Flu Vaccination in Children 2008 Samar Musmar MD















- Slides: 24

Flu Vaccination in Children 2008 Samar Musmar, MD, FAAFP Vice Dean for Clinical Affairs An-Najah National University Faculty of Medicine Head, Medicine and Society Dep.

Objectives • • • Biology of Influenza complications Influenza epidemiology Influenza Control Influenza vaccine Effectiveness of vaccine SE of TIV flu vaccine LAIV Recommendations for flu Vaccine in Children • Antiviral medications

Human Influenza Virus (Flu) Influenza • Highly transmissible respiratory illness caused by influenza viruses • 3 types A, B, C • Yearly winter epidemics (seasonal or interpandemic influenza) • Sporadic, unpredictable pandemics

Influenza virus type A • Subtyped based on surface glycoproteins • 16 hemagglutinin (HA) and 9 neuraminidase (NA) • Current human subtypes: H 1 N 1 & H 3 N 2 • Capable of epidemics and pandemics • Antigenic shift--pandemics and drift—yearly epidemic • Infects multiple other species and can jump between them • Birds, pigs, horses, dogs… • Birds are the reservoir for new subtypes: H 116

Influenza Virus Types B and C • Influenza B • • Humans only reservoir Less mortality in most years c/w type A Associated with epidemics, not pandemics One influenza B strain in the annual seasonal influenza vaccine • Influenza C • Causes mild disease, sporadic cases • Not included in vaccine

Why to use vaccine? • Complication in Children 1. Serious illnesses----hospitalization (Influenza pneumonia mainly) 2. Influenza-associated deaths – uncommon • data indicate -- although deaths are more common among children with risk factors for influenza complications, the majority of pediatric deaths occur among children of all age groups with no known high-risk conditions

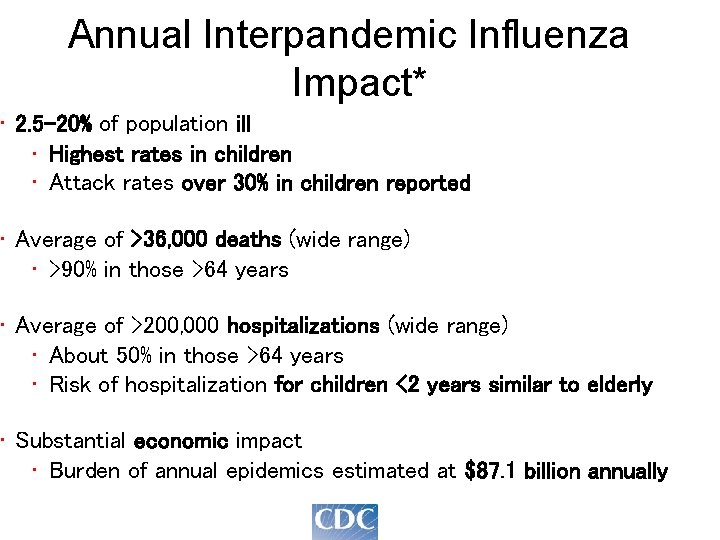
Annual Interpandemic Influenza Impact* • 2. 5 -20% of population ill • Highest rates in children • Attack rates over 30% in children reported • Average of >36, 000 deaths (wide range) • >90% in those >64 years • Average of >200, 000 hospitalizations (wide range) • About 50% in those >64 years • Risk of hospitalization for children <2 years similar to elderly • Substantial economic impact • Burden of annual epidemics estimated at $87. 1 billion annually

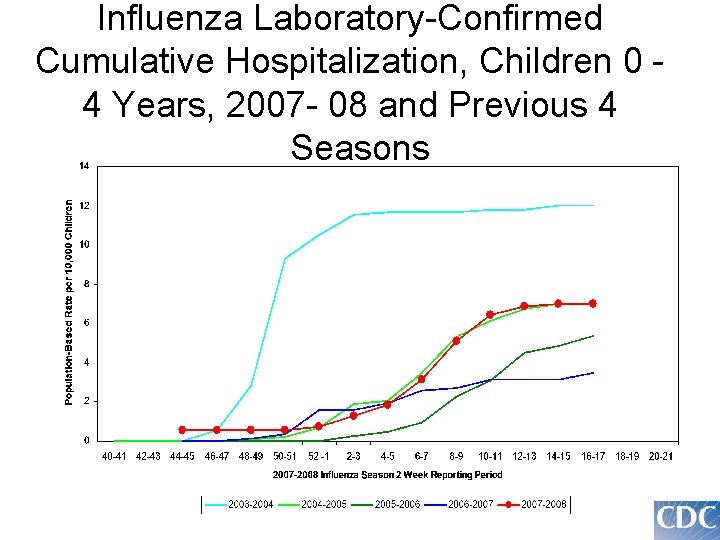
Influenza Laboratory-Confirmed Cumulative Hospitalization, Children 0 4 Years, 2007 - 08 and Previous 4 Seasons

