Seasonal flu vaccination programme for children 202021 Part

























- Slides: 31

Seasonal flu vaccination programme for children: 2020/21 Part 2 Administration of the intranasal flu vaccine by Healthcare Support Workers (Level 3 and 4 on NHS Career Framework) to children August 2020 NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Part 2 NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Contents Part 1 • Overview of the current flu vaccination programme in Scotland • What is flu? • Why include children in the seasonal Flu vaccination programme? Part 2 • Use of Live Attenuated Influenza Vaccine: Fluenz™ Tetra • Administration of Live Attenuated Influenza Vaccine: Fluenz™ Tetra • Storage and handling of flu vaccines Part 3 • Legislation governing the administration of vaccines • Anaphylaxis, basic life support and adverse reactions • Documentation, record keeping and reporting NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Learning outcomes – Part 2 • Describe safe and effective practice in the administration of the intranasal flu vaccine • Discuss contraindications, precautions and adverse reactions to the intranasal flu vaccine in children • Describe the correct procedures for storage and handling of the intranasal flu vaccine NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Use of Fluenz™ Tetra NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Live Attenuated Intranasal Vaccine: Fluenz™ Tetra • In 2020/21 season the intranasal vaccine Fluenz™ Tetra will be continue to be used for the majority of children • It is a live attenuated vaccine which means that during the manufacturing process the virus has been weakened and cannot cause flu, but protects against flu in the future • Fluenz™ Tetra protects against two strains of influenza A and two strains of influenza B NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Use of Fluenz™ Tetra: • • Generic name: influenza vaccine (live attenuated, nasal) Brand name: Fluenz™ Tetra Marketed by Astra. Zeneca Licensed from 24 months to less than 18 years of age Nasal Spray (suspension) in a prefilled nasal applicator Supplied as pack containing 10 individual prefilled applicators Container dimensions: 117. 5 x 115. 5 x 36 mm. Contains some substances necessary for the manufacturing process including porcine gelatin, egg proteins and traces of the antibiotic gentamicin NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Use of Fluenz™ Tetra (cont. ) Fluenz™ Tetra presentation • Prefilled nasal applicator • Nasal spray (suspension) • Each applicator contains 0. 2 ml NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

