Enoxaparin in primary PCI From FINESSE to ATOLL



- Slides: 18

Enoxaparin in primary PCI From FINESSE to ATOLL G. Montalescot Institut de Cardiologie Pitié-Salpêtrière Hospital Paris, France The FINESSE Trial is supported by Eli Lilly and Co and Centocor. G. Montalescot, disclosure: Institutional research grant, consulting and speaker fees from Daiichi Sankyo, Eli Lilly, Sanofi Aventis, BMS.

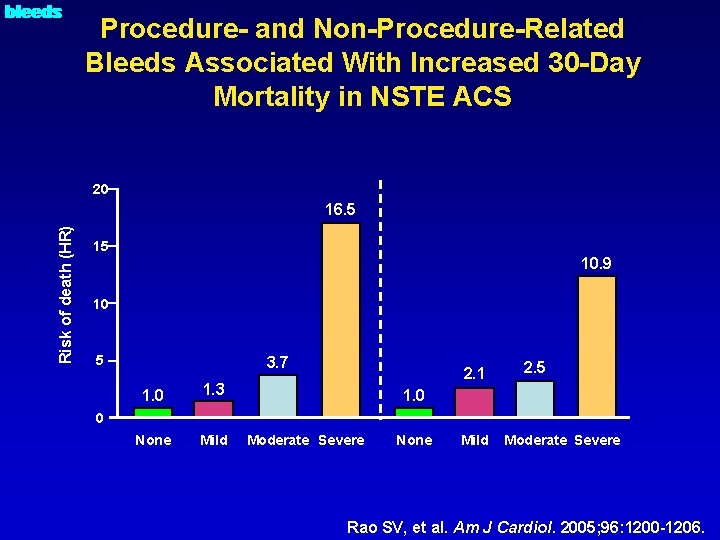
O bleeds Procedure- and Non-Procedure-Related Bleeds Associated With Increased 30 -Day Mortality in NSTE ACS 20 Risk of death (HR) 16. 5 15 10. 9 10 5 3. 7 1. 0 1. 3 None Mild 2. 1 2. 5 1. 0 0 Moderate Severe None Mild Moderate Severe Rao SV, et al. Am J Cardiol. 2005; 96: 1200 -1206.

Can we improve safety in PCI with enoxaparin?

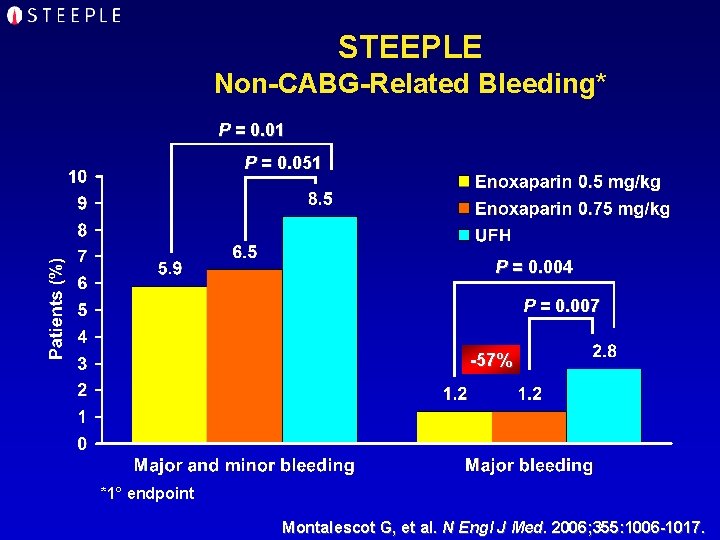
STEEPLE Non-CABG-Related Bleeding* P = 0. 01 P = 0. 051 P = 0. 004 P = 0. 007 -57% *1° endpoint Montalescot G, et al. N Engl J Med. 2006; 355: 1006 -1017.

