Faculty of Medical Sciences Postgraduate Development Programme Research































































![– reasonable/prudent person standard • Bolitho v. City & Hackney Health Authority [1998] A. – reasonable/prudent person standard • Bolitho v. City & Hackney Health Authority [1998] A.](https://slidetodoc.com/presentation_image_h2/c45d8523a31eb8dec39fee11ba60fa7d/image-65.jpg)










- Slides: 75

Faculty of Medical Sciences Postgraduate Development Programme: Research Ethics: theory Jan Deckers School of Medical Education 1

Objectives • To reflect on what ethics is. • To develop your skills as an ethical researcher. • To prepare yourself to apply for ethical approval from research ethics committees. 2

What is ‘ethics’? • Ethics ‘deals with the standards and principles of moral reasoning’. (Rachels, 1998, 15) • Internet Encyclopedia of Philosophy: ‘ethics (or moral philosophy) involves systematizing, defending, and recommending concepts of right and wrong behaviour’ • Let us compare a few statements: – ‘David will be here in 10 minutes. ’ – ‘David should be here in 10 minutes. ’ • The latter may express an expectation of something that may happen, but this expectation may not be an ethical expectation: e. g. you may simply express that, given that he has no more than 1 mile left to walk and that you know that he intends to walk this way, he will be here soon. • The latter could be an ethical statement if what is meant is: ‘David ought to be here in 10 mins. ’ Unlike what is the case for the above interpretation, either you or David may feel that David had acted wrongly if he would not be here in 10 mins. The expectation is no longer an expectation of what is likely to happen, without reference to a theory of good/bad behaviour. If David won’t be here in 10 mins, he will have behaved badly, unless he could invoke a particularly good reason why he was not here. 3

So, what is ‘ethics’? • a theory of – how we ought/ought not to act – which values or principles ought (not) to guide our actions • ethics is about evaluating/justifying particular actions 4

What is ‘meta-ethics’? • the study of the status/meaning of ethical theories • the study of moral justification 5

Three meta-ethical positions: e. g. ‘X is wrong’ • Moral absolutism (‘ethics from the pulpit’) – ‘I know that X is wrong (and anyone who disagrees is wrong). ’ • Moral relativism (‘ethics from the restaurant’) – ‘X might seem wrong to you, but what is right and wrong is entirely subjective (nothing but a matter of taste). ’ • Pyrrhonian moral scepticism – ‘I believe/think that X is wrong (but those who disagree may be right). ’ 6

‘Pyrrhonian’ moral scepticism • A school of thinking named after Pyrrho – a Greek philosopher who lived from c. 360 to c. 270 BCE 7

Why do we need ethics? • Many people feel the need to: = subjective aspect – justify their behaviour – to explain why their behaviour is (un)acceptable • These explanations often relate actions to principles. • A theory is an account of which principles should be followed, and of how to balance them against each other. • Many (or all? ) things deserve moral consideration = objective aspect 8

objective aspect: how much value should we give to different things? subatomic particle atom molecule single -celled organism human being nonhuman ape table chair dog locust cow earthworm 9

in this exercise, the key questions are: • Which things are proper objects of ‘moral consideration’? • How much ‘relative moral significance’ should we give to different things? • key distinction: – Intrinsic value: value for oneself ≠ – Instrumental/use value: value for others 10

Different answers to these questions lead to different theories of value • Strong anthropocentrism (strong ‘speciesism’) • Animal-centred approaches: Pathocentrism (‘animal welfarism’) and animal rights ethics • Biocentrism • Ecocentrism • Weak anthropocentrism/speciesism

strong anthropocentrism Illustratred with a picture: Goose pulling in 19 th-century West Virginia, as depicted by Frederic Remington, "A Gander-Pull“, 3 February 1894, Harper's Weekly

strong anthropocentrism Or illustratred with: Painter of the burial chamber of Sennedjem; The Yorck Project: 10. 000 Meisterwerke der Malerei. DVD-ROM, 2002. ISBN 3936122202. Distributed by DIRECTMEDIA Publishing Gmb. H.

