Review Conversion Formula of particles of Particles Moles



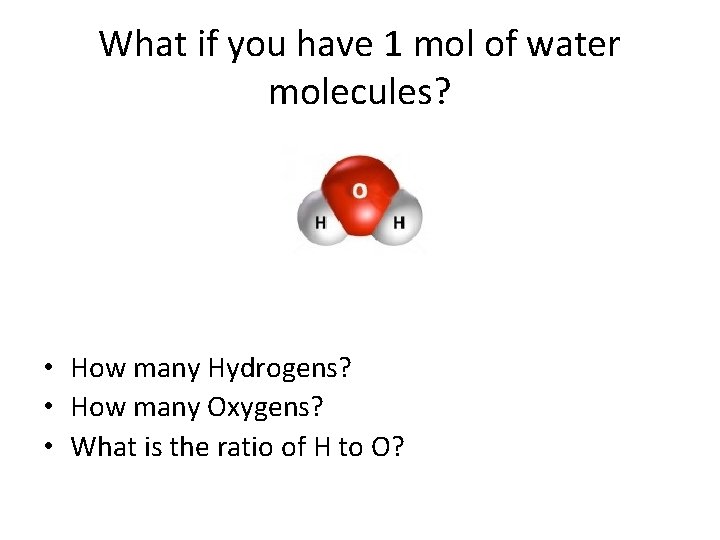

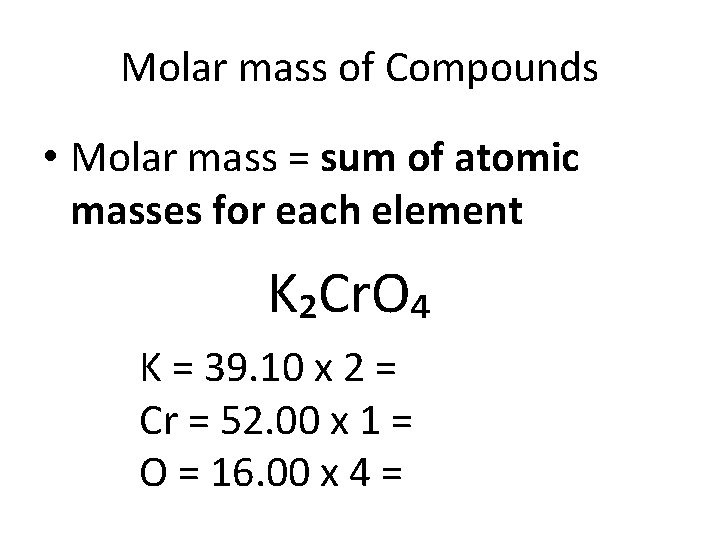



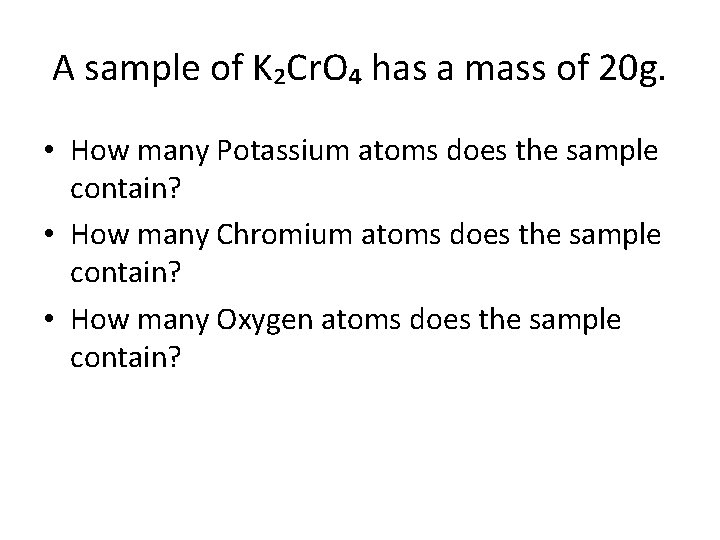
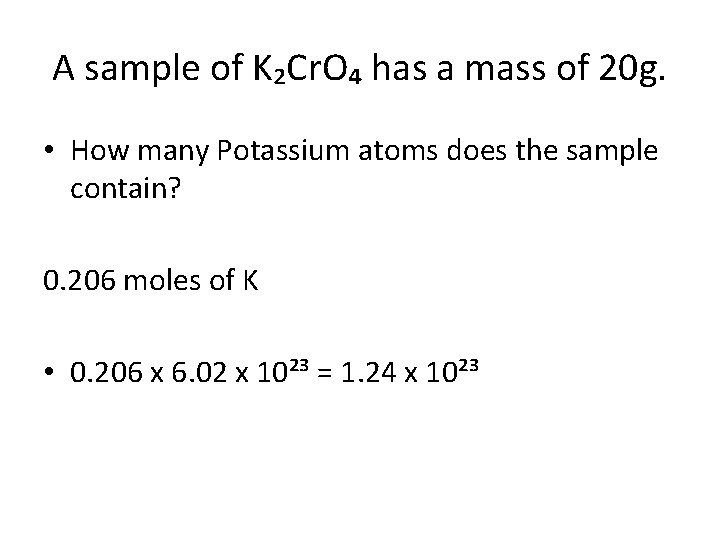
- Slides: 28

