Export Control Overview Office of Regulatory Compliance Export























































- Slides: 62

Export Control Overview Office of Regulatory Compliance

Export Control Laws § Prohibits the unlicensed “export” of certain controlled technologies to foreign persons for reasons of national security and trade protection § Export is broad and includes oral or written disclosure of information, visual inspection, or actual shipment outside the U. S. of technology, software/code, or equipment to a foreign person. § Any method will do: » E-mail » Mail » In-person » Website » Visual inspection that reveals technical data » Conference » Hand-carried, i. e. , laptop, memory devices

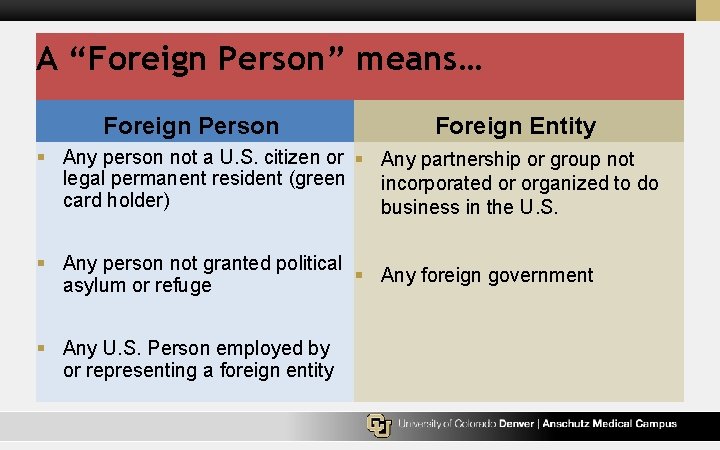
A “Foreign Person” means… Foreign Person Foreign Entity § Any person not a U. S. citizen or § Any partnership or group not legal permanent resident (green incorporated or organized to do card holder) business in the U. S. § Any person not granted political § Any foreign government asylum or refuge § Any U. S. Person employed by or representing a foreign entity

“Deemed export” defined “Release of information or technology that is subject to export control to any foreign nationals in the U. S. Such a release of information is considered to be an export to the foreign national’s home country. ” § Info must be subject to EC regulations § Release is to a Foreign National § Occurring in the United States

Purpose of Export Control Laws § Prevent terrorism § Curtail export of technologies that assist the military potential of adversaries § Compliance with trade agreements § Prevent development of nuclear, chemical and biological weapons § Promote regional stability § Human rights considerations

Governing Regulations and Oversight Agency Department of Commerce – EAR: Technologies with “dual uses” but primarily commercial Department of State –ITAR: Technologies with inherently military properties Department of the Treasury-OFAC: Prohibits transactions of value with certain countries and individuals

Export Administration Regulations Enforced by the Department of Commerce through its Export Administration Regulations (EAR). Primarily covers technologies and technical information with both commercial and military applications, i. e. , “dual use” technologies (chemicals, satellites, software, computers, etc. ).

International Traffic in Arms Regulations Enforced by the Department of State under the International Traffic in Arms Regulations (ITAR). Controlled technologies of an inherently military nature: defense articles, defense services and related technical data listed on the Munitions Control List (MCL).

Economic Sanctions § The Office of Foreign Assets Control ("OFAC") of the US Department of the Treasury administers and enforces economic and trade sanctions against targeted foreign countries, terrorists, international narcotics traffickers, and those engaged in activities related to the proliferation of weapons of mass destruction. § Regulations target specific nations in controlling significant financial transactions or services. Countries currently sanctioned are the Balkans, Burma, Cuba, Iran, Iraq, Liberia, Libya, North Korea, Sudan, Syria, and Zimbabwe. http: //www. treas. gov/offices/enforcement/ofac/

Examples of activities prohibited in OFAC Embargoed Countries § Conducting surveys and interviews § Engaging the services of persons to develop new informational materials or support of research activities (i. e. , just hiring certain individuals from certain countries to work on an overseas trial may be prohibited!) § Providing marketing and business services

