End of Semester Review 2011 2012 Reminders Help
























- Slides: 35

End of Semester Review 2011 -2012

Reminders Help Session: Tuesday, January 31 5 pm – 7: 00 pm What to bring day of: Pencil Calculator Notes Page QUIET snack

Unit 1 (Chapter 5) • Conversions • Significant Digits • Scientific Notation

How many significant figures are in the answer to the following: 12. 6 m x 2. 0 m x 13. 84 m A. B. C. D. 2 1 6 4

How many significant figures are in the answer to the following: 11. 63 m. L – 1. 2 m. L A. B. C. D. 2 1 3 4

How many milliliters are there in 0. 0200 kiloliters? A. B. C. D. 2. 00 x 107 m. L 2. 00 x 104 m. L 2. 00 x 103 m. L 20. 0 m. L

If I can run 5. 00 km in 21. 3 min, how fast am I traveling in miles per hour? A. B. C. D. 10. 8 miles/hour 87. 5 miles/hour 0. 146 miles/hour 8. 75 miles/hour

Write the number 623000 in the correct scientific notation. A. B. C. D. 6. 23 x 105 6. 23 x 10 -5 6. 23 x 103 623 x 103

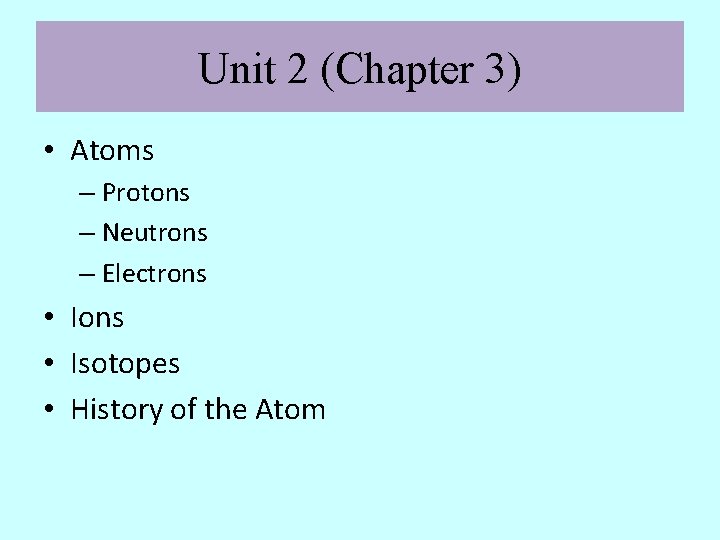
Unit 2 (Chapter 3) • Atoms – Protons – Neutrons – Electrons • Ions • Isotopes • History of the Atom

The first scientist to propose a model of the atom based on scientific knowledge and experimentation. A. B. C. D. Dmitri Mendeleev Isaac Newton John Dalton Neils Bohr

An ion is formed by the loss or gain of _________ A. Protons B. Neutrons C. Electrons

An isotope is formed by the loss of gain of ________ A. Protons B. Neutrons C. Electrons

The mass number is determined by A. Adding up the numbers from the periodic table B. Adding the number of protons to the number of electrons C. Adding the number of electrons to the number of neutrons D. Adding the number of protons to the number of neutrons

Unit 3 (Chapter 11) • Light • Electron Configuration

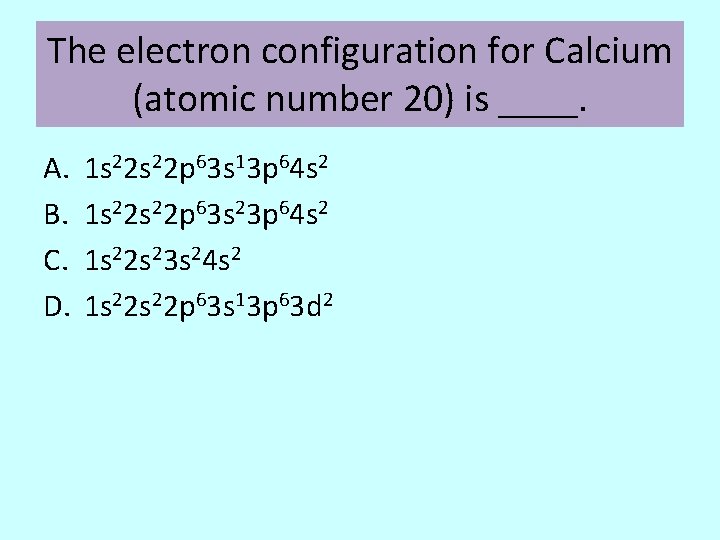
The electron configuration for Calcium (atomic number 20) is ____. A. B. C. D. 1 s 22 p 63 s 13 p 64 s 2 1 s 22 p 63 s 23 p 64 s 2 1 s 22 s 23 s 24 s 2 1 s 22 p 63 s 13 p 63 d 2

