Electron Transport and Oxidative Phosphorylation Dr sadia haroon






- Slides: 27

Electron Transport and Oxidative Phosphorylation Dr. sadia haroon Department of biochemistry Peshawar medical college.

Mitochondria • outer membrane relatively permeable • inner membrane permeable only to those things with specific transporters – Impermeable to NADH and FADH 2 – Permeable to pyruvate • Compartmentalization – Kreb's and β-oxidation in matrix – Glycolysis in cytosol

Most energy from Redox • electrons during metabolic reactions sent to NAD and FAD – Glycolysis • In cytosol • produces 2 NADH – Pyruvate dehydrogenase reaction • In mitochondrial matrix • 2 NADH / glucose – Krebs • In mitochondrial matrix • 6 NADH and 2 FADH 2 / glucose

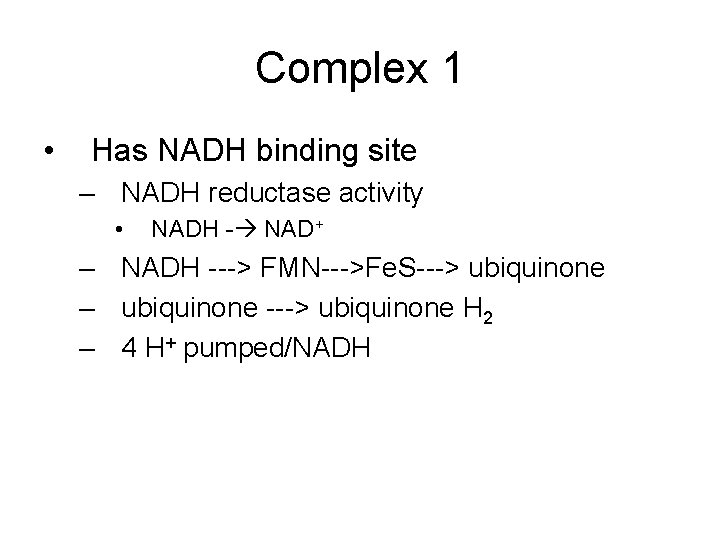
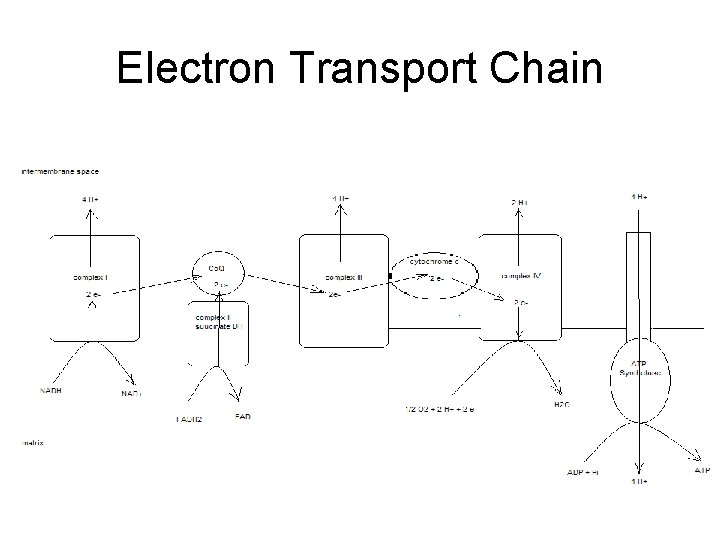
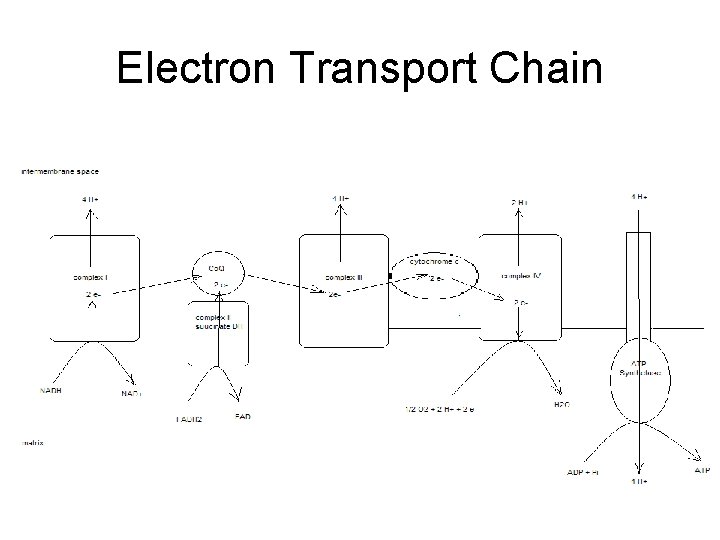
Electron Transport Chain • Groups of redox proteins – On inner mitochondrial membrane – Binding sites for NADH and FADH 2 • • On matrix side of membrane Electrons transferred to redox proteins NADH reoxidized to NAD+ FADH 2 reoxidized to FAD