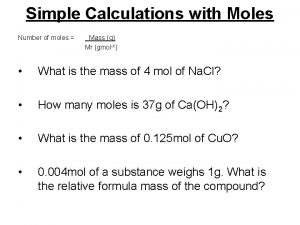
Review

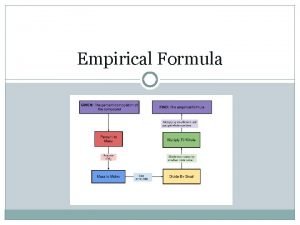
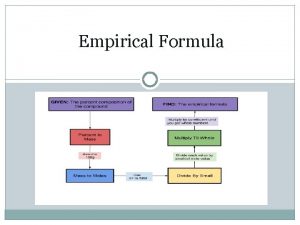
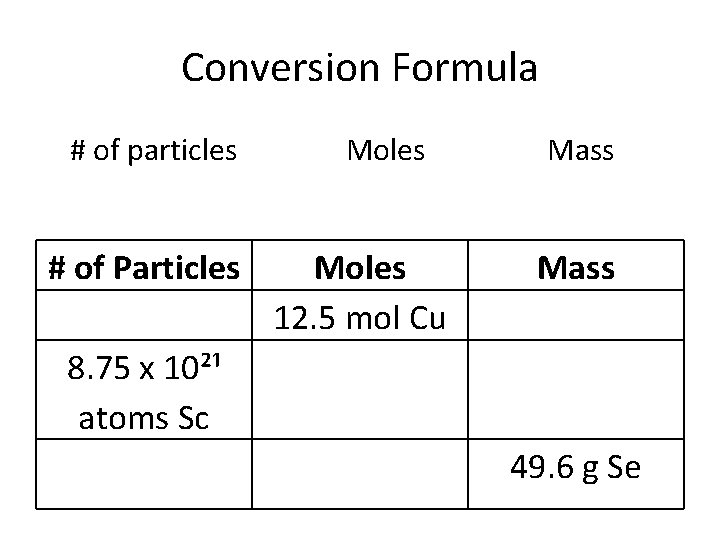
Conversion Formula # of particles # of Particles Moles 12. 5 mol Cu Mass 8. 75 x 10²¹ atoms Sc 49. 6 g Se

Moles of Compounds

Chemical Formulas • Tell the number and types of atoms that make up the formula unit of the compound 1 Water molecule= H₂O How many Hydrogens? How many Oxygens? What is the ratio of H to O?

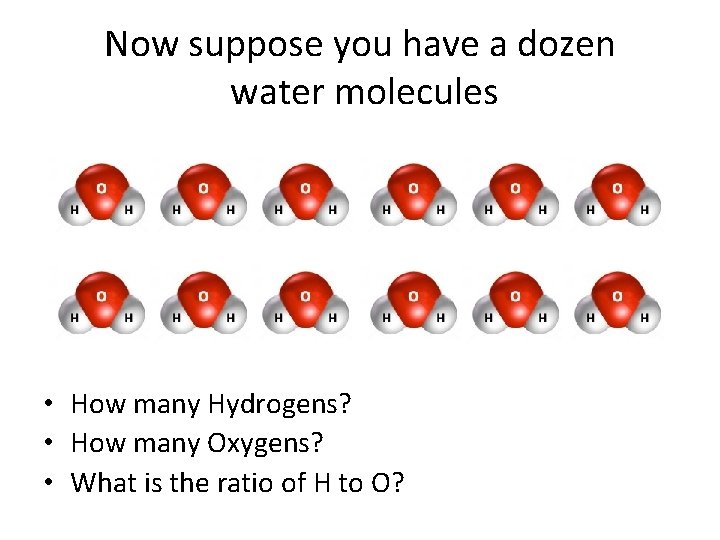
Now suppose you have a dozen water molecules • How many Hydrogens? • How many Oxygens? • What is the ratio of H to O?
What if you have 1 mol of water molecules? • How many Hydrogens? • How many Oxygens? • What is the ratio of H to O?