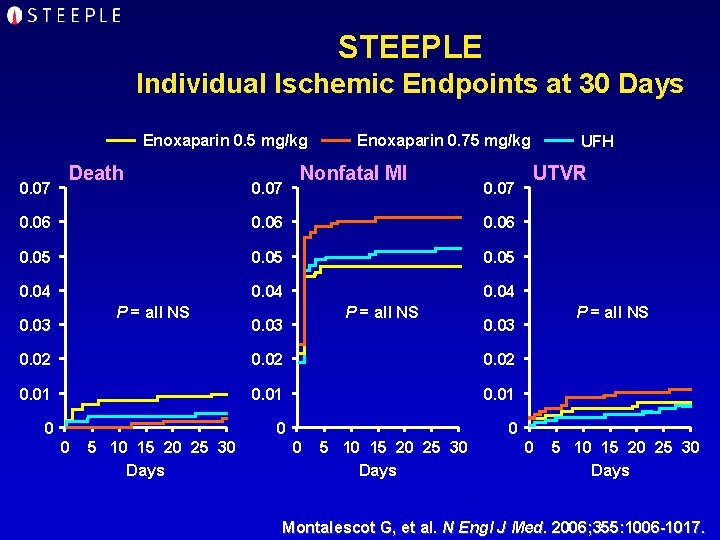
STEEPLE Individual Ischemic Endpoints at 30 Days Enoxaparin 0. 5 mg/kg 0. 07 Death 0. 07 Enoxaparin 0. 75 mg/kg Nonfatal MI 0. 07 0. 06 0. 05 0. 04 P = all NS 0. 03 0. 02 0. 01 0 0 0 5 10 15 20 25 30 Days UTVR P = all NS 0. 03 0. 02 0 UFH 0 5 10 15 20 25 30 Days Montalescot G, et al. N Engl J Med. 2006; 355: 1006 -1017.

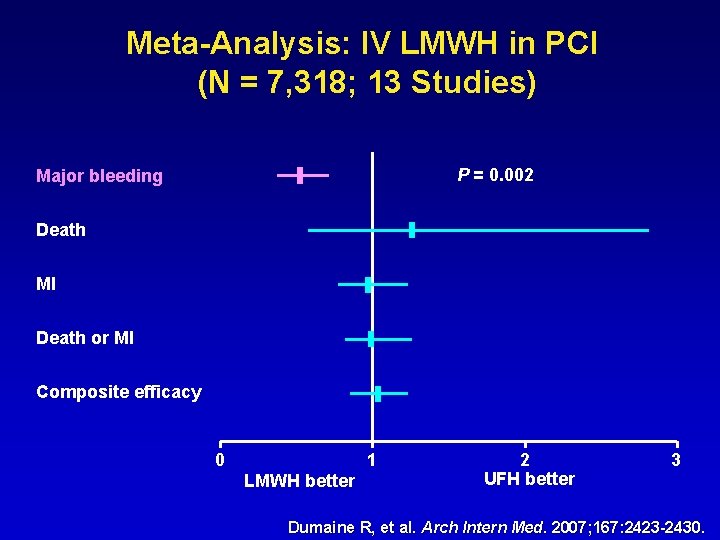
Meta-Analysis: IV LMWH in PCI (N = 7, 318; 13 Studies) P = 0. 002 Major bleeding Death MI Death or MI Composite efficacy 0 1 LMWH better 2 UFH better 3 Dumaine R, et al. Arch Intern Med. 2007; 167: 2423 -2430.

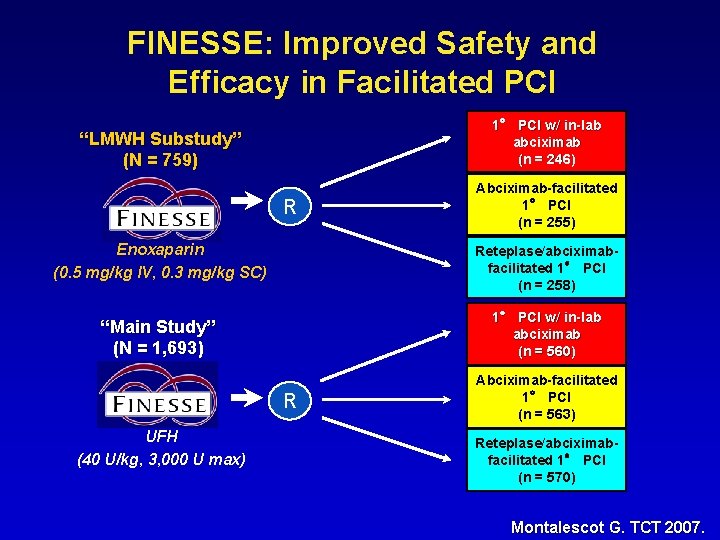
FINESSE: Improved Safety and Efficacy in Facilitated PCI 1° PCI w/ in-lab abciximab (n = 246) “LMWH Substudy” (N = 759) R Abciximab-facilitated 1° PCI (n = 255) Enoxaparin (0. 5 mg/kg IV, 0. 3 mg/kg SC) Reteplase/abciximabfacilitated 1° PCI (n = 258) “Main Study” (N = 1, 693) 1° PCI w/ in-lab abciximab (n = 560) R UFH (40 U/kg, 3, 000 U max) Abciximab-facilitated 1° PCI (n = 563) Reteplase/abciximabfacilitated 1° PCI (n = 570) Montalescot G. TCT 2007.