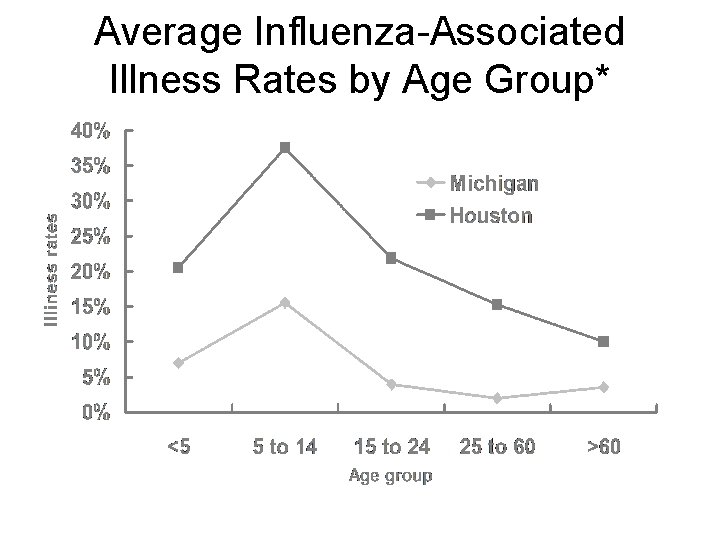
Average Influenza-Associated Illness Rates by Age Group* Sources: Monto J Infect Dis Glezen N Engl J Med

Types of Flu vaccine • Trivalent inactivated influenza vaccine (TIV) 1. Annual 2. Any person aged >6 months • Live, attenuated influenza vaccine (LAIV) 1. Annual 2. Healthy, nonpregnant persons aged 2 --49 years


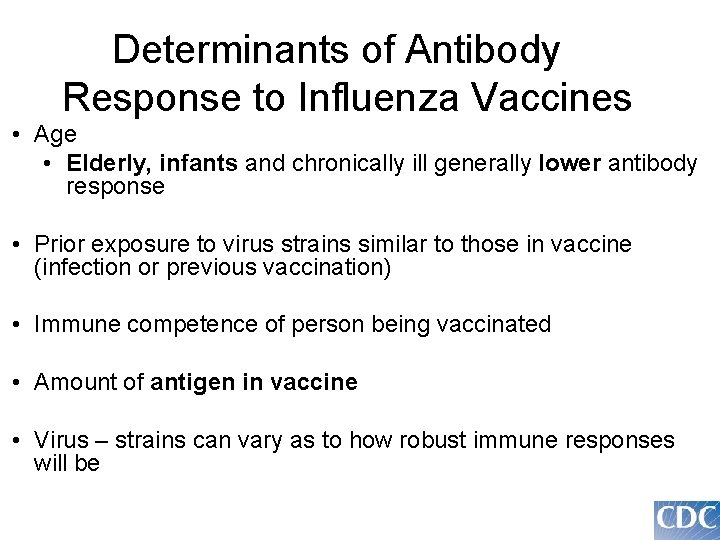
Determinants of Antibody Response to Influenza Vaccines • Age • Elderly, infants and chronically ill generally lower antibody response • Prior exposure to virus strains similar to those in vaccine (infection or previous vaccination) • Immune competence of person being vaccinated • Amount of antigen in vaccine • Virus – strains can vary as to how robust immune responses will be

