I V Enoxaparin or Unfractionated Heparin in Primary










![Independent correlates of death at 6 months Variables HR[95%CI] Pvalue Beta blockers, yes vs. Independent correlates of death at 6 months Variables HR[95%CI] Pvalue Beta blockers, yes vs.](https://slidetodoc.com/presentation_image_h2/3b4448baa9fa251343390adf4b421a56/image-18.jpg)

- Slides: 20

I. V. Enoxaparin or Unfractionated Heparin in Primary PCI: Acute and Long-term results G. Montalescot, M. Cohen, P. Goldstein, K. Huber, C. Pollack, U. Zeymer, E. Vicaut for the ATOLL investigators ATOLL: Acute STEMI Treated with primary PCI and intravenous enoxaparin Or UFH to Lower ischemic and bleeding events at short- and Long-term follow-up (Investigator-driven study) G. MONTALESCOT, DISCLOSURE: Research Grants to the Institution or Consulting/Lecture Fees from Abbott Vascular, Astra-Zeneca, Bayer, Biotronik, Boehringer-Ingelheim, Boston Scientific, Cleveland Clinic Foundation, Cardiovascular Research Foundation, Cordis, Daiichi-Sankyo, Duke institute, Eli-Lilly, Europa, Fédération Française de Cardiologie, Fondation de France, GSK, ICM, INSERM, Lead-up, Medtronic, Menarini, Nanospheres, Novartis, Pfizer, Sanofi-Aventis Group, Servier, Société Française de Cardiologie, The Medicines Company, TIMI group.

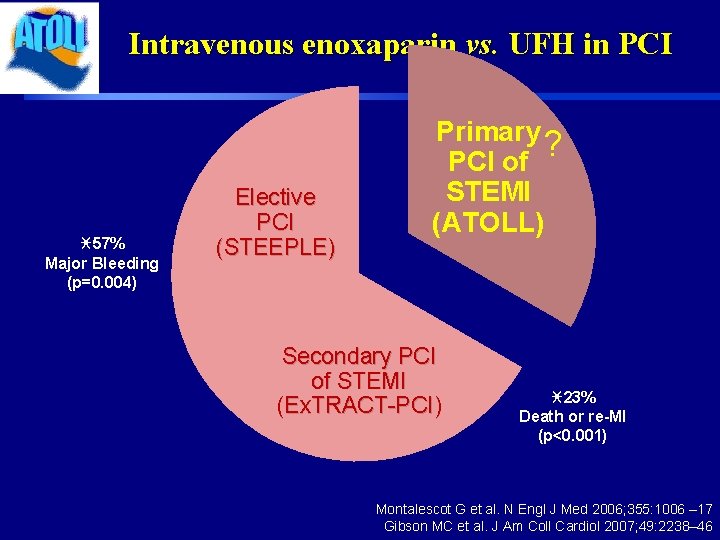
Intravenous enoxaparin vs. UFH in PCI i 57% Major Bleeding (p=0. 004) Elective PCI (STEEPLE) Primary ? PCI of STEMI (ATOLL) Secondary PCI of STEMI (Ex. TRACT-PCI) i 23% Death or re-MI (p<0. 001) Montalescot G et al. N Engl J Med 2006; 355: 1006 – 17 Gibson MC et al. J Am Coll Cardiol 2007; 49: 2238– 46

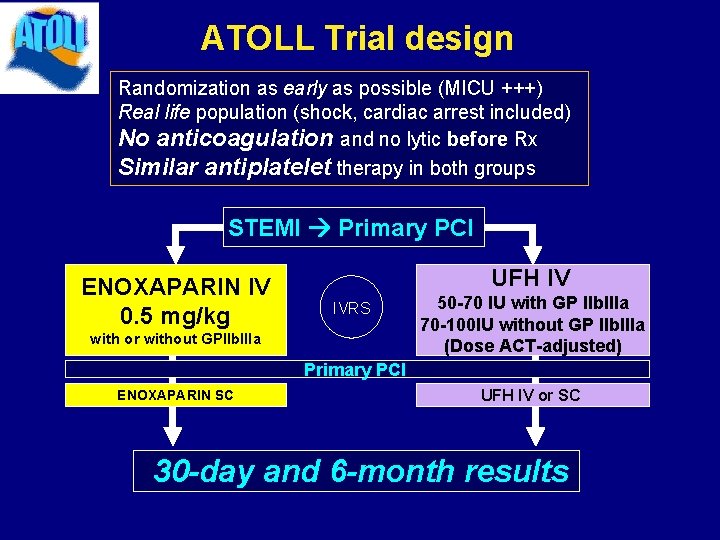
ATOLL Trial design Randomization as early as possible (MICU +++) Real life population (shock, cardiac arrest included) No anticoagulation and no lytic before Rx Similar antiplatelet therapy in both groups STEMI Primary PCI ENOXAPARIN IV 0. 5 mg/kg UFH IV IVRS with or without GPIIb. IIIa 50 -70 IU with GP IIb. IIIa 70 -100 IU without GP IIb. IIIa (Dose ACT-adjusted) Primary PCI ENOXAPARIN SC UFH IV or SC 30 -day and 6 -month results