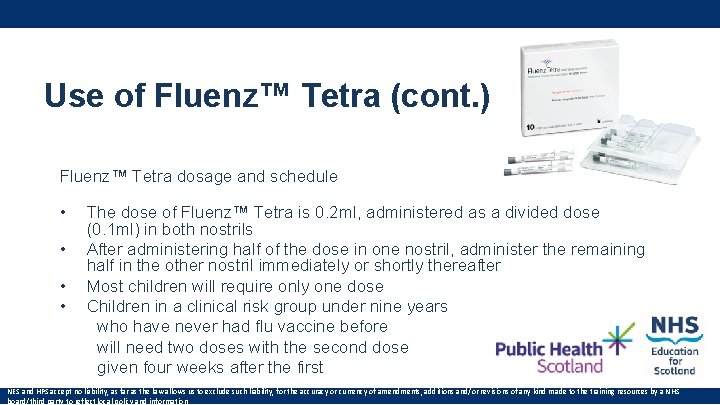
Use of Fluenz™ Tetra (cont. ) Fluenz™ Tetra dosage and schedule • The dose of Fluenz™ Tetra is 0. 2 ml, administered as a divided dose (0. 1 ml) in both nostrils • After administering half of the dose in one nostril, administer the remaining half in the other nostril immediately or shortly thereafter • Most children will require only one dose • Children in a clinical risk group under nine years who have never had flu vaccine before will need two doses with the second dose given four weeks after the first NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Use of Fluenz™ Tetra (cont. ) • Fluenz™ Tetra has been manufactured for nasal use and must not be injected • Fluenz™ Tetra can be administered at the same time as other vaccines including live vaccines • Child should breathe normally - no need to actively inhale or sniff • No need to repeat either half of dose if patient sneezes, blows their nose or their nose drips following administration NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Use of Fluenz™ Tetra (cont. ) Contraindications • • Age under 2 years Age 18 years or above Confirmed anaphylactic reaction to a previous dose of flu vaccine Confirmed anaphylactic reaction to any component of the vaccine (except for ovalbumin [egg protein]) There are very few individuals who cannot receive any flu vaccine. When there is any doubt you must refer to a Registered Healthcare Practitioner NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Use of Fluenz™ Tetra Important reasons not to give Fluenz™ Tetra include: • Severe reduced immunity due to conditions or some medications: – Acute and chronic leukaemias – Lymphoma – HIV positive patient not on highly active antiretroviral therapy – Cellular immune deficiencies – High dose steroids • Individuals receiving salicylate therapy (other than for topical treatment of localised conditions) • Known to be pregnant You must refer to a Registered Healthcare Practitioner if you have any doubt NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Severe Asthma and active wheezing Fluenz™ Tetra is not recommended in children currently experiencing symptoms including: -those who have had increased wheezing in the past 72 hrs -children who have increased their use of medications in previous 72 hrs Fluenz™ Tetra should only be given on the advice of a specialist for children who require regular oral steroids for maintenance of asthma control, or have previously required intensive care for asthma exacerbation NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Fluenz™ Tetra - Egg Allergy • Except for those with severe anaphylaxis to egg which has previously required intensive care, children with an egg allergy can be safely vaccinated with Fluenz™ Tetra in any setting (including primary care and schools)* • Where there any concerns, Healthcare Support Workers must, at all times, consult/defer to a Registered Healthcare Practitioner NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Fluenz™ Tetra and pork gelatin Fluenz™ Tetra contains pork (porcine) gelatin, an essential ingredient in many medicines, including some vaccines • Many faith groups have approved the use of gelatin-containing vaccines • It is, however, an individual choice whether or not to receive this vaccine and we recognise there will be diversity of thought within different communities NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

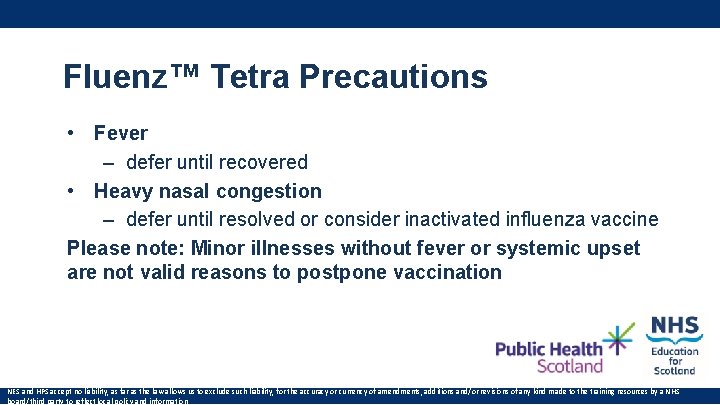
Fluenz™ Tetra Precautions • Fever – defer until recovered • Heavy nasal congestion – defer until resolved or consider inactivated influenza vaccine Please note: Minor illnesses without fever or systemic upset are not valid reasons to postpone vaccination NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Fluenz™ Tetra Precautions (cont. ) • Fluenz™ Tetra should not be administered at the same time as use with some medications for flu • Fluenz™ Tetra should not be administered within 48 hours of cessation of treatment with some flu medications • Administration of some flu mediations within two weeks of administration of Fluenz™ Tetra may adversely affect the effectiveness of the vaccine NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Administration of Fluenz™ Tetra NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Administration of Fluenz™ Tetra Video clip showing administration Click on the image to access the video clip showing how to administer Fluenz™ Tetra vaccine: NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