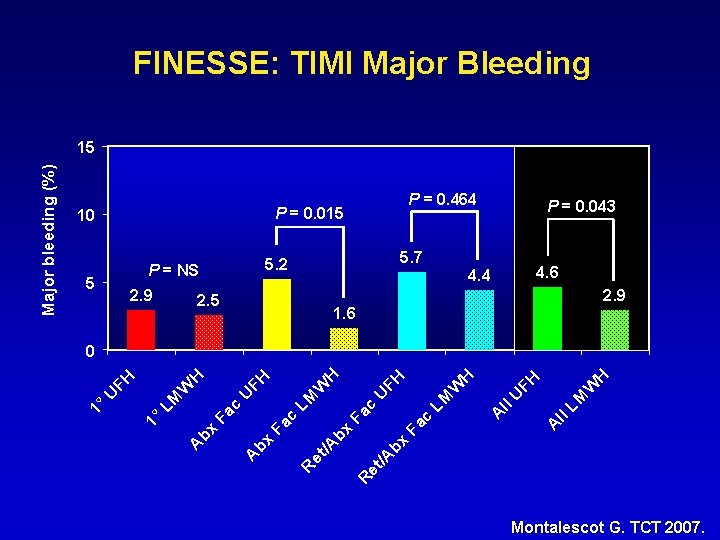
FINESSE: TIMI Major Bleeding P = 0. 464 P = 0. 015 10 5 2. 9 5. 7 5. 2 P = NS 2. 5 P = 0. 043 4. 6 4. 4 2. 9 1. 6 H W LM ll A A ll U FH H W c Fa bx bx /A /A R et et R LM U c Fa bx A FH H LM c Fa bx A W FH U W LM 1° U FH H 0 1° Major bleeding (%) 15 Montalescot G. TCT 2007.

Can we improve safety and efficacy in primary PCI?

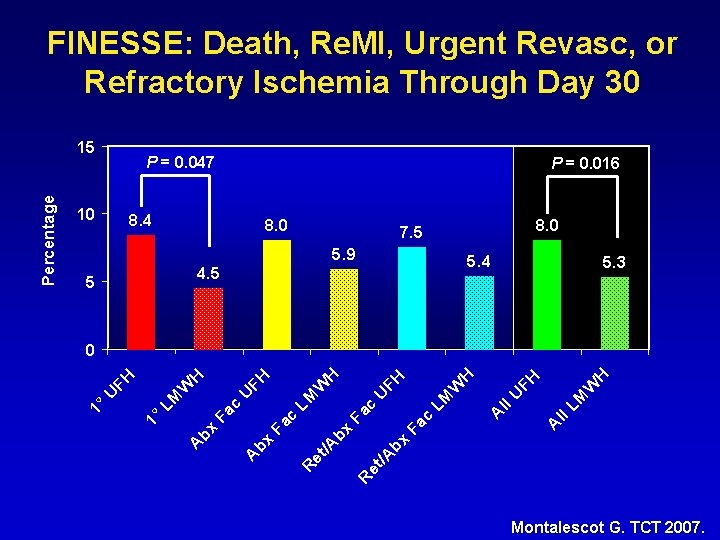
FINESSE: Death, Re. MI, Urgent Revasc, or Refractory Ischemia Through Day 30 P = 0. 047 10 P = 0. 016 8. 4 8. 0 7. 5 5. 9 5. 4 4. 5 5 5. 3 H W LM ll A A ll U FH H W c Fa bx bx /A /A R et et R LM U c Fa bx A FH H LM c Fa bx A W FH U W LM 1° U FH H 0 1° Percentage 15 Montalescot G. TCT 2007.