Trial organization ACTION Study Group (Academic Research Organization, Paris): 1 -Coordinating Center: Institute of Cardiology, Pitié-Salpêtrière Hospital, Paris 2 -Sponsor: AP-HP (Assistance Publique-Hôpitaux de Paris) 3 -Data center, Statistics: Unité Recherche Clinique, Lariboisière Hospital, Paris 4 -International CRO: Pierrel-Hyperphar 5 -Funding: AP-HP and unrestricted research grant from Sanofi-Aventis Group Steering Committee: G. Montalescot (Chair, France), M. Cohen (USA), P. Goldstein (France), K. Huber (Austria), C. Pollack (USA), E. Vicaut (France), U. Zeymer (Germany) Data Safety Monitoring Board: A. Cohen (Chair, France), M. Cucherat (France), A. Gitt (Germany) Core Laboratory: R. Dumaine, A. Samadi Clinical Event Committee: F. Philippe, P. Sabouret, F. Boccara, A. Bellemain, O. Gournay

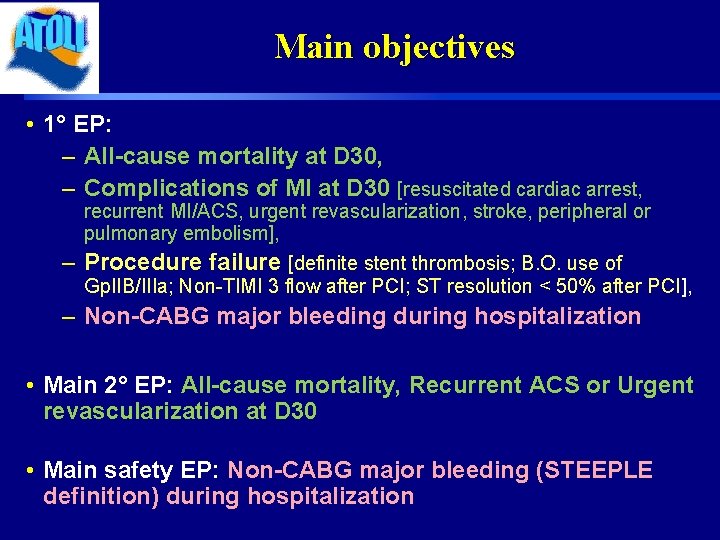
Main objectives • 1° EP: – All-cause mortality at D 30, – Complications of MI at D 30 [resuscitated cardiac arrest, recurrent MI/ACS, urgent revascularization, stroke, peripheral or pulmonary embolism], – Procedure failure [definite stent thrombosis; B. O. use of Gp. IIB/IIIa; Non-TIMI 3 flow after PCI; ST resolution < 50% after PCI], – Non-CABG major bleeding during hospitalization • Main 2° EP: All-cause mortality, Recurrent ACS or Urgent revascularization at D 30 • Main safety EP: Non-CABG major bleeding (STEEPLE definition) during hospitalization