4 Complexes • • proteins in specific order Transfers 2 electrons in specific order – Proteins localized in complexes • • Embedded in membrane Ease of electron transfer – Electrons ultimately reduce oxygen to water • 2 H+ + 2 e- + ½ O 2 -- H 2 O

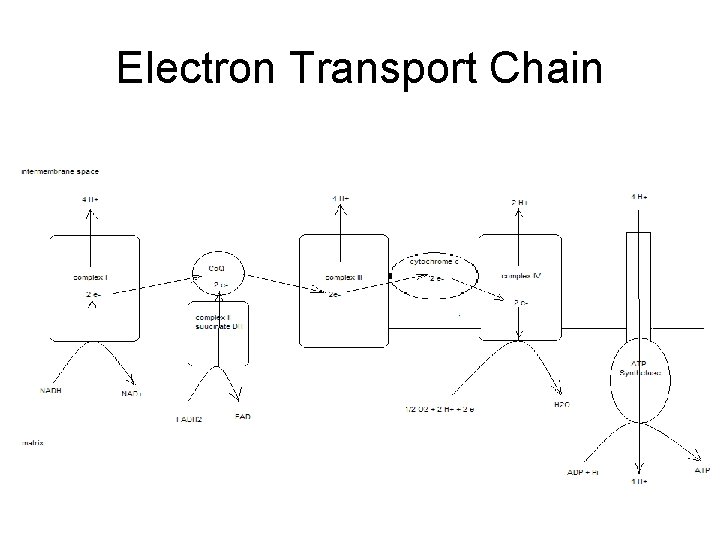
Electron Transport Chain

Complex 1 • Has NADH binding site – NADH reductase activity • NADH - NAD+ – NADH ---> FMN--->Fe. S---> ubiquinone – ubiquinone ---> ubiquinone H 2 – 4 H+ pumped/NADH

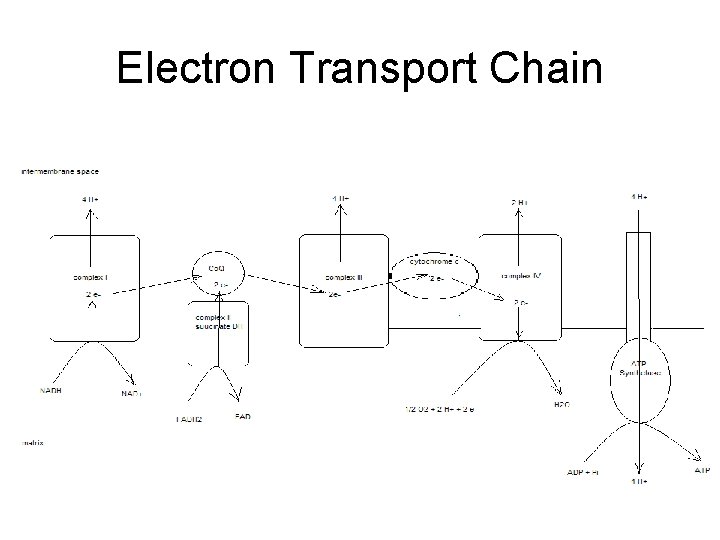
Electron Transport Chain

Complex II • succinate ---FAD—ubiquinone – Contains coenzyme Q – FADH 2 binding site • • FAD reductase activity FADH 2 -- FAD