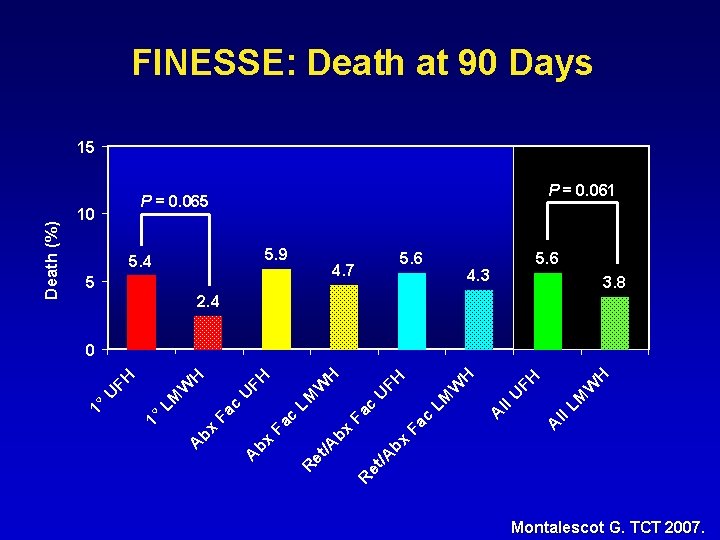
FINESSE: Death at 90 Days P = 0. 061 P = 0. 065 10 5. 9 5. 4 5 5. 6 4. 7 5. 6 4. 3 3. 8 2. 4 H W LM ll A A ll U FH H W c Fa bx bx /A /A R et et R LM U c Fa bx A FH H LM c Fa bx A W FH U W LM 1° U FH H 0 1° Death (%) 15 Montalescot G. TCT 2007.

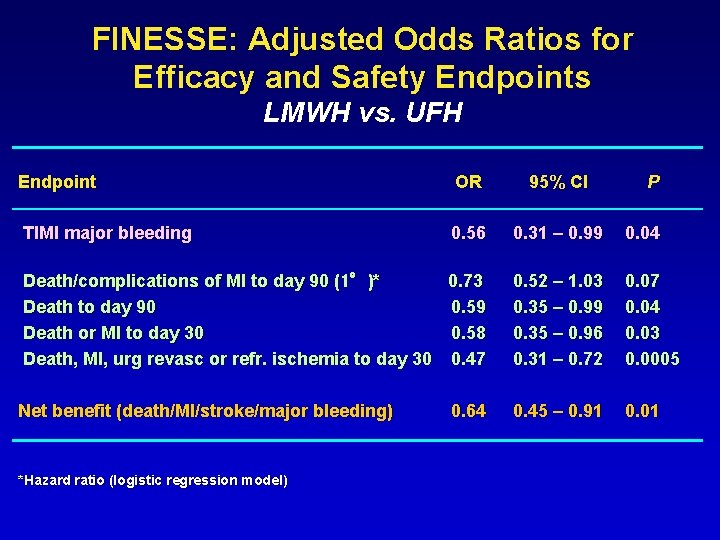
FINESSE: Adjusted Odds Ratios for Efficacy and Safety Endpoints LMWH vs. UFH Endpoint OR 95% CI TIMI major bleeding 0. 56 0. 31 – 0. 99 0. 04 Death/complications of MI to day 90 (1°)* 0. 73 Death to day 90 0. 59 Death or MI to day 30 0. 58 Death, MI, urg revasc or refr. ischemia to day 30 0. 47 0. 52 – 1. 03 0. 35 – 0. 99 0. 35 – 0. 96 0. 31 – 0. 72 0. 07 0. 04 0. 03 0. 0005 Net benefit (death/MI/stroke/major bleeding) 0. 45 – 0. 91 0. 01 *Hazard ratio (logistic regression model) 0. 64 P