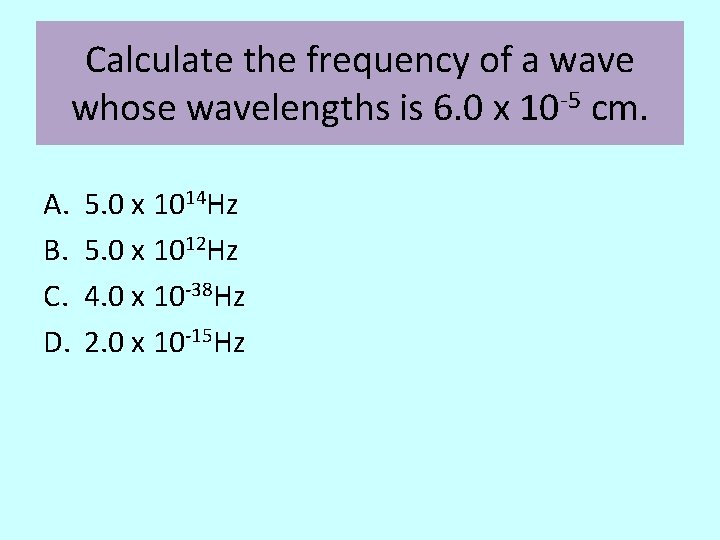
Calculate the frequency of a wave whose wavelengths is 6. 0 x 10 -5 cm. A. B. C. D. 5. 0 x 1014 Hz 5. 0 x 1012 Hz 4. 0 x 10 -38 Hz 2. 0 x 10 -15 Hz

A line-emission spectra of an atom is caused by the energy released when electrons A. absorb energy B. jump to a higher energy level C. fall to a lower energy level

Unit 4 (Chapter 4) • Naming – Binary vs. Ternary Compounds • Transition Metals – Polyatomic Ions – Molecular Compounds

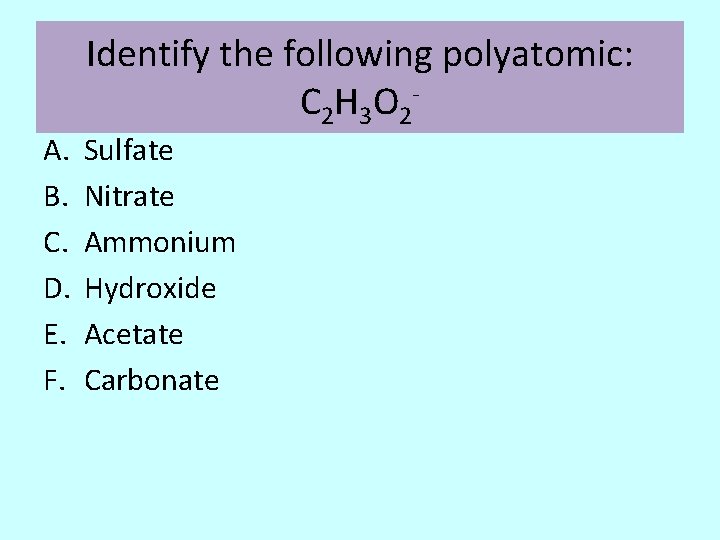
A. B. C. D. E. F. Identify the following polyatomic: C 2 H 3 O 2 - Sulfate Nitrate Ammonium Hydroxide Acetate Carbonate

Pick the correct formula: Aluminum sulfide A. B. C. D. Al. S Al 2(SO 4)3 Al. SO 4 Al 2 S 3