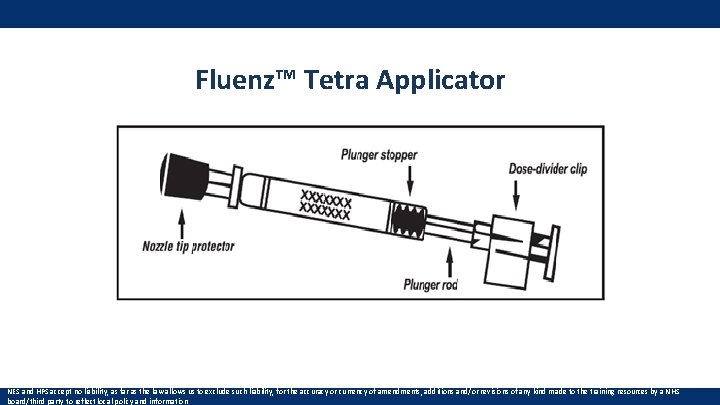
Fluenz™ Tetra Applicator NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

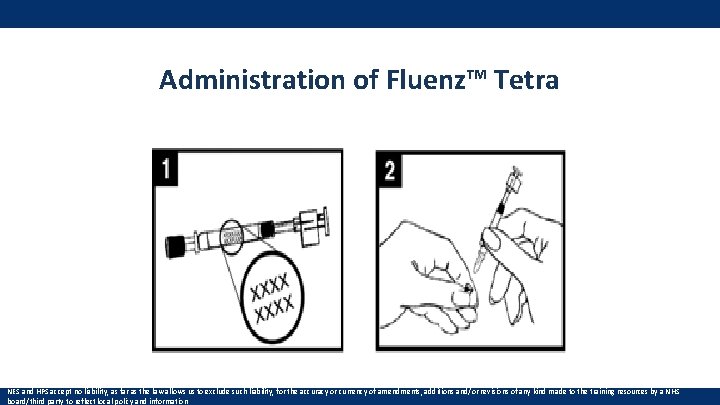
Administration of Fluenz™ Tetra NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

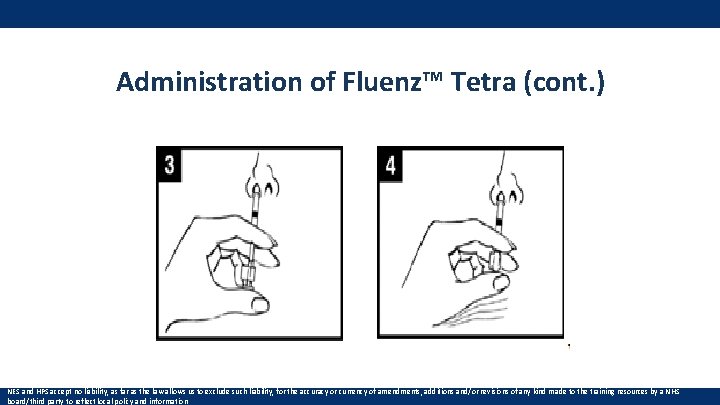
Administration of Fluenz™ Tetra (cont. ) NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Administration of Fluenz™ Tetra (cont. ) NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Common side effects following Fluenz™ Tetra administration The most common side-effects following administration of Fluenz™ Tetra were: – blocked/runny nose – reduced appetite – weakness – headache NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Fluenz™ Tetra: Risk of transmission • There is a theoretical potential for transmission of live attenuated influenza virus in Fluenz ™ Tetra to immunocompromised contacts for one to two weeks following vaccination • However, where close contact with very severely immunocompromised patients (e. g. bone marrow transplant patients requiring isolation) is likely or unavoidable (e. g. household members) an appropriate alternative inactivated influenza vaccines should be considered NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Storage and handling of flu vaccines NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Storage and handling of vaccines: The Cold Chain • The cold chain is the system of transporting and storing vaccines within the safe temperature range of 2 -8°C at all times • Vaccines are biological products and become less potent with time if not stored correctly effectiveness may be lost NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Storage and handling of the intranasal vaccine Fluenz™ Tetra • • Fluenz™ Tetra must be stored in accordance with manufacturer’s instructions Store between 2 -8°C Store in original packaging Protect from light Before use, Fluenz™ Tetra intranasal vaccine may be taken out of the refrigerator, without being replaced, for a maximum period of 12 hours at a temperature not above 25°C If the vaccine has not been used after this 12 hour period, it should be disposed of in accordance with local procedures for disposal of clinical waste Check expiry dates regularly NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Reporting suspected adverse reactions Yellow card scheme • Voluntary reporting system for suspected adverse reaction to medicines/vaccines • Success depends on early, complete and accurate reporting • Report to the registered healthcare practitioner even if uncertain about whether vaccine caused condition • See the Yellow Card reporting scheme • See chapter 8 of Green Book for details NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