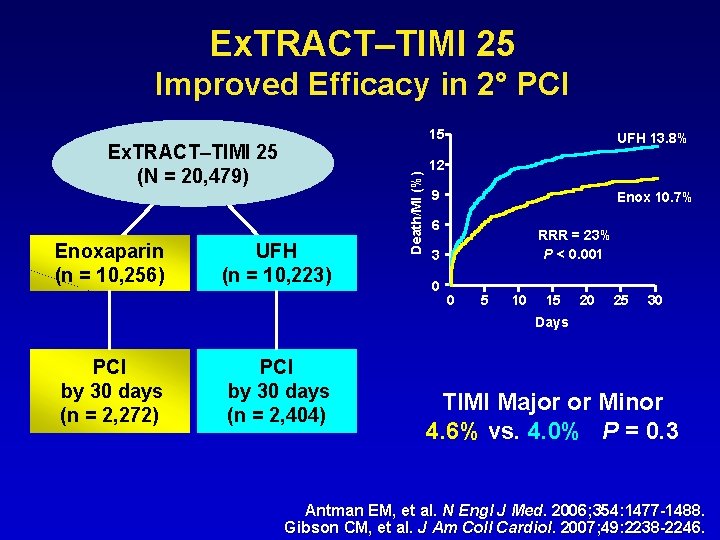
Ex. TRACT–TIMI 25 Improved Efficacy in 2° PCI Ex. TRACT–TIMI 25 (N = 20, 479) Enoxaparin (n = 10, 256) UFH (n = 10, 223) Death/MI (%) 15 UFH 13. 8% 12 9 Enox 10. 7% 6 RRR = 23% P < 0. 001 3 0 0 5 10 15 20 25 30 Days PCI by 30 days (n = 2, 272) PCI by 30 days (n = 2, 404) TIMI Major or Minor 4. 6% vs. 4. 0% P = 0. 3 Antman EM, et al. N Engl J Med. 2006; 354: 1477 -1488. Gibson CM, et al. J Am Coll Cardiol. 2007; 49: 2238 -2246.

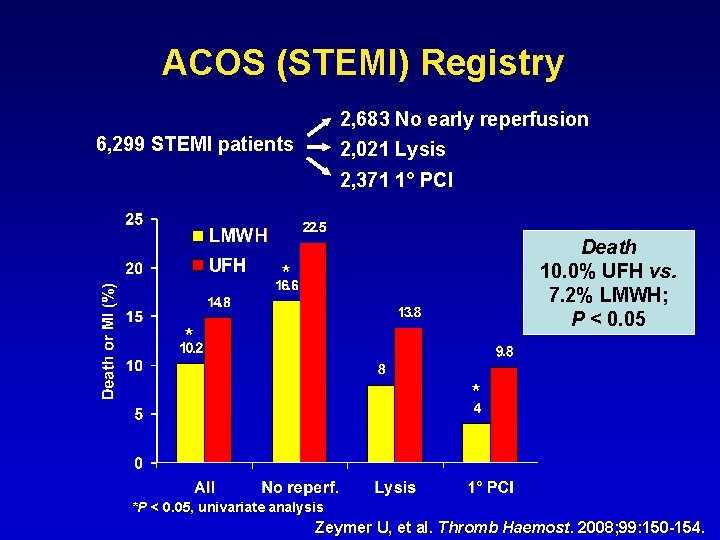
ACOS (STEMI) Registry 2, 683 No early reperfusion 6, 299 STEMI patients 2, 021 Lysis 2, 371 1° PCI Death 10. 0% UFH vs. 7. 2% LMWH; P < 0. 05 * *P < 0. 05, univariate analysis Zeymer U, et al. Thromb Haemost. 2008; 99: 150 -154.

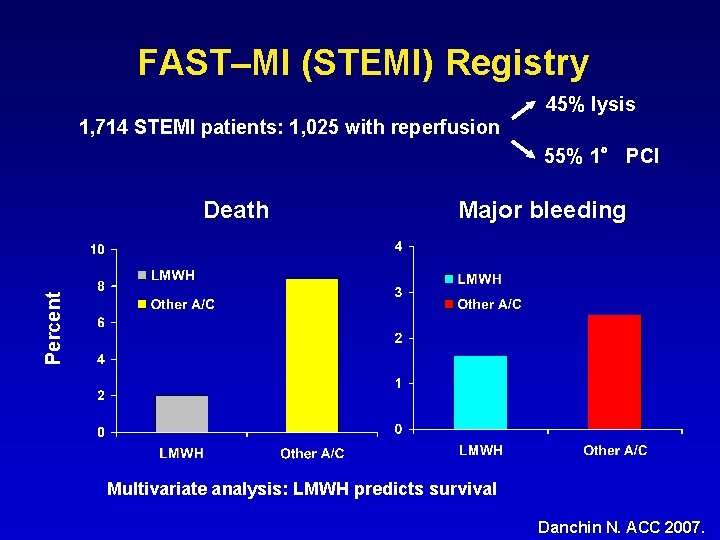
FAST–MI (STEMI) Registry 45% lysis 1, 714 STEMI patients: 1, 025 with reperfusion 55% 1° PCI Major bleeding Percent Death Multivariate analysis: LMWH predicts survival Danchin N. ACC 2007.