Pick the correct formula: Magnesium Carbonate A. B. C. D. Mg 2 CO 3 Mg. C Mn 2 CO 3

Choose the correct name: Na. Cl A. B. C. D. Sodium Chlorate Sodium (I) Chlorite Sodium Chloride Sodium Carbide

Choose the correct name: Cu. SO 4 A. B. C. D. Copper Sulfate Copper (I) Sulfate Copper (II) Sulfate Copper Sulfite

Unit 5 (Chapter 7 and 8) • • Reaction Types Balancing Reactions Predicting Products Solubility

When copper (II) nitrate reacts with sodium hydroxide, it is this kind of reaction: A. B. C. D. E. Synthesis Decomposition Single Displacement Double Displacement Combustion

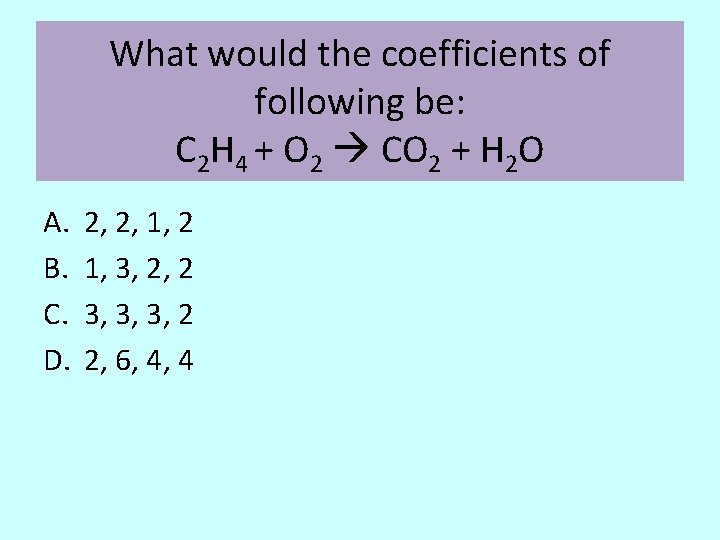
What would the coefficients of following be: C 2 H 4 + O 2 CO 2 + H 2 O A. B. C. D. 2, 2, 1, 2 1, 3, 2, 2 3, 3, 3, 2 2, 6, 4, 4

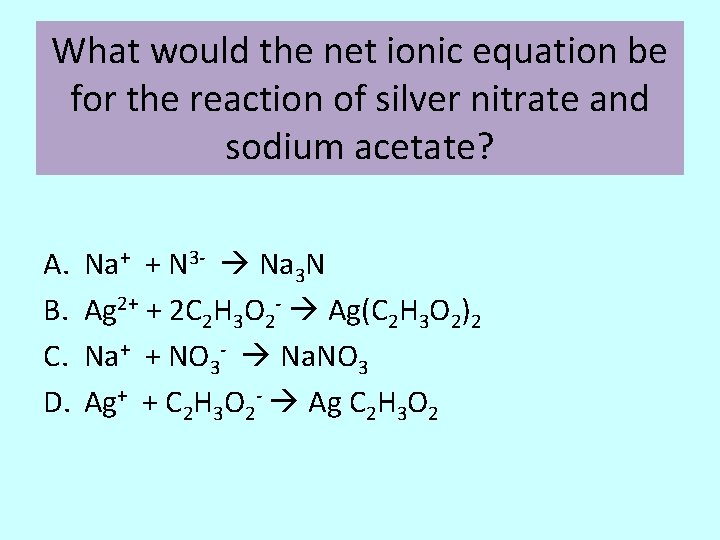
What would the net ionic equation be for the reaction of silver nitrate and sodium acetate? A. B. C. D. Na+ + N 3 - Na 3 N Ag 2+ + 2 C 2 H 3 O 2 - Ag(C 2 H 3 O 2)2 Na+ + NO 3 - Na. NO 3 Ag+ + C 2 H 3 O 2 - Ag C 2 H 3 O 2

Which of the following is insoluble in water? A. B. C. D. Na. OH Cu. SO 4 Ba. NO 3 (NH 4)2 S