animal-centred approaches; illustrated with a picture of Melanie Joy, a scholar who promotes animal rights; https: //vimeo. com/81491557

biocentrism, illustrated with a picture of someone reacting to ‘breatharianism’; by Healty. Life. Begins. With. Healty. Mind

ecocentrism, illustrated with a picture of Aldo Leopold, founder of the ‘land ethic’ (ethics = about preserving the beauty, stability, and integrity of ecosystems; picture by USFS Region 5, https: //www. flickr. com/photos/usfsregion 5/5441676907)

weak anthropocentrism/speciesism (illustrated with a picture of the book cover of Animal (De)liberation; https: //doi. org/10. 5334/bay)

What is bioethics, and what is research ethics? • bioethics = about how moral agents ought to relate to biological organisms • research ethics = about ethical issues that pertain to research. 18

two dimensions • 1. law and professional guidelines • 2. reflection 19

How does it work? • 1. establishing knowledge of the relevant legal and professional guidelines • 2. exercising your ability to reflect. . . . – how? 20

the tools of the trade • clarity • the principle of non-contradiction • the use of analogies and thought experiments 21

clarity: the importance of defining concepts • reportive definitions – = lexical definitions – to reflect the existing meaning of a term • stipulative definitions – to assign new meaning to a term • clarifying definitions – may combine the above – to give more precise meaning to a term 22

rationale for emphasising clarity • the ‘what do you mean? ’ question may resolve a lot of moral disputes 23

example: a simple, apparent disagreement between James and John • James: Animal research is unacceptable. – P (P= premise) 1 (major): Inflicting pain is unacceptable. – P 2 (minor): Animal research causes pain. – conclusion: Animal research is unacceptable. • John: Animal research is not necessarily unacceptable. – agrees with James about P 1, but does not accept P 2 as not all research inflicts pain. – conclusion: Animal research is not necessarily unacceptable. 24

How to resolve this apparent dispute? • James may now agree with John that not all animal research is unacceptable, as he may question P 2, e. g. after John has explained to him that his definition of ‘animal research’ includes studies that merely observe animals in nature. • James’s definition of ‘animal research’ shifted, which may have resolved what looked like a moral dispute. 25

the importance of defining concepts = the virtue of clarity • aiming for clarity may help you to: – scrutinise what you think before you say something. – scrutinise what others say before you decide to (dis)agree with it. – make yourself understood. 26

the principle of non-contradiction or the principle of consistency • example: a researcher carries out research on patients who have lost most of their cognitive capacities due to a stroke. She says: “I believe that researchers who want to carry out research on patients should only proceed if patients give their voluntary, informed consent to taking part in the research. ” 27

arguing by reductio ad absurdum (reduction to absurdity) • 1. start from the assumption that X is right. • 2. try to find a logical conclusion that follows from X, but that you cannot agree to. • 3. If you can find such a conclusion, you must then also reject X. 28

example • Premise A: Research that involves research subjects should not happen unless subjects give their consent. • Premise B: People with severely reduced cognitive capacities have lost the capacity to give consent. • Conclusion: No research should be done with people with severely reduced cognitive capacities. • You may then decide that you cannot accept this conclusion (as you do not wish to exclude people with severely reduced cognitive capacities), which means that your premise A should be rejected/modified. 29

but … what if? • Should we accept that sometimes it may be necessary to make people act inconsistently? (when allowing them to do otherwise leads to immoral behaviour) 30

the use of analogies • Example: – When a research project is likely to kill me (healthy), research should not proceed. ↓ – When a research project is likely to kill healthy others, research should not proceed. • what is at work here is the principle of universalisability – Is this a valid analogy? 31

the use of analogies • Example: – When a research project is likely to kill human research subjects, research should not proceed. ↓ – When a research project is likely to kill nonhuman research subjects, research should not proceed. • Is this a valid analogy? 32

the use of thought experiments • A thought experiment is an analogy between a real case and an imaginary case whereby the latter is claimed to shed light on how to handle the former. 33

the use of thought experiments • Example: Julian Savulescu (2002)’s leukaemia case: – Imagine: A nuclear reactor has exploded, leaving your child exposed to nuclear fall out. Numerous children develop leukaemia, including your own. Bone marrow can be generated most successfully by reprogramming brain cells from children. Unfortunately, a child must be killed, as no brains from those who have deceased are available. The extracted stem cells could then be reprogrammed to treat ten children. 34