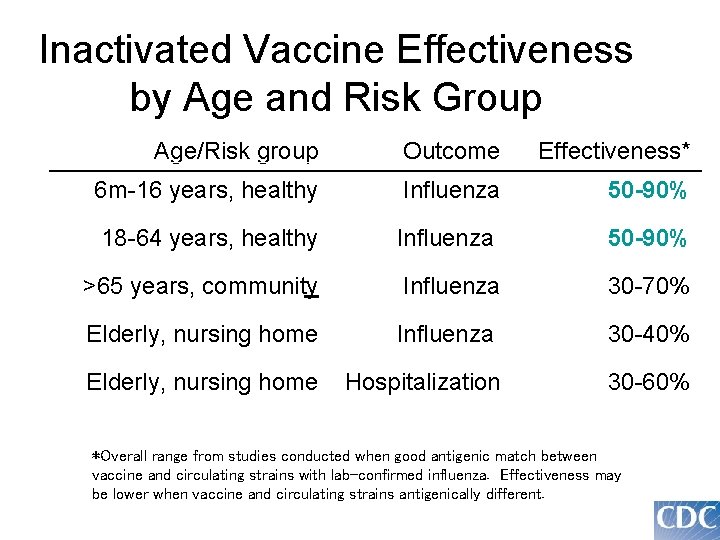
Inactivated Vaccine Effectiveness by Age and Risk Group Age/Risk group Outcome Effectiveness* 6 m-16 years, healthy Influenza 50 -90% 18 -64 years, healthy Influenza 50 -90% >65 years, community Influenza 30 -70% Elderly, nursing home Influenza 30 -40% Elderly, nursing home Hospitalization 30 -60% *Overall range from studies conducted when good antigenic match between vaccine and circulating strains with lab-confirmed influenza. Effectiveness may be lower when vaccine and circulating strains antigenically different.

New and Updated Recommendations from the Advisory Committee on Immunization Practices (ACIP)

New from the ACIP: Influenza Vaccination Recommendations for Children All children aged 6 months through 18 years should receive annual influenza vaccination, beginning in 2008 if feasible, and beginning no later than during the 2009 -2010 influenza season

Timeline of ACIP Recommendation Changes for use of Influenza Vaccine Before 2000: Persons aged 65 or older Persons with chronic medical conditions that make them more likely to have complications of influenza Pregnant women in the second or third trimester Contacts (household and out of home caregivers) of the above groups Healthcare workers 2000: Adults 50 and older 2004: Children aged 6 --23 months Contacts (household and out of home caregivers) of children aged 0 --23 months Women who will be pregnant during influenza season 2006: Children aged 6 --59 months Contacts (household and out of home caregivers) of children aged 0 -59 months 2008: All children aged 6 months— 18 years

Rationale for Expanding Vaccination Recommendations to Include all Schoolage Children and Adolescents* Rationale • Evidence that influenza has substantial adverse impacts among school age children and their contacts (e. g. , increased school absenteeism, antibiotic use, medical care visits, and parental work loss) • Evidence that influenza vaccine is effective and safe for school-age children • The expectation that a simple age-based influenza vaccine recommendation will improve current low vaccine coverage levels among the approximately 50% of school-age children who already had a risk- or contact-based indication for annual influenza vaccination Also noted • The potential for the indirect effect of reducing influenza among persons who have close contact with children, and reducing overall transmission within communities, if sufficient vaccination coverage among children can be achieved *Approved at February 27, 2008 ACIP meeting. Begins in 2008 -09 influenza season

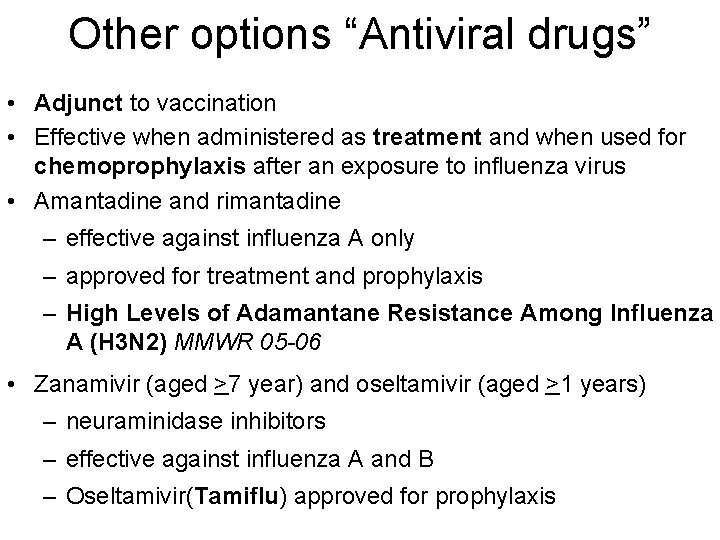
Other options “Antiviral drugs” • Adjunct to vaccination • Effective when administered as treatment and when used for chemoprophylaxis after an exposure to influenza virus • Amantadine and rimantadine – effective against influenza A only – approved for treatment and prophylaxis – High Levels of Adamantane Resistance Among Influenza A (H 3 N 2) MMWR 05 -06 • Zanamivir (aged >7 year) and oseltamivir (aged >1 years) – neuraminidase inhibitors – effective against influenza A and B – Oseltamivir(Tamiflu) approved for prophylaxis