Unit 6 (Chapter 6) • The Mole • Molar Mass

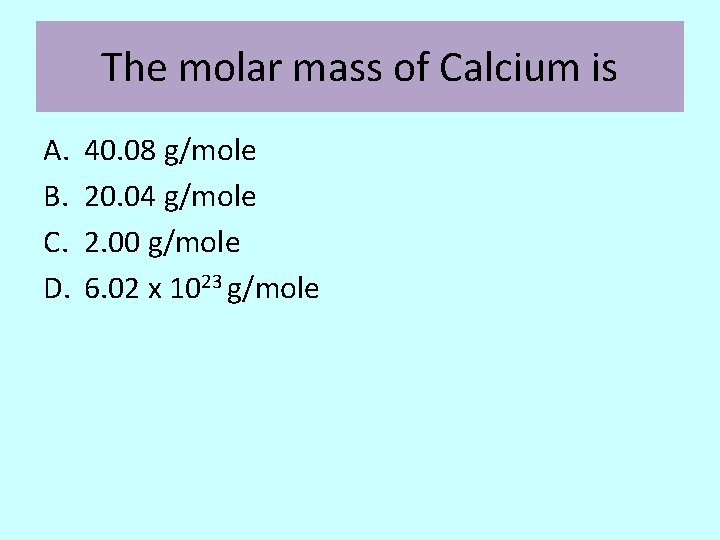
The molar mass of Calcium is A. B. C. D. 40. 08 g/mole 20. 04 g/mole 2. 00 g/mole 6. 02 x 1023 g/mole

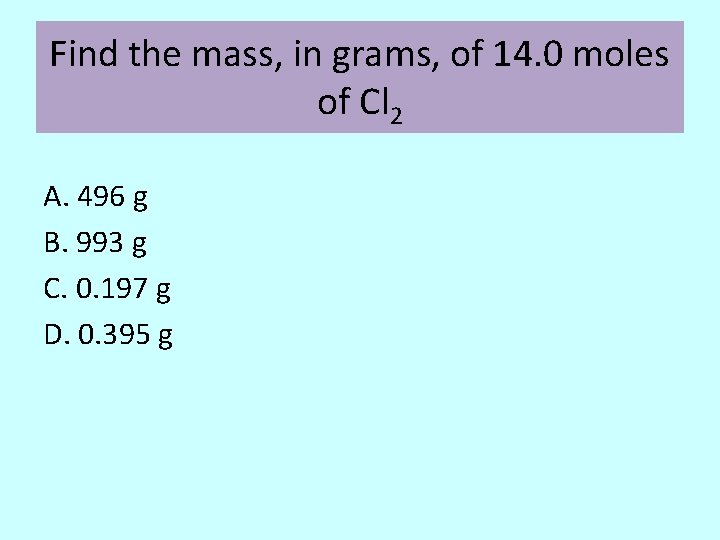
Find the mass, in grams, of 14. 0 moles of Cl 2 A. 496 g B. 993 g C. 0. 197 g D. 0. 395 g

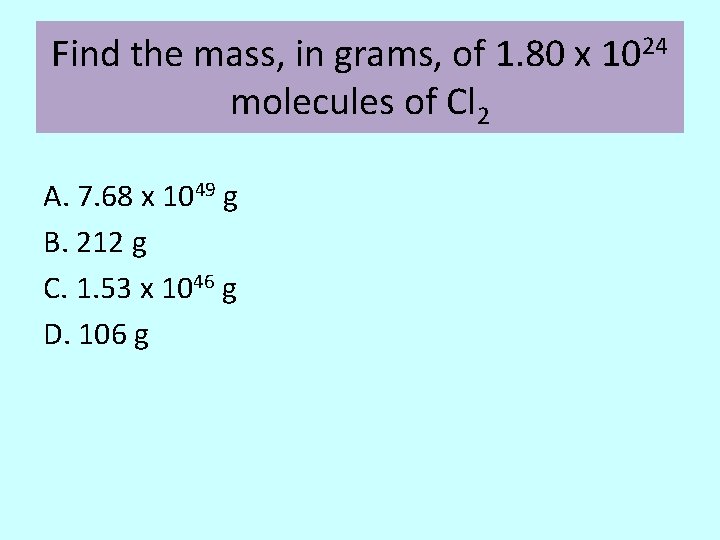
Find the mass, in grams, of 1. 80 x 1024 molecules of Cl 2 A. 7. 68 x 1049 g B. 212 g C. 1. 53 x 1046 g D. 106 g