• Since a one in eleven chance of certain death seems preferable to a one hundred percent chance of imminent death, the question is: – Would you enter your child into a lottery and risk a 1/11 chance of your child being sacrificed (by being killed to treat others), or refrain from entering your child into such a lottery (which would mean certain death for your child)? – If you do, Savulescu claims that you should also enter all human embryos into a similar lottery as the real world would be similar to this imaginary world: the real world is a world that lacks the great benefits that we would have had by now if only there had been less public opposition to embryonic stem cell research. 35

Why use analogies and thought experiments? • Rachels (1998, p. 22): ‘like cases should be treated alike and different cases differently’ 36

some common ethical theories • consequentialism • deontology • principlism 37

consequentialism • focus on consequences – example: utilitarianism: consequences are measured in terms of whether or not they produce happiness 38

deontology / deontological ethics • focus on rules and motives 39

principlism, also known as the ‘four principles’ approach: 1. autonomy (self-determination) 2. beneficence (well-being) 3. non-maleficence (no harm) 4. justice (1&4 are deontological? ; 2&3 are consequentialist? ) = a very popular approach in bioethics Ref: Beauchamp & Childress, 2001. 40

Why is research ethics important? • Many people would agree that ‘not everything goes’ in relation to research. • some examples: – Tuskegee Syphilis Experiment • For 40 years (between 1932 and 1972) 399 black men with advanced syphilis were told they were treated for ‘bad blood’ by the US Public Health Service. They were never told they had syphilis, and never received treatment for it. The aim of the study was to examine the effect of syphilis on the human body. The data were collected from the autopsies. – The TGN 1412 trial 41

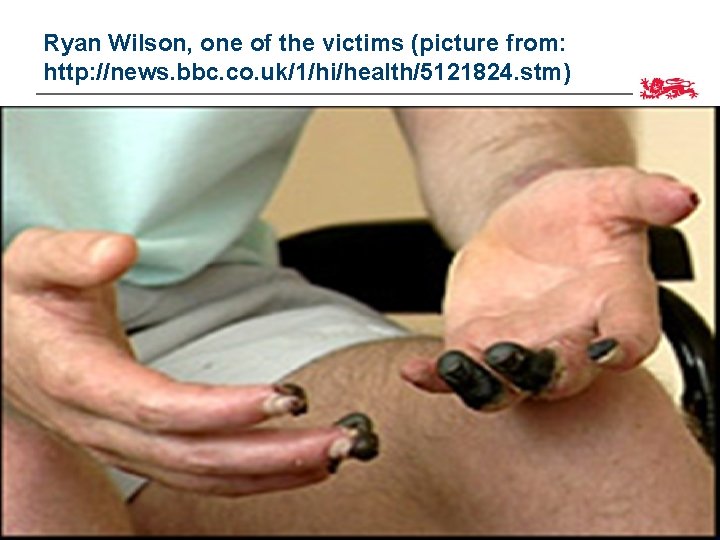
Ryan Wilson, one of the victims (picture from: http: //news. bbc. co. uk/1/hi/health/5121824. stm) 42

the TGN 1412 trial • a phase 1 clinical trial conducted in 2006 by Parexel, an independent clinical trials unit based in London • £ 2000 paid to 6 healthy volunteers, who were given the drug within the span of 1 hr • All ended up in hospital. • 4 developed multiple organ dysfunction and suffered from a cytokine storm 43

applying the four principles to this trial • 1. Did the researchers respect the autonomy of the research participants? – Could the fact that a financial incentive was provided have undermined their autonomy? (e. g. : Sandel, 2012) • 2. Did it promote beneficence? – There may be little doubt that the research was done with the aim to promote the well-being of future patients, but how important was this motive compared to the motive to do well for the company (i. e. to discover something new or to make a profit)? Could this potential ‘conflict of interests’ have been avoided? 44