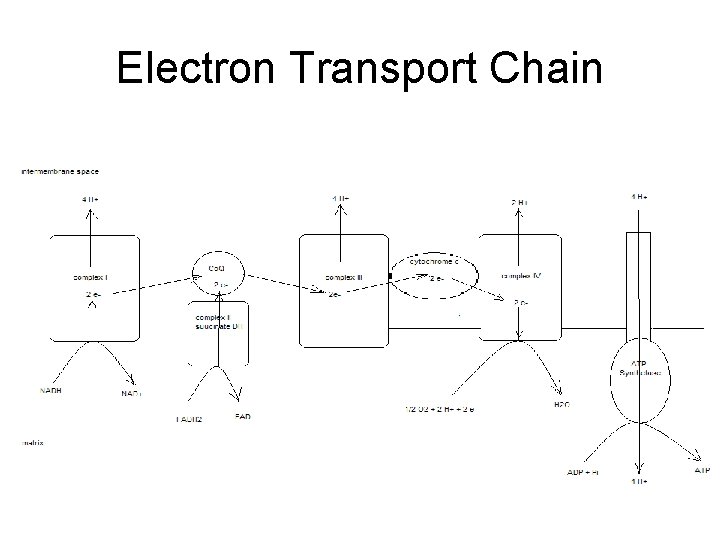
Electron Transport Chain

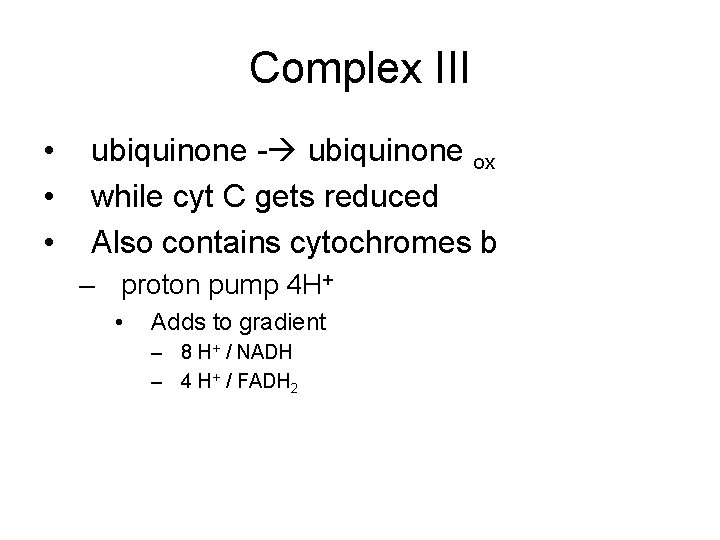
Complex III • • • ubiquinone - ubiquinone ox while cyt C gets reduced Also contains cytochromes b – proton pump 4 H+ • Adds to gradient – 8 H+ / NADH – 4 H+ / FADH 2

Electron Transport Chain

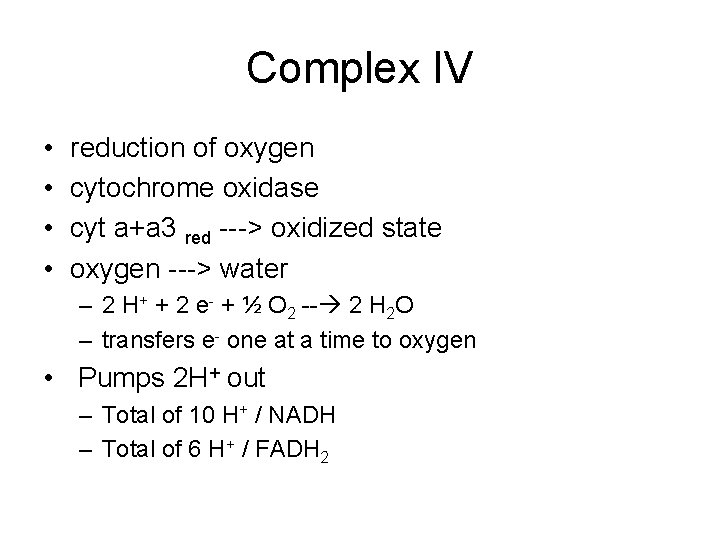
Complex IV • • reduction of oxygen cytochrome oxidase cyt a+a 3 red ---> oxidized state oxygen ---> water – 2 H+ + 2 e- + ½ O 2 -- 2 H 2 O – transfers e- one at a time to oxygen • Pumps 2 H+ out – Total of 10 H+ / NADH – Total of 6 H+ / FADH 2

Totals • Proton gradient created as electrons transferred to oxygen forming water – 10 H+ / NADH – 6 H+ / FADH 2