How do these laws impact research at the University? § If research involves controlled technologies, it may be necessary to receive government approval (a license) before allowing: » Certain foreign researchers and students in the U. S. (including on campus at UCD & Anschutz) or foreign persons outside the U. S. to participate in research involving the controlled technologies » The sharing of research results with foreign persons » Providing training and other services to foreign persons » Sending equipment or software outside the U. S. § Certain other activities may require an license: » Performing certain “services” in an OFAC sanctioned country: e. g. , giving a presentation in Iran

Export Control Laws and Research at Denver | Anschutz General Rule: The University of Colorado Denver | Anschutz Medical Campus, its faculty and Employees, may not export certain materials and information to foreign persons without a license from the U. S. Government, unless an exclusion applies. Fortunately, the majority of research at Denver | Anschutz will be covered under an exclusion to the ECL requirements. WHAT ARE THE EXCEPTIONS?

Export Control Law Exclusions § § § Public Domain: Information and research results already published and publicly available Education Exclusion: General scientific, mathematical or engineering principles commonly taught in universities (ITAR); Educational information released by instruction in catalog courses and associated teaching laboratories (EAR) Employment Exclusion: License is not needed to share EC materials if the foreign national is a full-time employee, not a national of certain countries, and with a permanent address in the U. S. while employed at UC Denver

Fundamental Research Exclusion (FRE) § Basic and applied research in science and engineering § Occurring at an accredited institution of higher learning in the U. S. § Which results in the ordinarily publishing and broad sharing of the information with the scientific community § Different from “proprietary research”, the results of which are restricted for proprietary or national security reasons § The “Bread and Butter” exception that the institution will seek to protect.

Keepin’ is “F’Real” § There are no publication restrictions; and § There are no sponsor prohibitions on foreign national participation; and § The research is conducted in the United States

How do you destroy the FRE? The FUNDAMENTAL RESEARCH EXCLUSION (FRE) would be destroyed by a contract clause that: § Gives a sponsor a right to approve publications § Forbids the participation of foreign nationals in the research effort AND § The subject matter is export controlled. *These limitations are applicable to any sponsor, whether federal, private or not-for-profit*

How are sponsored projects Impacted by ECLs? § Important Federal funding opportunities (Homeland Security, NSF, NIH, DOD) directly linked to ECLs § Terms and conditions restricting access by foreign nationals or removing research from fundamental research exclusion § Contract requirements from Corporate Sponsors on ECLs § Tech Transfer Issues: disclosure/licensing of technologies and material transfer agreements to foreign nationals

Sponsored Projects AND ECL’S: What to Look For Proposals and contracts where: § There is a shipment of equipment to a foreign country § Training or collaboration with foreign nationals § Any work with or travel to an OFAC controlled country § Any reference to export controlled technologies in the award

Routing Form § Dual Use Research of Concern (DURC) and Export Control Sections § Specific “export control” language in PA, other document; restrictions on publications; restrict participation based on country of origin/citizenship; foreign collaborations; shipping to a foreign country; use of proprietary information. § Automatic trigger from Info. Ed to “Yes” answers resulting in review § Does “Yes” mean it’s DURC or EC? § No-additional analysis required § BUT-negative answers does not mean no Export Control

FY 2019 National Defense Authorization Act § Concerns involving national security issues related to international talent recruitment programs § Requires DOD to establish an initiative to work with academic institutions performing defense research and engineering activities § Support protection of IP, controlled info, key personnel, information about critical technologies § Limit undue influence, including through foreign talent programs, by countries wanting to exploit US technology § Support efforts towards development of domestic talent in relevant scientific and engineering fields

NIH Statement on Protecting Integrity of US Biomedical Research Identify methods to: 1. Improve accurate reporting of all sources of research support, financial interests, and affiliations 2. Mitigate the risk to intellectual property security while continuing NIH’s long tradition of collaborations, including with foreign scientists and institutions; and 3. Explore additional steps to protect the integrity of peer review.

CONFLICT OF INTEREST AND COMMITMENT WHY DO I HAVE TO COMPLY? AN OVERVIEW


COI Policy Statement Maintenance of the public trust is critical to the mission and reputation of the University of Colorado Denver I Anschutz Medical Campus (UCD-Anschutz), which is committed to upholding the principles of transparency, integrity and accountability. UCD-Anschutz encourages its employees to interact with business and industry, public and private organizations, and government agencies in ways that support the institution’s missions. Notwithstanding the foregoing, teaching, research, outreach and other activities shall not be compromised, or perceived as compromised, by financial and/or personal benefit.