• 3. Was it non-maleficent? – The research caused harm. Could this harm have been avoided? • 4. Was it just? – Was it legal? – other considerations, e. g. moral rights: – Would I like to be treated like that? (The “how would you feel. . ” test, or “don’t do unto others what you would not want others to do unto you”) 45

Case 1 • James Strong is a researcher who has drafted an information sheet that he would like to use to recruit research participants. He includes the following: – ‘This research will not expose you to any risks as previous research has not found any evidence that the D drug is harmful. This research merely repeats what has already been shown by other studies, so you can trust what we are going to do. This research has already been funded and it has also been granted ethical approval from Newcastle University’s Research Ethics Committee. ’ 46

Case 2 • Claire is working on a research project aimed at making tomato plants more resistant to frost. She hopes that isolating a gene from a flounder fish which can resist very cold temperatures and inserting this gene into tomato plants will make these plants resistant to frost. 47

Case 3 • David does not support animal experimentation. Newcastle University is building a new facility to develop its research on animals. David wants to stop the University from building this facility, and speak up for the plight of animals. David decides to organise a protest in Newcastle with some like-minded people. They take to the streets and shout ‘stop the animal lab’. Afterwards, they go to the pub for a bar meal. David orders a pint of lager and a lamb pie with chips. [see Deckers 2016 for more on ‘animal ethics’] 48

Appendix 1: further information in relation to the principle of autonomy: • ‘consent’, a cornerstone of good research? – consent = closely related to ‘autonomy’ • providing consent = giving permission • Research often requires legal consent from those who participate in it. – why? 49

Legal context • common law duty to obtain consent • statutory laws, e. g. – Mental Capacity Act 2005 (MCA) 50

What if consent is compromised? • criminal law: – Researchers may be liable to assault/battery: • battery: ‘an act that directly and either intentionally or negligently causes some physical contact with the person of another without that person’s consent’ (Mc. Hale & Fox, 2007, p. 352. ) – Researchers may be liable to criminal (gross/extreme) negligence in criminal law (= rare). • civil law: – Researchers may be liable to trespass to the person (infringing the bodily integrity or liberty of another) and negligence. 51

the tort of negligence • a person claiming negligence (= a claimant) must satisfy: – that the defendant owed him or her a duty of care – that this duty was breached, and – that the claimant was harmed by this breach and that this harm was not too remote ref: Pattinson, 2009, p. 68, 78. 52

three requirements in relation to consent • The research subject: – has capacity – is informed adequately – gives consent without being coerced (voluntariness) 53

three questions • What is capacity and what to do if it is lacking? • What does it mean to be adequately informed? • Can research subjects give voluntary consent to participate in research? 54

first question: What is capacity? • necessary condition for valid consent = the person in question ‘has capacity’ (is competent) • legal presumption: every person has capacity from the age of 16 • if in doubt: assess – how? 55

Mental Capacity Act 2005 • section 3. 1: a person lacks capacity if a person is unable: – ‘(a) to understand the information relevant to the decision, – (b) to retain that information, – (c) to use or weigh that information as part of the process of making the decision, or – (d) to communicate his decision (whether by talking, using sign language or any other means). ’ • What should be done in situations where adults lack capacity? – distinguish between clinical and other research 56

the law related to clinical research • Medicines for Human Use (Clinical Trials) Regulations 2004 • The patient’s legal representative can give permission on behalf of the patient if the clinical trial has gained ethical approval from a Research Ethics Committee. – This person can be – ‘the doctor primarily responsible for the medical treatment provided to that adult’ or ‘a person nominated by the relevant health care provider’, provided they are not connected with the trial (Schedule 1, part 1, section 2) 57