Acknowledgments • Fluenz™ Tetra vaccine pack images and Fluenz™ Tetra administration step-by-step images courtesy of Astra. Zeneca UK • Images from electronic Medicines Compendium. Sm. PC (summary of product characteristics) Fluenz™ Tetra. https: //www. medicines. org. uk/emc/product/3296/smpc Accessed 18 th July 2018 NES and HPS accept no liability, as far as the law allows us to exclude such liability, for the accuracy or currency of amendments, additions and/or revisions of any kind made to the training resources by a NHS board/third party to reflect local policy and information

 National programme for child health and welfare
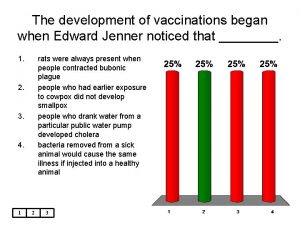
National programme for child health and welfare A vaccination for smallpox was developed in 1796 by ____
A vaccination for smallpox was developed in 1796 by ____ Vaccination bruxelles
Vaccination bruxelles Diseases with vaccines
Diseases with vaccines Vaccination schedule in palestine
Vaccination schedule in palestine Encephalitis lethargica
Encephalitis lethargica Mandatory vaccination
Mandatory vaccination Vaccination dose
Vaccination dose Spay perry county
Spay perry county Bankeryds vårdcentral influensavaccin
Bankeryds vårdcentral influensavaccin Niccolo paganini powerpoint
Niccolo paganini powerpoint Vaccine vial monitors
Vaccine vial monitors Epi schedule
Epi schedule Defaulter vaccination schedule
Defaulter vaccination schedule Difference between spermatogenesis and oogenesis
Difference between spermatogenesis and oogenesis Poultry vaccination schedule
Poultry vaccination schedule Spanish flu
Spanish flu Oraciones con xa xe xi xo xu
Oraciones con xa xe xi xo xu Spanish flu
Spanish flu Flu timeline
Flu timeline Decoding the flu case study answers
Decoding the flu case study answers Homophone for jeans
Homophone for jeans Oraciones con fla fle fli flo flu
Oraciones con fla fle fli flo flu Mec flu
Mec flu Flu produit chimique
Flu produit chimique Stomach flu vs influenza
Stomach flu vs influenza Spanish flu
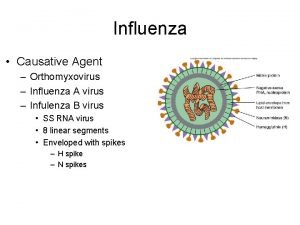
Spanish flu Causative agent
Causative agent Flu watch canada
Flu watch canada Cuento con fla fle fli flo flu
Cuento con fla fle fli flo flu Root word flu
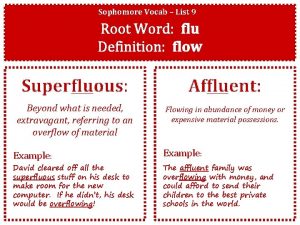
Root word flu Suppose a student carrying a flu virus
Suppose a student carrying a flu virus