What is conflict of interest? Situations in which financial or other personal consideration may conflict, or have the appearance of conflicting, with an employee’s professional judgment in exercising any university duty or responsibility in administration, management, instruction, research or other professional activities.

What is a conflict of commitment? Outside relationships or activities (such as professional consulting for a fee) which adversely affect, or have the appearance of adversely affecting, an employee’s commitment to his/her UCD-Anschutz duties or responsibilities.

Who is Required to Disclose Potential Conflicts of interest and/or Commitment? * • All UC Denver I Anschutz Medical Campus (UCD-Anschutz) faculty • UCD-Anschutz officers and others who have a fiscal responsibility at UC Denver • All principal investigators, co-investigators, research coordinators, PRAs or others designated as key personnel on grants, contracts or human subjects protocols • IRB and other regulatory/research committee members • All individuals who may potentially bias the design, conduct or analysis of the research • Others upon request of COI Office *Note: Instructors, lecturers, adjunct professor, retirees, clinical faculty exempt from disclosing unless there is research or grant

Disclosure Period § Annual basis (Mid-August– October 31) § New employees who meet the disclosure criteria are required to complete a disclosure within 60 days of their hire date § Update within 30 days of any change § Travel must be disclosed via travel form

What is the dollar amount for disclosure ? UCD-Anschutz has a zero dollar threshold for financial disclosures.

What is a “Significant Financial Interest? § Equity value greater than $5, 000 or 5% ownership in a single entity. § Serving as a director, officer or other decision-maker for a commercial sponsor of human subjects research. § Receiving compensation for services as a consultant or advisor to a commercial (including a not for profit) sponsor of research in excess of $5, 000 annually. § Royalty income or the right to receive future royalties under a patent, license or copyright, where the research is directly related to the licensed technology or work of any amount. § Personally accepting payment from the human subjects research sponsor, for non-research related travel or gifts.

What is not considered a Significant Financial Interest? § Salary, royalties, or other remuneration from UC Denver or its affiliates (Veteran’s Administration, Children’s Hospital Colorado, University of Colorado Hospital, National Jewish Hospital or Denver Health and Hospital Authority). § Interests held in a diversified, independently managed mutual fund. § Income from seminars, lectures or teaching engagements sponsored by public or nonprofit entities. § Income from service on an advisory committee or review panel for a public or nonprofit entity.

PHS Policy Application § A grant cannot be submitted to PHS unless the PI and any persons who may potentially bias the design, conduct or analysis of the study have completed a disclosure § All eligible personnel must also have COI training on file prior to any PHS grant submission § For PHS regulated research, a copy of the management plan must be submitted to NIH prior to any funds being used for research purposes § A copy of any management plan must be made available to members of the public within 5 days of the request § Failure to disclose a significant financial interest will result in a remediation review

PHS and Travel* § Faculty or employees disclosure, within 30 days, for of each trip of any reimbursed or sponsored travel unless the travel is sponsored by the following: » A federal, state or local government agency » An institution of higher education » An academic teaching hospital » A research institute that is affiliated with an institution of higher education *Travel form is available at COI website

Conflict of Interest and Commitment Committee § The COICC reviews the SFIs disclosed by researchers. COIC also reviews related projects to determine if a FCOI exists § If a FCOI exists then the committee reviews and reviews a COI management plan § The management plan must be signed by the investigator within 10 days to take effect § Summary of the COI must be submitted to NIH if the study is PHS funded prior to any funds on the effected grant been drawn

COI Management Strategies § Full disclosure of researcher’s financial interests to any human research participants § Disclosure of financial relationship in all written and oral presentations, publications, and abstracts § Modification of the research plan § Monitoring of research by independent reviewers, including oversight of participant recruitment and enrollment; ICF process; analysis of the study data, reporting to the sponsor § Divestiture of significant financial interests § Disqualification of the researcher from part or all of the research project