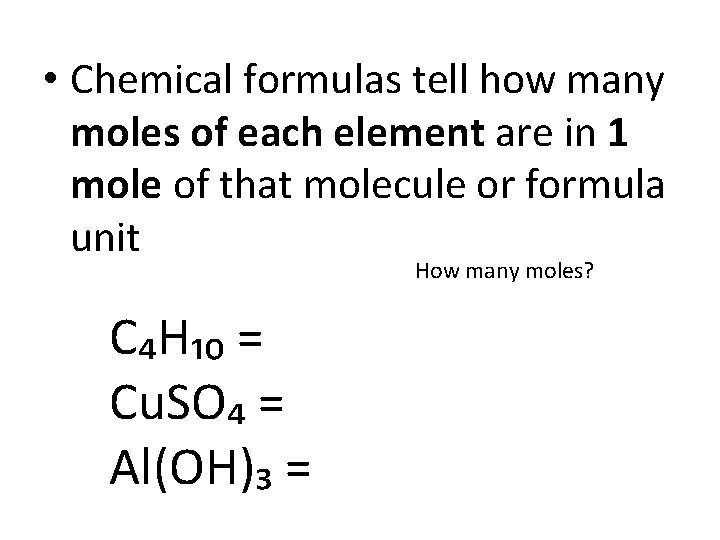
• Chemical formulas tell how many moles of each element are in 1 mole of that molecule or formula unit How many moles? C₄H₁₀ = Cu. SO₄ = Al(OH)₃ =

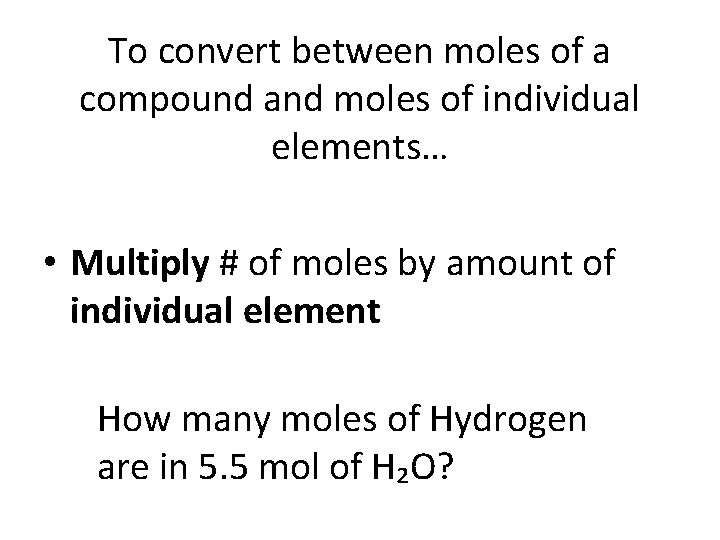
To convert between moles of a compound and moles of individual elements… • Multiply # of moles by amount of individual element How many moles of Hydrogen are in 5. 5 mol of H₂O?

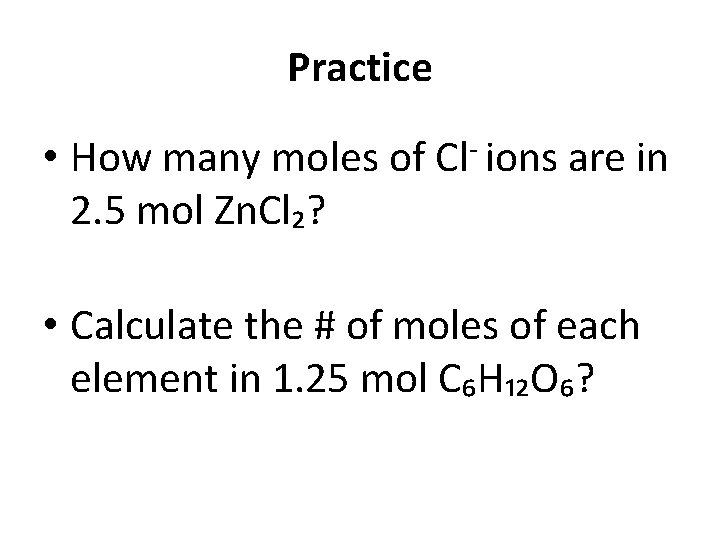
Practice • How many moles of Cl- ions are in 2. 5 mol Zn. Cl₂? • Calculate the # of moles of each element in 1. 25 mol C₆H₁₂O₆?