Antiviral Resistance 2008 USA • Neuraminidase Inhibitor Resistance : – (11%) A (H 1 N 1) viruses resistant to oseltamivir • Was (0. 7%) in 2005 -2006 – 0 (H 3 N 2) viruses resistant to oseltamivir – 0 B viruses resistant to oseltamivir – All tested viruses remain sensitive to zanamivir – Given type/subtype prevalence in the United States, low rate of overall resistance (~2%) • Adamantane Resistance – 14% influenza A (H 1 N 1) viruses tested were resistant – (99%) influenza A (H 3 N 2) viruses tested were resistant

Other options Nonpharmacologic interventions • Advising frequent hand washing and improved respiratory hygiene • Reasonable and inexpensive • Have been demonstrated to reduce respiratory diseases but • Have not been studied adequately to determine if they reduce transmission of influenza virus


Universal flu vaccine • intended to provide protection against all ‘A’ strains of the virus that causes human influenza, including pandemic strains • Will not need to be renewed annually • targets M 2 e(matrix protien 2 ectodomain) a relatively invariant viral determinant • Successful clinical trials, animal , phase I human

Other Key Updates in the 2008 ACIP Influenza Vaccine Recommendations • Either TIV or LAIV should be used when vaccinating healthy persons aged 2 --49 years • 2 doses separated by >4 weeks for children aged 6 months— 8 years receiving vaccination for the first time • Children 2— 4 years old should be screened for reactive airways disease before receiving LAIV (MMWR Nov 23 2007) • “Healthy” means no underlying chronic illness that confers increased risk for influenza complications • All new 2008– 2009 trivalent vaccine virus strains • A/Brisbane/59/2007 (H 1 N 1)-like • A/Brisbane/10/2007 (H 3 N 2)-like • B/Florida/4/2006 -like – Neuraminidase inhibitors (oseltamivir or zanamivir) remain drugs of choice in prevention or treatment • High levels of resistance among adamantane class drugs

Thank You for your Attention
 2008 2008
2008 2008 Asad samar
Asad samar Sina samar
Sina samar Contoh hukum bacaan alif lam syamsiyah
Contoh hukum bacaan alif lam syamsiyah Samar haque
Samar haque Samar agnihotri
Samar agnihotri Poultry vaccination schedule
Poultry vaccination schedule Bankeryds vårdcentral influensavaccin
Bankeryds vårdcentral influensavaccin Vaccination bruxelles
Vaccination bruxelles A vaccination for smallpox was developed in 1796 by ____
A vaccination for smallpox was developed in 1796 by ____ Advantages and disadvantages of vaccines ppt
Advantages and disadvantages of vaccines ppt Vaccination schedule in palestine
Vaccination schedule in palestine Mandatory vaccination
Mandatory vaccination Sleeping princesses
Sleeping princesses Dog vaccination perry county
Dog vaccination perry county Intervax bcg
Intervax bcg Pcv vaccine dose
Pcv vaccine dose Niccolo paganini fingers
Niccolo paganini fingers 10 rights of medication administration
10 rights of medication administration Conclusion of immunization
Conclusion of immunization Difference between spermatogenesis and oogenesis
Difference between spermatogenesis and oogenesis Flu produit chimique
Flu produit chimique Stomach flu vs influenza
Stomach flu vs influenza Homophone of waste
Homophone of waste Mec flu
Mec flu