Pharma Policy (CU-AMC) Students, residents and faculty may not accept: Gifts, monetary stipends, paid travel or honoraria solely for attendance at industry-sponsored dinners, lectures or sales presentations. Exceptions § For professional development courses or educational programs, activity may occur with prior approval § Educational programs designed to demonstrate proper use of medical or surgical devices or techniques § Travel related to research or technology transfer § Academic unit can create a central fund to support tuition, travel or participation in educational activities

Speakers’ Bureau May not participate or receive compensation talks through a speaker’s bureau if: a) The content of the slides, lecture or educational handouts need approval by industry reps; or b) If the content of the material to be presented does not represent a balanced and objective assessment of treatment options; or c) The compensation offered is above fair-market value; or d) The company provides honoraria or gifts to the attendees; or e) The overall purpose of the lecture or course is marketing

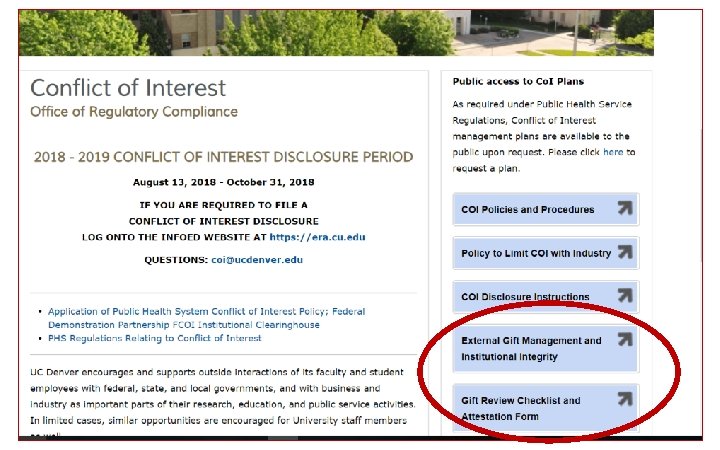
External Gift Management and Institutional Integrity (CU-AMC) § § New policy effective July 1, 2018 § § Protect the academic freedom of University faculty and staff § Does not address Individual Conflicts of Interest or funding for IRBapproved research § Policy and checklist links: Ensure the integrity of our university and the professionals who work here Support receipt of gifts that are fully consistent with the core values and mission of the University » Policy: https: //bit. ly/2 Mmy. Kfb » Checklist: https: //bit. ly/2 MI 2 AId

Gift Policy Guidance § The Advancement office or any faculty member may ask for PIIRC guidance on any gift, of any size § Some gifts will receive “heightened scrutiny” § Specifically, gifts from individuals or organizations that… Ø Produce products that claim to offer a health benefit, especially when the proposed activities might be perceived as an endorsement of the funder’s product(s) Ø Produce products/services that have harmful health impacts, Ø Have been the subject of substantial negative publicity, Ø Have also provided research support to the same faculty member receiving the non-research gift


When do you need to disclose gifts? Gift size thresholds § <$5, 000 or <$25, 000 and received during fundraising events (galas, luncheons, etc. ) – no change. § $5, 000 -$50, 000: faculty member should complete attestation form § $50, 000 -$100, 000: Faculty member and supervisor should sign form § $100, 000 -$1, 000: should receive PIIRC review and must have a written gift agreement § >$1, 000: must receive PIIRC review, have written gift agreement and Chancellor/designee approval

Governing Policies § Federal/NIH: 42 CFR Part 50, Subpart F, Objectivity of Research § Administrative Policy Statement 5012, Conflicts of Interest and Commitment » University of Colorado System Policy § University of Colorado Denver. IAnschutz Medical Campus, Procedures for Evaluating Conflicts of Interest and Commitment » UC Denver I Anschutz Campus Policy § University of Colorado Denver, Policy to Limit Conflicts of Interest Between Health Care Professionals and Industry Representatives » Applicable to UC Anschutz Schools of Dental Medicine, Nursing, Public Heath and Pharmacy, and the Health Sciences Library § SBIR/STTR Policy (forthcoming)

Questions? Website: ucdenver. edu/coi E-mail: coi@ucdenver. edu Christine Ahearn, christine. ahearn@ucdenver. edu