– This person must – speak to a member of the research team about the risks and objectives; – be provided with a contact point; – be told that the subject can be withdrawn at any time; – either consent or refuse to the subject’s participation. 58

further conditions for participation: • par. 6: ‘The subject has received information according to his capacity of understanding regarding the trial, its risks and its benefits. ’ • par 9: ‘There are grounds for expecting that administering the medicinal product to be tested in the trial will produce a benefit to the subject outweighing the risks or produce no risk at all. ’ 59

• par. 11: ‘The clinical trial relates directly to a lifethreatening or debilitating clinical condition from which the subject suffers. ’ (Schedule 1, part 5) • Subjects who refuse to participate when they still have capacity cannot be entered once they have lost capacity. 60

the law related to other research • for non-clinical research with adults, the Mental Capacity Act 2005 should be applied: Research on adults who lack capacity is possible if: – It involves minimal risk; – relates to the person’s condition; – cannot be done as effectively on people who have capacity; – (unpaid) carers or nominated third parties are consulted; – the person is not entered or is withdrawn if any resistance is shown; – and the research project has been approved by an appropriate body, such as a Research Ethics Committee. 61

the law related to clinical research with children • normally, a person with parental responsibility should be asked to give consent. • legal representatives should only be used in emergencies. • consent from a person with parental responsibility is required even if children are deemed to have capacity. (Schedule 1, part 4) 62

the law related to non-clinical research with children • Apply common law: – Children may be able to give consent if they are ‘Gillick competent’. – Children who are not Gillick competent may be able to give their assent. – Children must be informed, even if they lack competency. 63

second question: What does it mean to be informed adequately? • three different views: – professional practice standard • Bolam test: ‘a doctor is not guilty of negligence if he has acted in accordance with a practice accepted as proper by a responsible body of medical men’ (Bolam v. Friern Hospital Management Committee [1957] 1 W. L. R. 538) 64
![reasonableprudent person standard Bolitho v City Hackney Health Authority 1998 A – reasonable/prudent person standard • Bolitho v. City & Hackney Health Authority [1998] A.](https://slidetodoc.com/presentation_image_h2/c45d8523a31eb8dec39fee11ba60fa7d/image-65.jpg)
– reasonable/prudent person standard • Bolitho v. City & Hackney Health Authority [1998] A. C. 232 (HL) at 243: a court can reject medical opinion if it is not ‘reasonable or responsible’ (emboldening mine) • Chester v Afshar [2004] UKHL 41; [2005] 1 AC 134. 65

– individual person standard • J. Bridson et al. , 2003 (p. 1160; emboldening mine): ‘The professional standard is paternalistic, emphasising what clinicians consider appropriate to disclose rather than what patients want disclosed. The reasonable patient standard is inherently hypothetical, upholding the autonomy only of patients who behave like the typical patient. It asks what risks should be disclosed to a ‘reasonable’ patient in the particular patient’s position, not what risks the particular patient would regard as important. ’ 66

Are we moving towards the individual patient standard? • The “test of materiality is no longer restricted to what the reasonable person in the patient's position would consider significant: it now includes the added refinement that a risk is also material if ‘the doctor is or should reasonably be aware that the particular patient would be likely to attach significance to it’. ” (Heywood, R. 2015. RIP Sidaway: Patient-oriented disclosure – a standard waiting for? Montgomery v Lanarkshire Health Board [2015] UKSC 11. Medical Law Review, 23 (3): 455 -466, p. 460: with reference to Montgomery [2015] UKSC 11 at 87) 67

third question: how to ensure research subjects give their voluntary consent? A case: • Miss Jackson is paid by a drug company for participation in their trial of the new Alzheimer’s drug. She is keen to contribute to the possibility of making a scientific breakthrough that could much improve the quality of life of patients suffering from Alzheimer’s. Miss Jackson thinks it is appropriate that her patients are paid a small amount as well. • Are there any ethical issues here? 68

Appendix 2: Some practical advice for your research projects • Some useful questions to ask in relation to personal research projects – What is the aim of my research? – What are the implicit assumptions about what science is or should be? – Whose interests will be served by my research? – What are the risks? – What are the opportunity costs? • The answers to these questions must feed into the overall ethical evaluation of your research. 69