FINAL 30 -DAY RESULTS

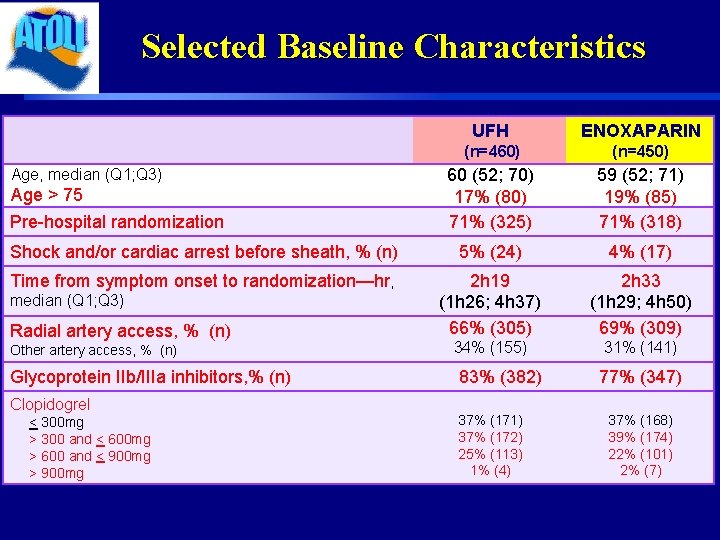
Selected Baseline Characteristics UFH ENOXAPARIN (n=460) (n=450) 60 (52; 70) 17% (80) 71% (325) 59 (52; 71) 19% (85) 71% (318) Shock and/or cardiac arrest before sheath, % (n) 5% (24) 4% (17) Time from symptom onset to randomization—hr, 2 h 19 (1 h 26; 4 h 37) 66% (305) 2 h 33 (1 h 29; 4 h 50) 69% (309) 34% (155) 31% (141) Age, median (Q 1; Q 3) Age > 75 Pre-hospital randomization median (Q 1; Q 3) Radial artery access, % (n) Other artery access, % (n) Glycoprotein IIb/IIIa inhibitors, % (n) Clopidogrel < 300 mg > 300 and < 600 mg > 600 and < 900 mg > 900 mg 83% (382) 37% (171) 37% (172) 25% (113) 1% (4) 77% (347) 37% (168) 39% (174) 22% (101) 2% (7)

Primary Endpoint Death, Complication of MI, Procedure Failure or Major Bleeding

Main Secondary Endpoint (ischemic) Death, Recurrent ACS or Urgent Revascularization

Consistent therapy Pre-specified analysis: no protocol violation (88%)

Death or Complication of MI Death, resuscitated cardiac arrest, recurrent ACS, Urg Revasc, stroke, peripheral or pulmonary embolism

Death (any) Death or resuscitated cardiac arrest

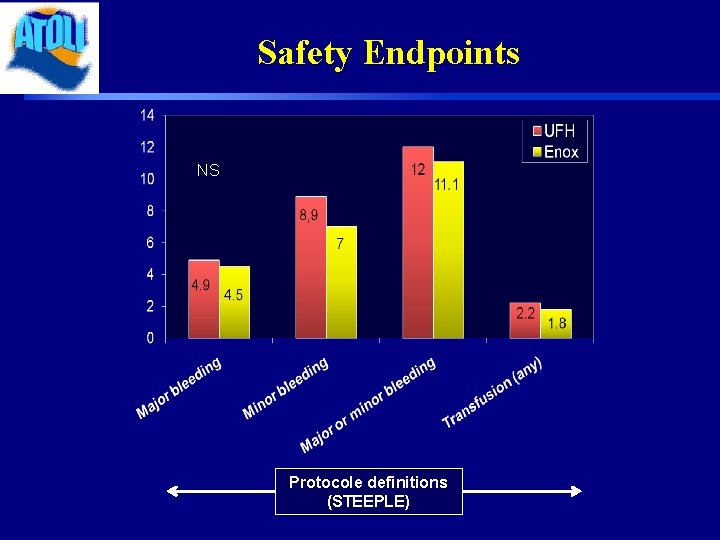
Safety Endpoints NS Protocole definitions (STEEPLE)