HIPAA Privacy and Security “Flags & Resources” Nov 2018 Lori Hopper, BA, CHC, CHPC Institutional Compliance & Privacy Officer Office for Regulatory Compliance 303 -724 -0983 | Lori. hopper@ucdenver. edu Deborah Geha, GSEC, HCISPP, ITIL Risk and Compliance Manager Office of Information Technology | Security & Compliance 303 -724 -9260 | deborah. geha@ucdenver. edu

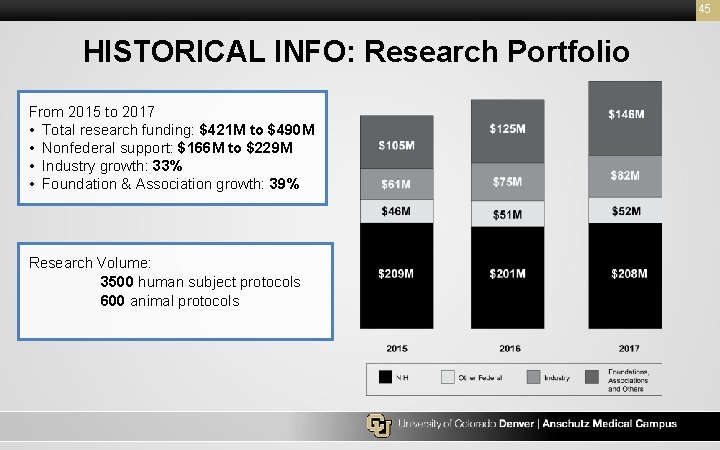
45 HISTORICAL INFO: Research Portfolio From 2015 to 2017 • Total research funding: $421 M to $490 M • Nonfederal support: $166 M to $229 M • Industry growth: 33% • Foundation & Association growth: 39% Research Volume: 3500 human subject protocols 600 animal protocols

46 CHALLENGES of an Academic Institution Decentralized hierarchical structure: Ø Challenges to Consistency Ø Devolved Responsibility Ø Challenges of Communication Ø How to be responsive to internal and external pressure or change? HELP with the “next right thing!”

47 HIPAA Policies & Procedures

48

HIPAA Privacy and Security Questions? Contact Us for Help Lori Hopper, BA, CHC, CHPC Institutional Compliance & Privacy Officer Office for Regulatory Compliance 303 -724 -0983 | Lori. hopper@ucdenver. edu Deborah Geha, GSEC, HCISPP, ITIL Risk and Compliance Manager Office of Information Technology | Security & Compliance 303 -724 -9260 | deborah. geha@ucdenver. edu

Human Subject Research Nov 2018

51 The Federal Regulations Governing Human Subjects Research § Department of Health and Human Services (DHHS): Federal department that generates regulations for human subject research and governing body for OHRP, FDA, CMS, NIH and others. § Office for Human Research Protections (OHRP): Human subjects regulations found at 45 CFR 46 § Food and Drug Administration (FDA): Human subjects regulations for drugs, biologics, and devices found at 21 CFR 11, 50, 54, 56, 312, 600, 812

52 Human Subject Research Defined – HHS § Research (45 CFR 46. 102(d)) means a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge. § Human Subject (45 CFR 46. 102(f)) means a living individual about whom an investigator conducting research obtains data through intervention or interaction with the individual, or identifiable private information.

53 Human Subject Research Defined – FDA 21 CFR parts 50. 3 and 56. 102 § Clinical investigation means any experiment that involves a test article and one or more human subjects. FDA has defined "clinical investigation" to be synonymous with "research". § Human subject means an individual who is or becomes a participant in research, either as a recipient of the test article or as a control. A subject may be either a healthy human or a patient. § Test article means any drug (including a biological product for human use), medical device for human use, human food additive, color additive, or electronic product.