More Practice • What is the # of moles of sulfate ions found in 3 mol of Fe₂(SO₄)₃? • How many moles of O are in 5 mol diphosphorous pentoxide?
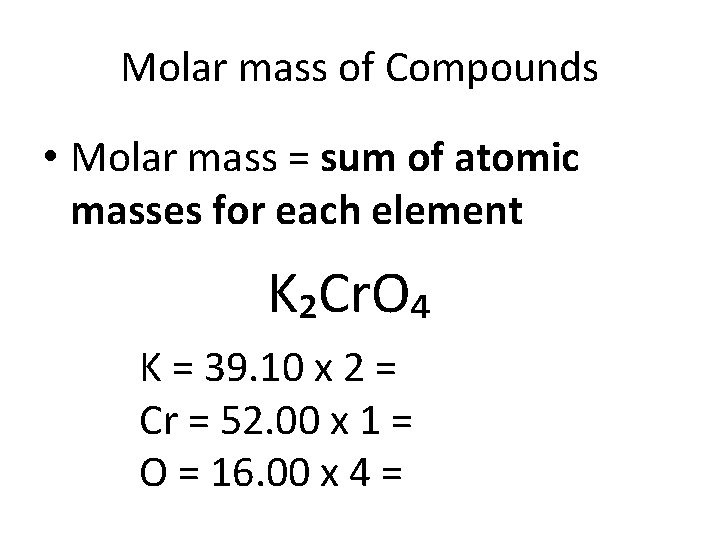
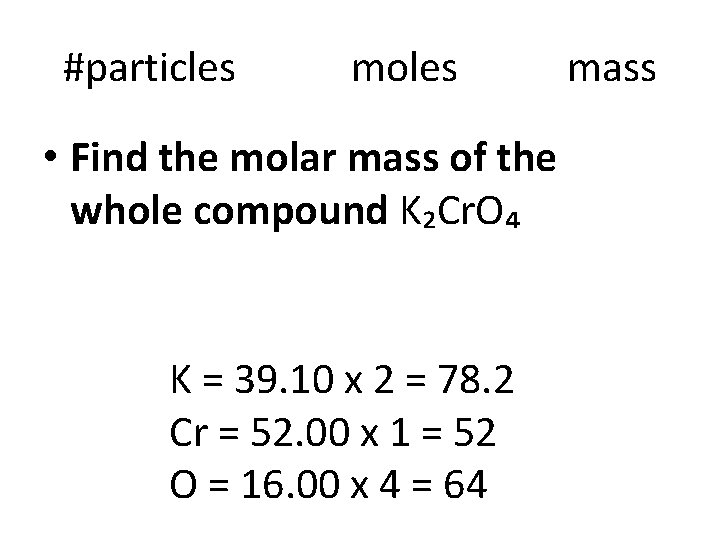
Molar mass of Compounds • Molar mass = sum of atomic masses for each element K₂Cr. O₄ K = 39. 10 x 2 = Cr = 52. 00 x 1 = O = 16. 00 x 4 =

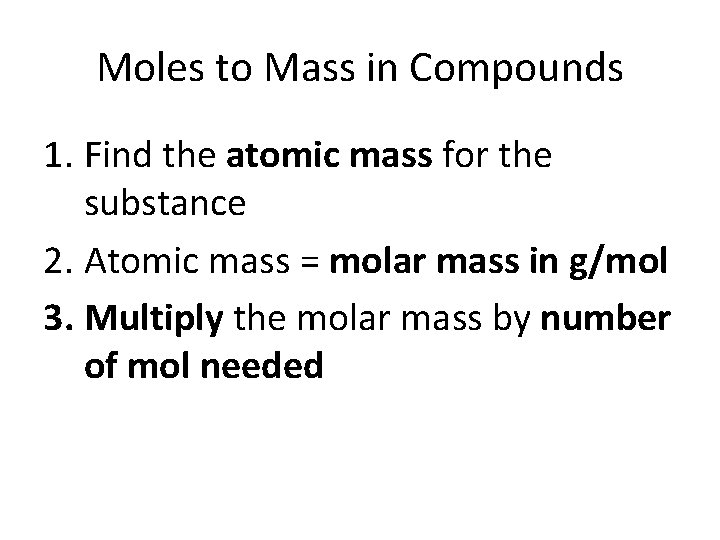
Moles to Mass in Compounds 1. Find the atomic mass for the substance 2. Atomic mass = molar mass in g/mol 3. Multiply the molar mass by number of mol needed