What do you still need to do to be ready for the final? Make Notes Page Eat! SLEEP

Reminders Help Session: Tuesday, January 31 5 pm – 7: 15 pm What to bring day of: Pencil Calculator Notes Page QUIET snack

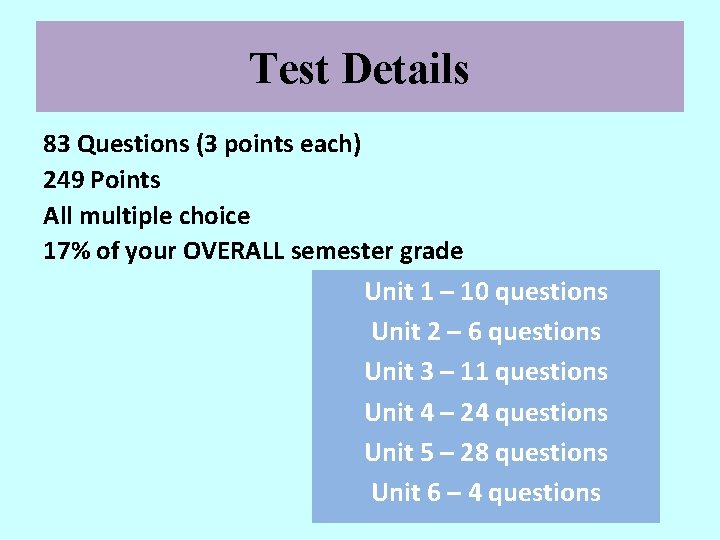
Test Details 83 Questions (3 points each) 249 Points All multiple choice 17% of your OVERALL semester grade Unit 1 – 10 questions Unit 2 – 6 questions Unit 3 – 11 questions Unit 4 – 24 questions Unit 5 – 28 questions Unit 6 – 4 questions
 Wcms dress code
Wcms dress code Interview etiquette definition
Interview etiquette definition Ehr alerts and reminders
Ehr alerts and reminders Staar reminders
Staar reminders Math reminders
Math reminders Environmental science a final exam
Environmental science a final exam Windows small business server 2011 end of life
Windows small business server 2011 end of life Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Physics semester 1 review
Physics semester 1 review World history semester 2 final review packet
World history semester 2 final review packet World history 1st semester midterm exam review answers
World history 1st semester midterm exam review answers Us history semester 2 final exam review
Us history semester 2 final exam review Algebra 1 semester 2 final review
Algebra 1 semester 2 final review Zoology final exam review
Zoology final exam review Geometry unit 5 review
Geometry unit 5 review Apes semester 1 final exam
Apes semester 1 final exam Physics fall semester exam review
Physics fall semester exam review American history final exam
American history final exam English 3 fall semester exam review
English 3 fall semester exam review English 3 fall semester exam review
English 3 fall semester exam review Chemistry
Chemistry Us history semester 1 final exam study guide answers
Us history semester 1 final exam study guide answers Biology semester 1 review 2018
Biology semester 1 review 2018 Chemistry 1 semester exam review
Chemistry 1 semester exam review World history 1st semester final review answers
World history 1st semester final review answers Freesurfer troubleshooting
Freesurfer troubleshooting Help help i'm being oppressed
Help help i'm being oppressed Helper chapter 1
Helper chapter 1 Education through self help is our motto
Education through self help is our motto My mother makes me chicken poem
My mother makes me chicken poem Master asl unit 2
Master asl unit 2 What is the volume of blood pumped per minute
What is the volume of blood pumped per minute End diastolic volume meaning
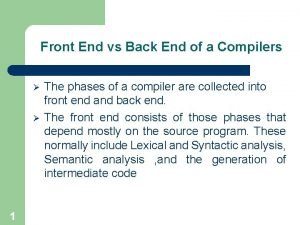
End diastolic volume meaning Front end and back end in compiler design
Front end and back end in compiler design Front end of compiler
Front end of compiler Zirkularstapler
Zirkularstapler