Electron Transport Chain

Generation of ATP • Proton dependant ATP synthetase – Uses proton gradient to make ATP – Protons pumped through channel on enzyme • From intermembrane space into matrix • ~4 H+ / ATP – Called chemiosmotic theory

Totals NADH 10 H+ X 1 ATP = 2. 5 ATP 4 H+ FADH 2 6 H+ X 1 ATP = 1. 5 ATP 4 H+

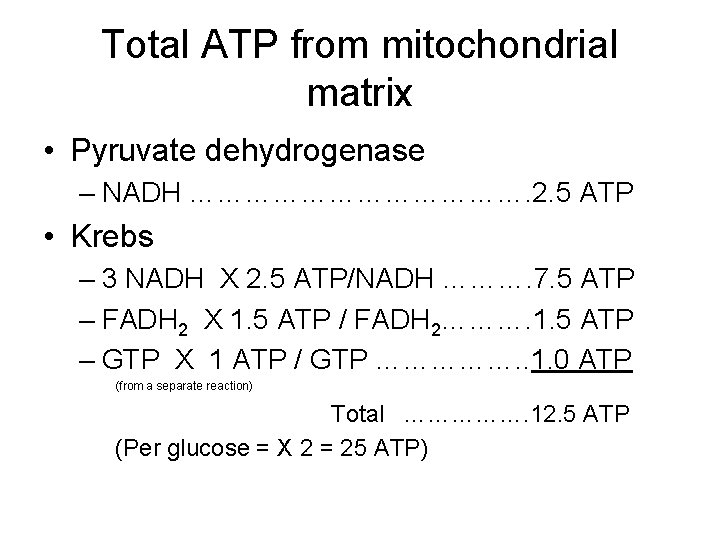
Total ATP from mitochondrial matrix • Pyruvate dehydrogenase – NADH ………………. 2. 5 ATP • Krebs – 3 NADH X 2. 5 ATP/NADH ………. 7. 5 ATP – FADH 2 X 1. 5 ATP / FADH 2………. 1. 5 ATP – GTP X 1 ATP / GTP ……………. . 1. 0 ATP (from a separate reaction) Total ……………. 12. 5 ATP (Per glucose = X 2 = 25 ATP)

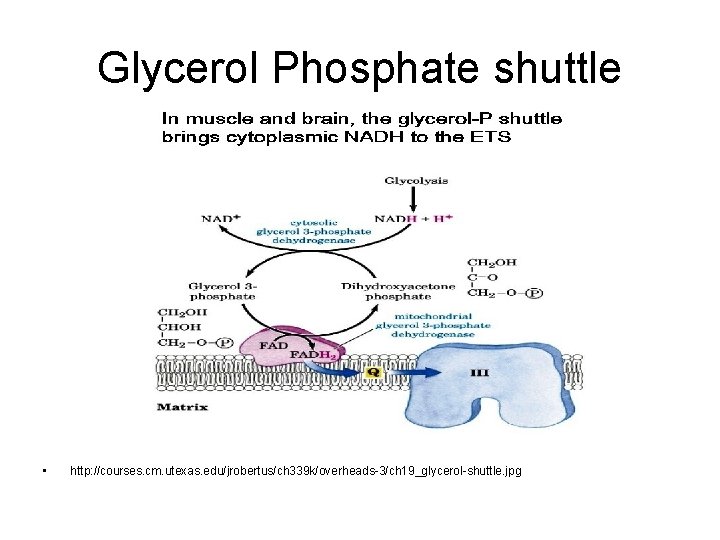
What about NADH from glycolysis? • NADH made in cytosol • Can’t get into matrix of mitochondrion • 2 mechanisms – In muscle and brain • Glycerol phosphate shuttle – In liver and heart • Malate / aspartate shuttle

Glycerol Phosphate shuttle • http: //courses. cm. utexas. edu/jrobertus/ch 339 k/overheads-3/ch 19_glycerol-shuttle. jpg

Glycerol phosphate shuttle • In muscle and brain • Each NADH converted to FADH 2 inside mitochondrion – FADH 2 enters later in the electron transport chain – Produces 1. 5 ATP