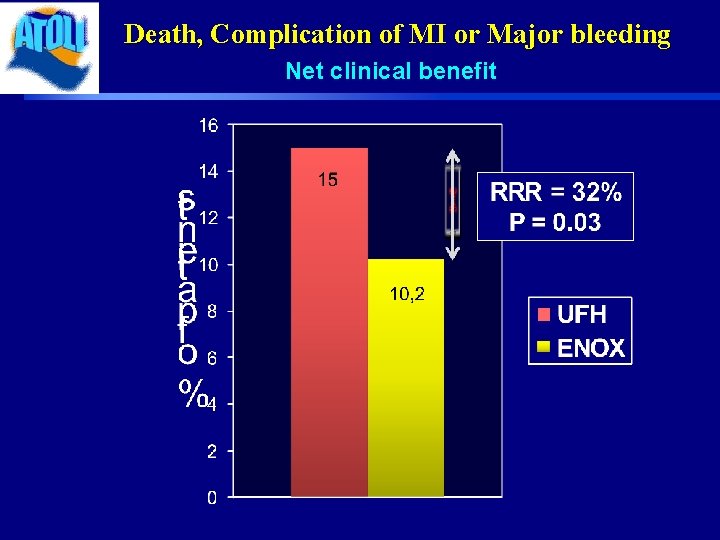
Death, Complication of MI or Major bleeding Net clinical benefit

6 -month Follow-up

6 -month results • Follow-up on mortality • 100% follow-up • We used a Cox regression model to identify independent predictors of death at 6 months. We firstly performed univariate analysis and significant variables were introduced into a stepwise cox regression model

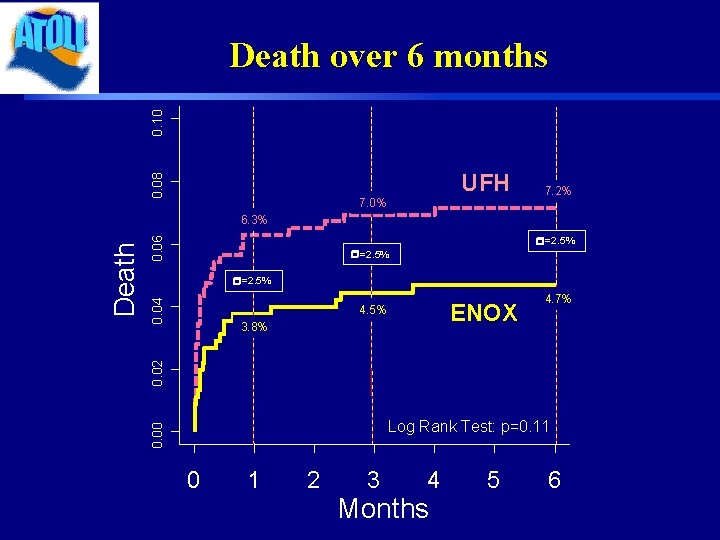
0. 08 0. 10 Death over 6 months UFH 7. 0% 7. 2% 0. 06 r=2. 5% 0. 04 r=2. 5% ENOX 4. 5% 4. 7% 0. 02 3. 8% Log Rank Test: p=0. 11 0. 00 Death 6. 3% 0 1 2 3 4 Months 5 6
![Independent correlates of death at 6 months Variables HR95CI Pvalue Beta blockers yes vs Independent correlates of death at 6 months Variables HR[95%CI] Pvalue Beta blockers, yes vs.](https://slidetodoc.com/presentation_image_h2/3b4448baa9fa251343390adf4b421a56/image-18.jpg)
Independent correlates of death at 6 months Variables HR[95%CI] Pvalue Beta blockers, yes vs. no 0. 16 [0. 08; 0. 32] <. 0001 KILLIP II, IV vs. I 3. 87 [2. 02; 7. 4] <. 0001 Age >75 vs. <75 4. 01 [2. 2; 7. 29] <. 0001 ACE yes vs. no 0. 32 [0. 16; 0. 66] 0. 0021 MI location, anterior vs. other 2. 24 [1. 27; 3. 94] 0. 0052 Prior heart failure, yes vs. no 4. 57 [1. 37; 15. 31] 0. 0137 Prior COPD, yes vs. no 3. 15 [1. 05; 9. 39] 0. 0401 Systolic BP [mm. Hg] (10 units increase) 0. 87 [0. 77; 0. 97] 0. 0149 Prior stroke, yes vs. no 3. 10 [1. 14; 8. 48] 0. 0273