54 Central Research Administration Resources:

55 UCD Research Resources http: //www. ucdenver. edu/research

56 Human Subject Research (HSR) Portal § Portal designed for researchers and study teams to submit their human subject research for review by » UCHealth » Children's Hospital Colorado » Clinical and Translational Research Center (CTRC) » University of Colorado § Administrators to facilitate institutional and affiliate review § Initiates build in On. Core

Centralized Clinical Research Support Center Regulatory Development • • Pre-Review • Human Research Portal • Pre-Review of Submissions • Consent writing assistance Education Study Monitoring • Clinical Trials training courses FDA Assistance • Audits by request • Responsible IND/IDE from study team Conduct of Application • For cause audits Research FDA Annual • Study monitoring • Post approval Reports Committee study start-up Clinical. Trials. gov assistance SUPPORT ACROSS THE LIFETIME OF A PROTOCOL Clinical. Research. Support. Center@ucdenver. edu

58 Clinical Research Administrative Operations Affiliate Partners Services University Services 1. Centralized research office for partners 1. 2. Facility Review/Approval of studies in partners’ facilities Negotiation clinical trial agreements for industry sponsored human studies 2. Centralized Administration for On. Core 3. Clinical Research Support Center (CRSC) 3. Badging/orientation training for new research personnel 4. Facilitation and regulatory support 4. Partners’ research policies and procedures 5. Education or research personnel 5. Compliance/QA for research billing 6. QA for clinical research 6. Epic for research 7. External IRB management 7. Billing for research services for partners 8. Clinical. Trials. gov registration and maintenance

59 Institutional Review Board (IRB) § All human subjects research at UCD and affiliates requires approval by Colorado Multiple Institutional Review Board (COMIRB), unless you are relying on an external IRB. This includes research using identifiable data about people, or human biological specimens. COMIRB § § § Acts as Privacy Board for HIPAA-related research at UCD, affiliate hospitals and for protocols under external IRB review (as appropriate) Maintains ‘Policies and Procedures for the Protection of Human Subjects’ Provides forms, instructions and guidance for researchers Holds weekly office hours Offers e. RA (Info. Ed) Training For questions and assistance: COMIRB Help Line: (303) 724 -1055 Email: COMIRB@ucdenver. edu External IRB Email: external. IRB@ucdenver. edu

60 Clinical Trials Contracting § Negotiates Clinical Trial Agreement (Contract) on behalf of the PI § Manages the process for reviewing, accepting and executing the Confidential Disclosure Agreement/Non-Disclosure Agreement § Material Transfer Agreements § Contact Information: Crao_contracts@ucdenver. edu http: //www. ucdenver. edu/research/OGC/Pages/MTA. aspx For further information, attend the CU: Budgeting for Clinical Research training series through Skill. Soft

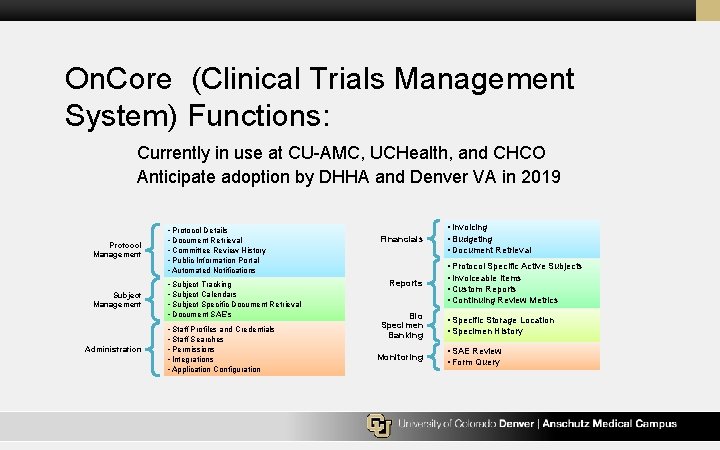
On. Core (Clinical Trials Management System) Functions: Currently in use at CU-AMC, UCHealth, and CHCO Anticipate adoption by DHHA and Denver VA in 2019 Protocol Management Subject Management Administration • Protocol Details • Document Retrieval • Committee Review History • Public Information Portal • Automated Notifications • Subject Tracking • Subject Calendars • Subject Specific Document Retrieval • Document SAE’s • Staff Profiles and Credentials • Staff Searches • Permissions • Integrations • Application Configuration Financials Reports Bio Specimen Banking Monitoring • Invoicing • Budgeting • Document Retrieval • Protocol Specific Active Subjects • Invoiceable Items • Custom Reports • Continuing Review Metrics • Specific Storage Location • Specimen History • SAE Review • Form Query