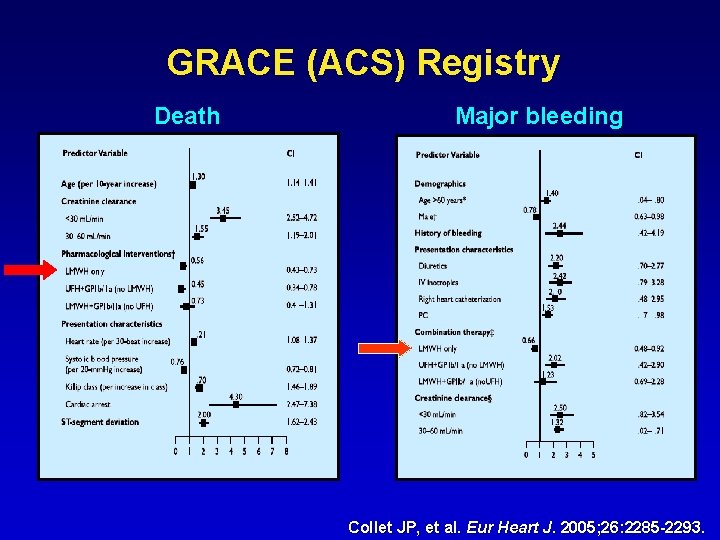
GRACE (ACS) Registry Death Major bleeding Collet JP, et al. Eur Heart J. 2005; 26: 2285 -2293.

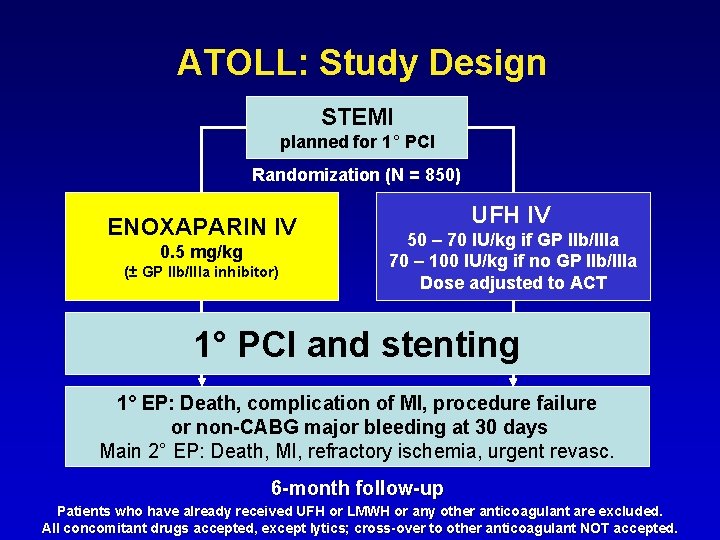
ATOLL: Study Design STEMI planned for 1° PCI Randomization (N = 850) ENOXAPARIN IV 0. 5 mg/kg (± GP IIb/IIIa inhibitor) UFH IV 50 – 70 IU/kg if GP IIb/IIIa 70 – 100 IU/kg if no GP IIb/IIIa Dose adjusted to ACT 1° PCI and stenting 1° EP: Death, complication of MI, procedure failure or non-CABG major bleeding at 30 days Main 2° EP: Death, MI, refractory ischemia, urgent revasc. 6 -month follow-up Patients who have already received UFH or LMWH or any other anticoagulant are excluded. All concomitant drugs accepted, except lytics; cross-over to other anticoagulant NOT accepted.

Conclusions • Time has come for a change of anticoagulation in p. PCI • Favorable data with IV enoxaparin in expeditive care of ACS, elective PCI, p. PCI • Same dose regimen of enoxaparin whatever the antiplatelet regimen • Optimal cost: benefit ratio
 Fondaparinux vs enoxaparin
Fondaparinux vs enoxaparin Enoxaparin
Enoxaparin Insulin aspart rob holland
Insulin aspart rob holland Heparin vs lovenox
Heparin vs lovenox Atoll tutorial
Atoll tutorial Darwin dana daly
Darwin dana daly Coral reef food web
Coral reef food web Nal game
Nal game Esprit de finesse pascal
Esprit de finesse pascal Finesse of a cavity
Finesse of a cavity Esprit de finesse pascal
Esprit de finesse pascal Finesse of a cavity
Finesse of a cavity Optical finesse
Optical finesse Finesse agent desktop
Finesse agent desktop Pci goal
Pci goal Pci magistrala
Pci magistrala Ranura agp
Ranura agp Pci compliance kiosk
Pci compliance kiosk Pci uex
Pci uex