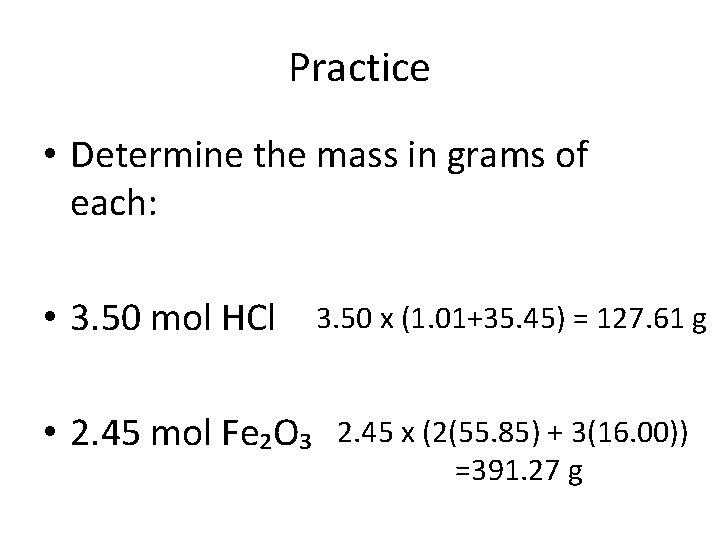
Practice • Determine the mass in grams of each: • 3. 50 mol HCl • 2. 45 mol Fe₂O₃ 3. 50 x (1. 01+35. 45) = 127. 61 g 2. 45 x (2(55. 85) + 3(16. 00)) =391. 27 g

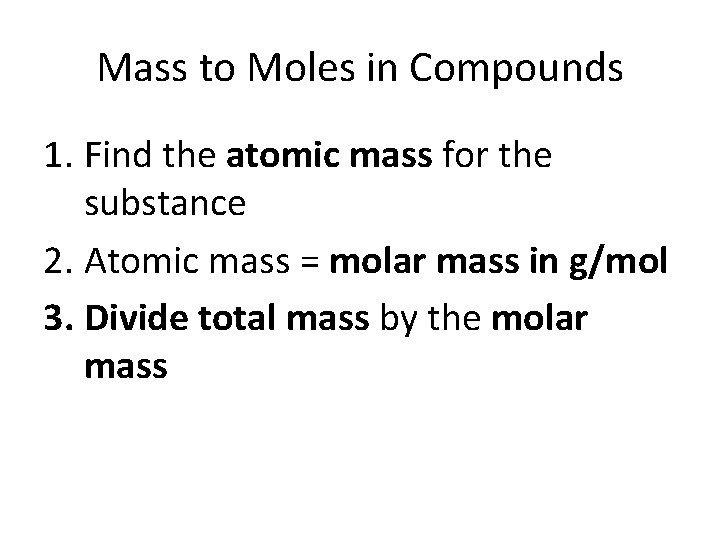
Mass to Moles in Compounds 1. Find the atomic mass for the substance 2. Atomic mass = molar mass in g/mol 3. Divide total mass by the molar mass

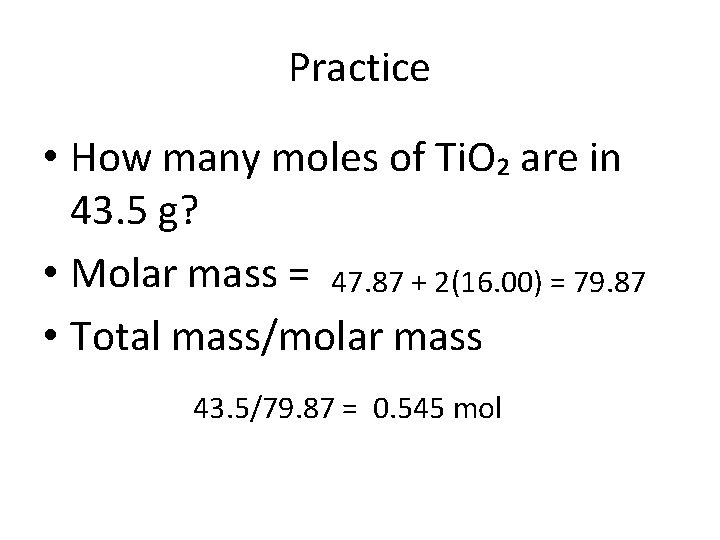
Practice • How many moles of Ti. O₂ are in 43. 5 g? • Molar mass = 47. 87 + 2(16. 00) = 79. 87 • Total mass/molar mass 43. 5/79. 87 = 0. 545 mol

Mass to # of Particles 1. Convert Mass to moles 2. Convert moles to # of particles

