 2) + (2. 02 x 2) = 171. 35 g/mol 15. 0 g Ba(OH)2 x 1 mol Ba(OH)2 171. 35 g Ba(OH)2 = 0. 0875 mol Ba(OH)2

Multi-step Mole Problems Using multiple conversion factors.

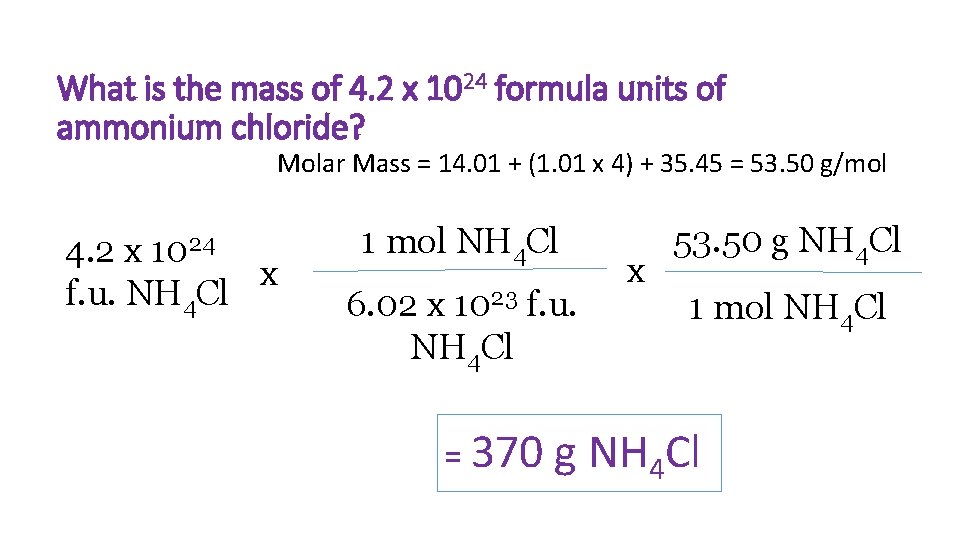
What is the mass of 4. 2 x 1024 formula units of ammonium chloride? Molar Mass = 14. 01 + (1. 01 x 4) + 35. 45 = 53. 50 g/mol 4. 2 x 1024 x f. u. NH 4 Cl 1 mol NH 4 Cl 6. 02 x 1023 f. u. NH 4 Cl = 370 x 53. 50 g NH 4 Cl 1 mol NH 4 Cl g NH 4 Cl
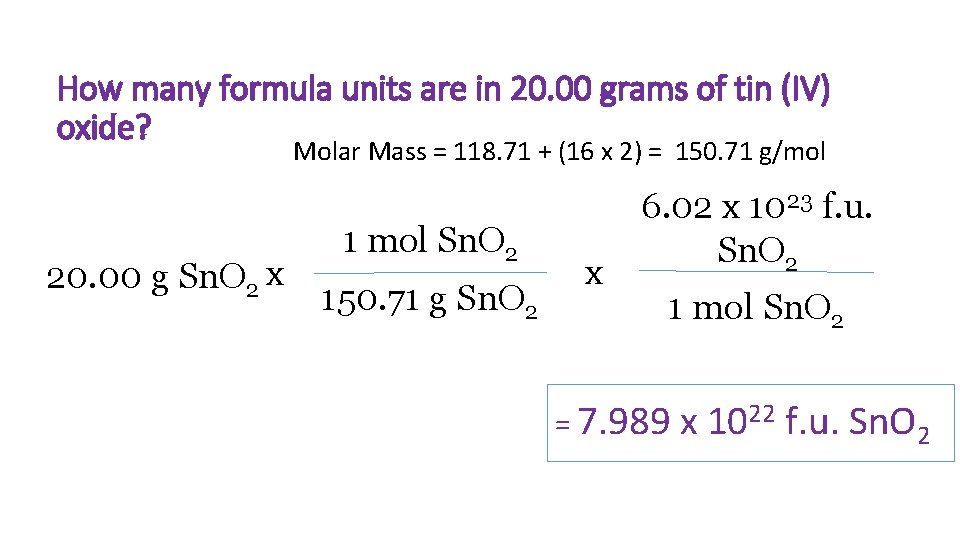
How many formula units are in 20. 00 grams of tin (IV) oxide? Molar Mass = 118. 71 + (16 x 2) = 150. 71 g/mol 20. 00 g Sn. O 2 x 1 mol Sn. O 2 150. 71 g Sn. O 2 x 6. 02 x 1023 f. u. Sn. O 2 1 mol Sn. O 2 = 7. 989 x 1022 f. u. Sn. O 2

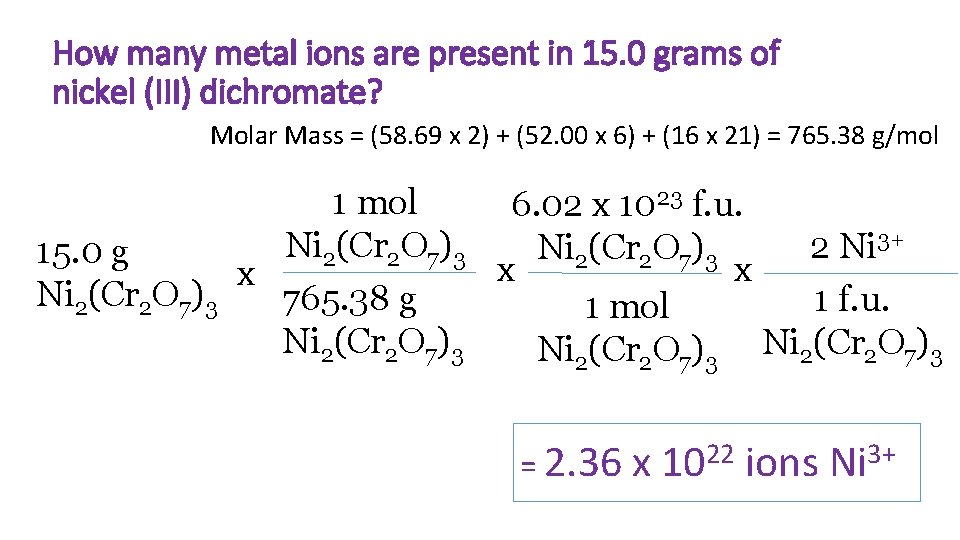
How many metal ions are present in 15. 0 grams of nickel (III) dichromate? Molar Mass = (58. 69 x 2) + (52. 00 x 6) + (16 x 21) = 765. 38 g/mol 1 mol 6. 02 x 1023 f. u. 3+ Ni (Cr O ) 2 Ni Ni (Cr O ) 15. 0 g 2 2 7 3 x x x Ni 2(Cr 2 O 7)3 765. 38 g 1 f. u. 1 mol Ni 2(Cr 2 O 7)3 = 2. 36 x 1022 ions Ni 3+