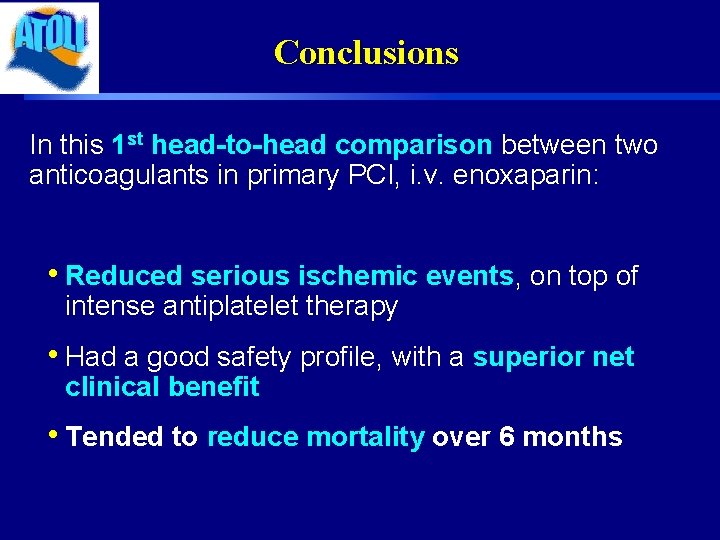
Conclusions In this 1 st head-to-head comparison between two anticoagulants in primary PCI, i. v. enoxaparin: • Reduced serious ischemic events, on top of intense antiplatelet therapy • Had a good safety profile, with a superior net clinical benefit • Tended to reduce mortality over 6 months

Special Thank to: INVESTIGATORS – Austria: WR. Benzer, K. Huber, F. Leisch, F. Weidinger – France: F. Adnet, M. Angioi, B. Barberon, JF. Benezet, JL. Bonnet, J. Boschat, B. Boulanger, D. Carrie, T. Chouihed, P. Coste, Y. Cottin, H. Courcoux, C. Cuvier, N. Danchin, JL. Ducasse, F. Duclos, P. Ecollan, S. Elhadad, E. Filippi, M. Freysz, F. Funck, S. Gallula, B. Gelée, A. Greffet, P. Henry, A. Jacquemin, T. Joseph, JM. Lablanche, H. Lardoux, H. Le Breton, B. Lederman, A. Margenet, G. Mehu, O. Nallet, F. Paganelli, M. Pansieri, L. Payot, C. Pouges, E. Salengro, C. Spaulding, G. Steg, O. Stibbe, E. Teiger, M. Thicoipe, C. Thuaire, J. Treuil, O. Wittenberg, O. Wolf – Germany: D. Andresen, C. Axthelm, Fischer, E. Girth, E. Hauptmann, U. Zeymer – USA: M. Cohen, F. Shamoon COMMITTEES – A Appaix-Bellemain, F Boccara, A Cohen, M Cucherat, R Dumaine, A Gitt, P Goldstein, O Gournay, K Huber, F Philippe, C Pollack, P Sabouret, A Samadi, E Vicaut, U Zeymer PIERREL Research– L. Basso, L. Merlini, M. Mazzoleni ACTION study Group – ME. Assossou, M. Aout, B. Bertin, D. Brugier, JP. Collet, M. Courreges-Viaud, V. Gallois, P. Gallula, V. Jouis, S. Kabla, C. Misse, G. Ngouala, A. Pena, S. Paulsrud, N. Vignolles
 Unfractionated heparin
Unfractionated heparin Robholland pantoprazole
Robholland pantoprazole Mechanism of action of clexane
Mechanism of action of clexane Fondaparinux vs enoxaparin
Fondaparinux vs enoxaparin Enoxaparin
Enoxaparin Immune thrombocytopenia
Immune thrombocytopenia Heparin
Heparin Heparin moa
Heparin moa Heparin mechanism of action
Heparin mechanism of action Cumarinderivate medikamente
Cumarinderivate medikamente Janna journeycake
Janna journeycake Koagülasyon kaskadı
Koagülasyon kaskadı Heparin drip nomogram
Heparin drip nomogram Heparin infüzyon protokolü
Heparin infüzyon protokolü Heparin warfarin
Heparin warfarin Heparin dosage calculation formula
Heparin dosage calculation formula Streptokinase mechanism of action
Streptokinase mechanism of action Hrombocytopenia
Hrombocytopenia Heparin inducirana trombocitopenija
Heparin inducirana trombocitopenija Anticoagulation medication chart
Anticoagulation medication chart Parenteral medication calculations
Parenteral medication calculations