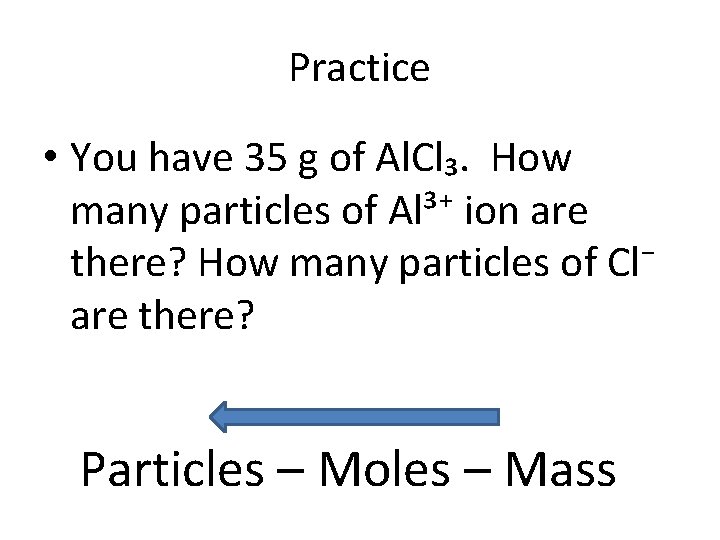
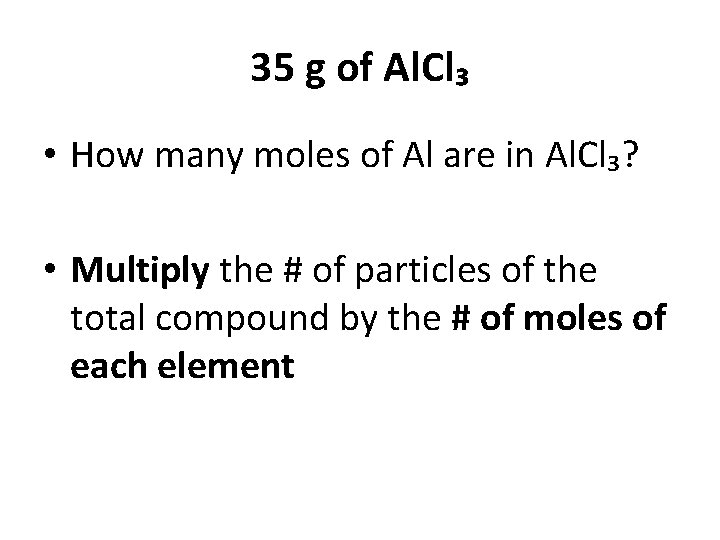
Practice • You have 35 g of Al. Cl₃. How many particles of Al³⁺ ion are there? How many particles of Cl⁻ are there? Particles – Moles – Mass

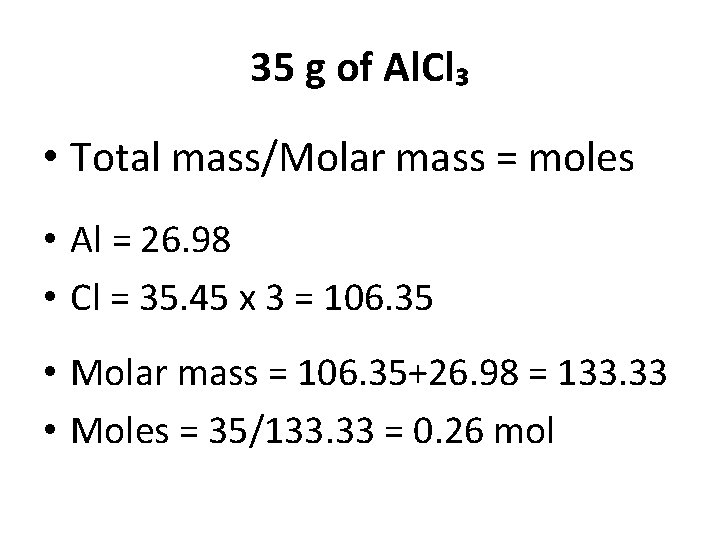
35 g of Al. Cl₃ • Total mass/Molar mass = moles • Al = 26. 98 • Cl = 35. 45 x 3 = 106. 35 • Molar mass = 106. 35+26. 98 = 133. 33 • Moles = 35/133. 33 = 0. 26 mol