Some golden advice? • ‘a/ write early and write often; • b/don’t get it right, get it written’ (Delamont, Atkinson, and Parry, 1997, p. 121) 70

In defence of ‘write early and write often’ • ‘ 1. The more you write, the easier it gets. • 2. If you write every day, it becomes a habit. • 3. Tiny bits of writing add up to a lot of writing. Break the writing up into small bits. Write 100 words on X, 200 words on Y, and file them safely. It all mounts up • 4. The longer you leave it unwritten the worse the task becomes. ’ (Delamont, Atkinson, & Parry, 1997, p. 121) 71

In defence of ‘don’t get it right, get it written’ • ‘ 1. Until it is on paper no one can help you to get it right. Draft, show the draft to people, redraft. • 2. Drafting is a vital stage in clarifying thought • 3. Start writing the bit that is clearest in your head: not the introduction, but Chapter 4, or the appendixes, or the conclusions, or the methods. As you draft, other bits become clear. • 4. Drafting reveals the places where ‘it’ isn’t right (yet) in ways that nothing else does. ’ (Delamont, Atkinson, & Parry, 1997, p. 121) 72

What makes for a good piece of written work? • The words ‘making a contribution’ and ‘originality’ appear frequently (Delamont, Atkinson, and Parry, 1997, p. 113114) • For the Newcastle Ph. D: ‘show distinct ability in conducting original investigations and in testing ideas, whether the candidate’s own or others’. (…) ‘The exposition of the work in thesis must be clear and must show that the candidate understands the relationship of the work embodied in thesis and theme of that work to a wider field of knowledge’ (Doctor of Philosophy by Thesis Examination Conventions, p. 149; http: //www. ncl. ac. uk/regulations/docs/Ph. DExam. Conv. Th esis 1112. pdf) 73

References and recommended further reading • • • • Beauchamp, T. and Childress, J. , Principles of Biomedical Ethics, 5 th edition, New York/Oxford: Oxford University Press, 2001. Bridson, J. , et al. , Making Consent Patient Centred, in BMJ 2003; 327: 1159 -1161. Deckers J. , Animal (De)liberation: Should the Consumption of Animal Products Be Banned? , London: Ubiquity Press, 2016 (http: //dx. doi. org/10. 5334/bay) Deckers J. , The New EU Directive on the Use of Animals for Research and the Value of Moral Consistency, in Journal of Bioethical Inquiry 2012; 9: 377 -379. Delamont, S. , Atkinson, P. , Parry, O. , Supervising the Ph. D: A Guide to Success, Bristol: Open University Press, 1997. Internet Encyclopedia of Philosophy (http: //www. iep. utm. edu/ethics/) Jamieson, D. , Method and Moral Theory, in Singer, P. (ed. ), A Companion to Ethics, Oxford: Blackwell, 1993, pp. 476 -490. Knight, A. The Costs and Benefits of Animal Experiments. Palgrave Mac. Millan, 2011. Mc. Hale, J. , & Fox, M. , Health Care Law, Second edition, Sweet and Maxwell, 2007. Nuffield Council on Bioethics, The Ethics of Research Involving Animals, London, 2005. Pattinson, S. , Medical Law and Ethics, Second edition, Sweet and Maxwell, 2009. Rachels, J. , Ethical Theory and Bioethics. In Kuhse, H. & P. Singer (eds. ), A Companion to Bioethics, Oxford: Blackwell, 1998, pp. 15 -23. Sandel, M. , What Money Can’t Buy. The Moral Limits of Markets, Allen Lane, 2012. Savulescu, J. , The Embryonic Stem Cell Lottery and the Cannibalization of Human Beings, in Bioethics 2002; 16: 508 -529. 74

Conclusions • Ethics is about the attempt to justify particular actions. • Logic and the making of valid analogies are the tools of the trade. • In bioethics, the focus is on biological organisms. • Research ethics focuses on ethical issues in research. • Various formal theories exist on what ethics should be about, including consequentialism, deontology, and the 4 principles approach. • Many institutions have ‘research ethics committees’ and demand approval from them before research projects can commence. 75