62 Thank you! Clinical Research Support Center § Clinical. Research. Support. Center@ucdenver. edu § (303) 724 -1111 Research website § ucdenver. edu/research
 Oig pharma compliance guidance
Oig pharma compliance guidance Cssd regulatory compliance
Cssd regulatory compliance Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress Regulatory compliance
Regulatory compliance Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress Missile launcher closed-loop system
Missile launcher closed-loop system Office of health standards compliance
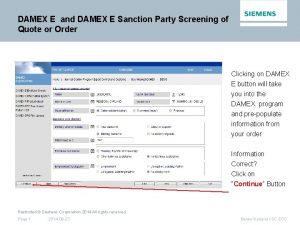
Office of health standards compliance Export control countries
Export control countries Damex prüfung siemens
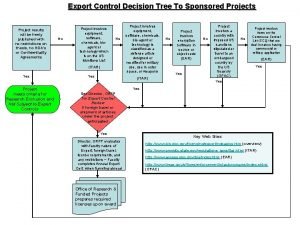
Damex prüfung siemens Export control decision tree
Export control decision tree Haddex
Haddex Factory office plan
Factory office plan Btec sport unit 3
Btec sport unit 3 Dispositional framework vs regulatory framework
Dispositional framework vs regulatory framework Traffic signs purpose
Traffic signs purpose Regulatory framework of financial services
Regulatory framework of financial services Chapter 22 regulatory and advisory agencies
Chapter 22 regulatory and advisory agencies Pharmacy law ethics and regulatory agencies
Pharmacy law ethics and regulatory agencies Disinfectants used in salons must be milady
Disinfectants used in salons must be milady Functions of sebi
Functions of sebi Regulatory institutions in indian financial system
Regulatory institutions in indian financial system Regulatory change management process
Regulatory change management process Objectives of irdai
Objectives of irdai Managing diversity and regulatory challenges
Managing diversity and regulatory challenges Signs signals and roadway markings
Signs signals and roadway markings Basle ii
Basle ii Regulatory capital vs economic capital
Regulatory capital vs economic capital Regulatory w automatyce
Regulatory w automatyce Gene regulatory network
Gene regulatory network State nuclear regulatory inspectorate of ukraine
State nuclear regulatory inspectorate of ukraine Ed ch
Ed ch Gene regulatory network
Gene regulatory network Regulatory reform fire safety order 2005
Regulatory reform fire safety order 2005 Film regulatory bodies
Film regulatory bodies Nespojité regulátory
Nespojité regulátory Minneapolis regulatory services
Minneapolis regulatory services Regulatory affairs kpi
Regulatory affairs kpi Post approval regulatory affairs
Post approval regulatory affairs Biologycorner.com
Biologycorner.com Fluorescent optic yellow sign
Fluorescent optic yellow sign Protective regulatory policy
Protective regulatory policy Regulatory framework accounting
Regulatory framework accounting Warehousing development and regulatory authority
Warehousing development and regulatory authority Regulatory creep
Regulatory creep California regulatory review course
California regulatory review course Regulatory reform fire safety order 2005 summary
Regulatory reform fire safety order 2005 summary National institute for food and drug surveillance
National institute for food and drug surveillance Regulatory readiness checklist
Regulatory readiness checklist Michigan department of licensing and regulatory affairs
Michigan department of licensing and regulatory affairs Ingegneria sicurezza antincendio
Ingegneria sicurezza antincendio Warning signs are what shape
Warning signs are what shape Regulatory system strengthening
Regulatory system strengthening Legal regulatory and political issues
Legal regulatory and political issues Dispositional framework vs regulatory framework
Dispositional framework vs regulatory framework Regulatory system strengthening
Regulatory system strengthening Regulatory agencies
Regulatory agencies Postal regulatory commission
Postal regulatory commission Sasra act
Sasra act Regulatory engagement meaning
Regulatory engagement meaning Regulatory motifs
Regulatory motifs Regulatory intelligence definition
Regulatory intelligence definition Good regulatory practice guidelines
Good regulatory practice guidelines