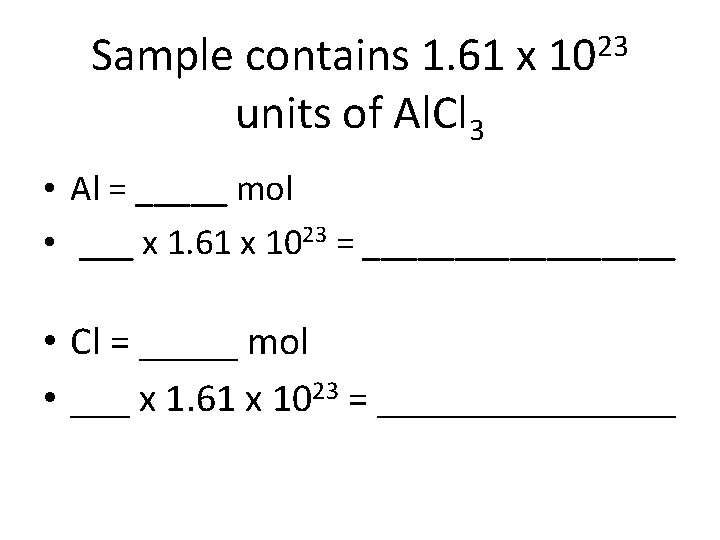
35 g of Al. Cl₃ • Moles x Avogadro’s # = particles • 0. 26 mol x (6. 02 x 10²³) = 1. 61 x 10²³ Now… How do you find out # of particles for Al and Cl?

35 g of Al. Cl₃ • How many moles of Al are in Al. Cl₃? • Multiply the # of particles of the total compound by the # of moles of each element

Sample contains 1. 61 x units of Al. Cl 3 23 10 • Al = _____ mol • ___ x 1. 61 x 1023 = _________ • Cl = _____ mol • ___ x 1. 61 x 1023 = ________

To find the mass of 1 formula unit of a compound… • Divide molar mass of the compound by Avogadro’s # • Molar mass of Al. Cl 3 is 133. • 133. 33/6. 02 x 1023 = 2. 21 x 10⁻²²

Practice Worksheet
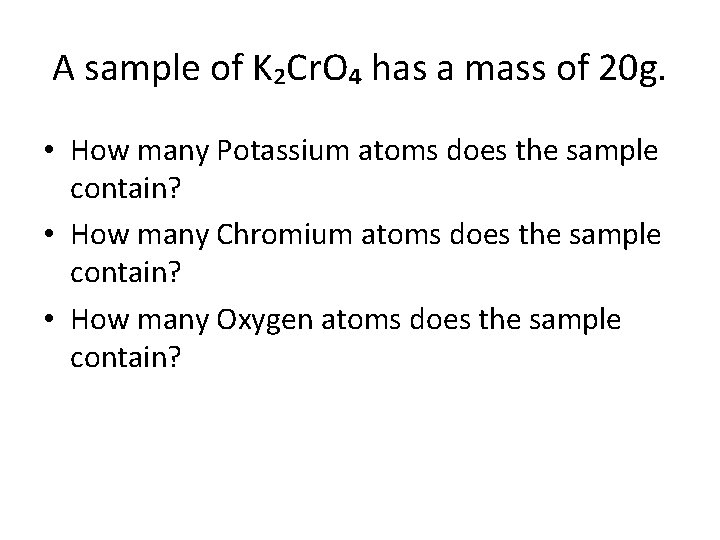
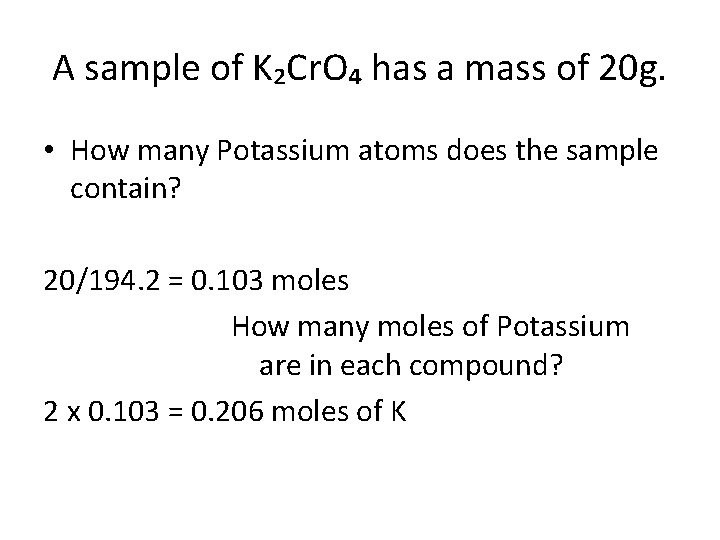
A sample of K₂Cr. O₄ has a mass of 20 g. • How many Potassium atoms does the sample contain? • How many Chromium atoms does the sample contain? • How many Oxygen atoms does the sample contain?

#particles moles • Find the molar mass of the whole compound K₂Cr. O₄ K = 39. 10 x 2 = 78. 2 Cr = 52. 00 x 1 = 52 O = 16. 00 x 4 = 64 mass

A sample of K₂Cr. O₄ has a mass of 20 g. • How many Potassium atoms does the sample contain? 20/194. 2 = 0. 103 moles How many moles of Potassium are in each compound? 2 x 0. 103 = 0. 206 moles of K
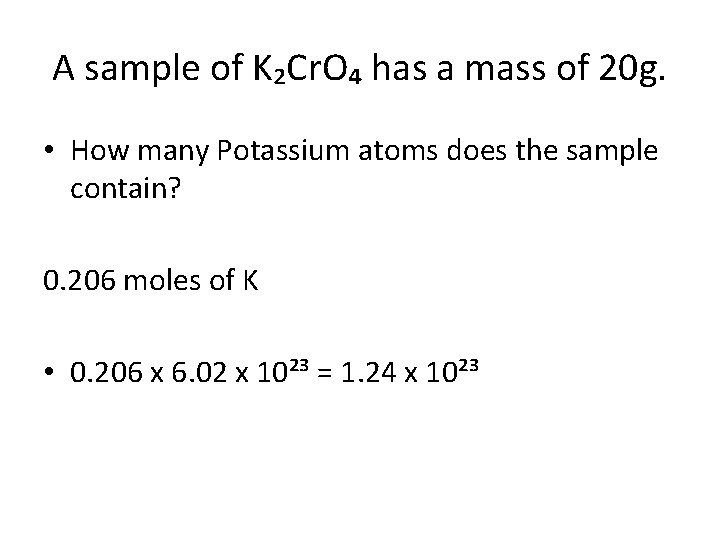
A sample of K₂Cr. O₄ has a mass of 20 g. • How many Potassium atoms does the sample contain? 0. 206 moles of K • 0. 206 x 6. 02 x 10²³ = 1. 24 x 10²³

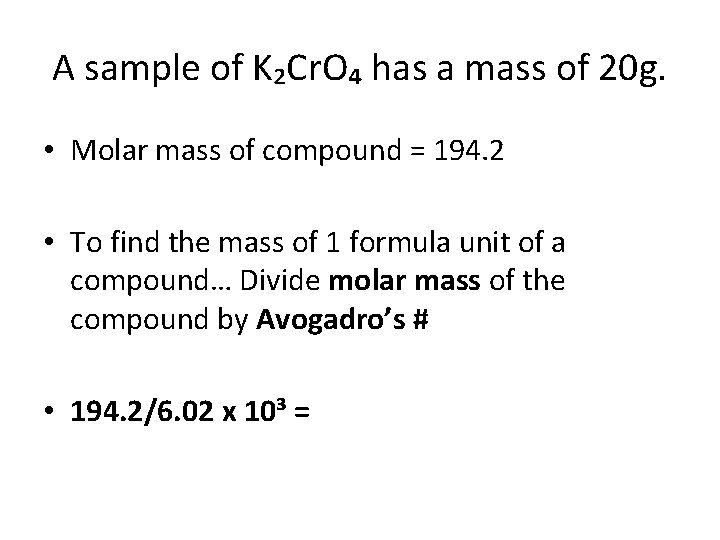
A sample of K₂Cr. O₄ has a mass of 20 g. • Molar mass of compound = 194. 2 • To find the mass of 1 formula unit of a compound… Divide molar mass of the compound by Avogadro’s # • 194. 2/6. 02 x 10³ =
 If i place 3 moles of n2 and 4 moles of o2 in a 35l
If i place 3 moles of n2 and 4 moles of o2 in a 35l Mol to particles
Mol to particles Moles conversion chart
Moles conversion chart Moles to grams
Moles to grams Moles to grams conversion
Moles to grams conversion Calculate moles from grams
Calculate moles from grams Mole island diagram
Mole island diagram What is a mol
What is a mol Grams to mole
Grams to mole 1 mole = grams
1 mole = grams Mol formula chemistry
Mol formula chemistry How to find moles from grams
How to find moles from grams Amu vs molar mass
Amu vs molar mass How to find the molar mass
How to find the molar mass Representative particle
Representative particle Why is the mole at the center of the road map
Why is the mole at the center of the road map Grams to mole
Grams to mole Formula units
Formula units Responde en el cuaderno
Responde en el cuaderno Molecules to moles
Molecules to moles Grams to moles formula
Grams to moles formula How many eggs is 5 dozen
How many eggs is 5 dozen Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Ap gov final review
Ap gov final review Narrative review vs systematic review
Narrative review vs systematic review Systematic review definition
Systematic review definition Narrative review vs systematic review
Narrative review vs systematic review Grid conversion factor radiology
Grid conversion factor radiology Process costing and hybrid product-costing systems
Process costing and hybrid product-costing systems