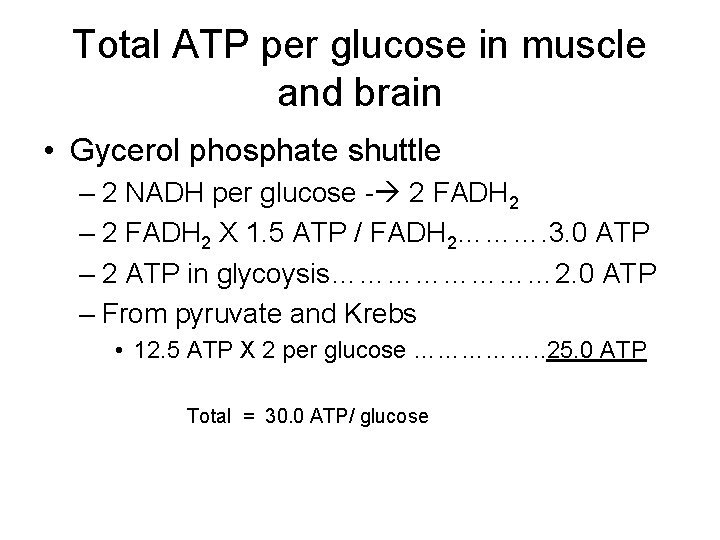
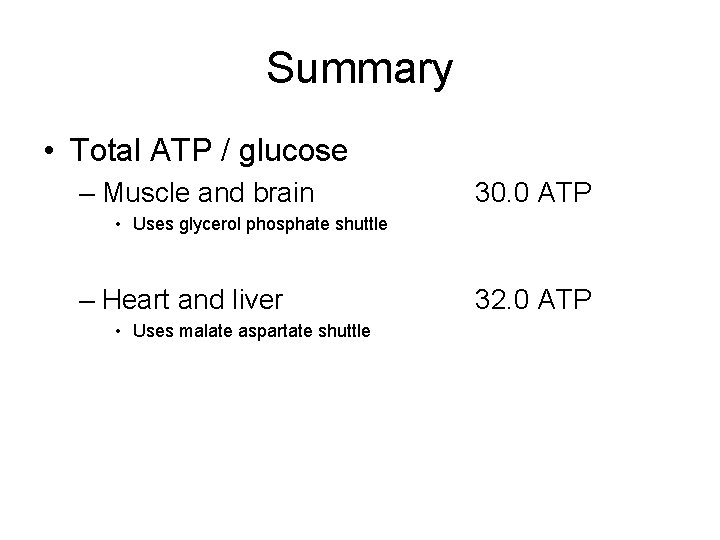
Total ATP per glucose in muscle and brain • Gycerol phosphate shuttle – 2 NADH per glucose - 2 FADH 2 – 2 FADH 2 X 1. 5 ATP / FADH 2………. 3. 0 ATP – 2 ATP in glycoysis………… 2. 0 ATP – From pyruvate and Krebs • 12. 5 ATP X 2 per glucose ……………. . 25. 0 ATP Total = 30. 0 ATP/ glucose

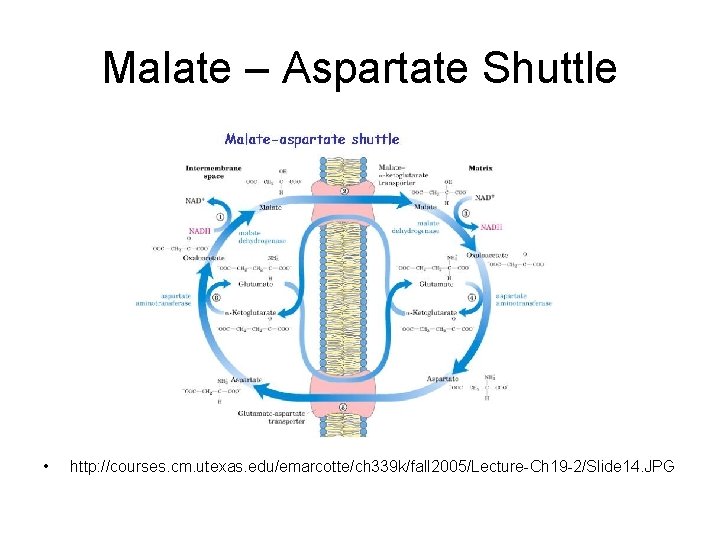
Malate – Aspartate Shuttle • http: //courses. cm. utexas. edu/emarcotte/ch 339 k/fall 2005/Lecture-Ch 19 -2/Slide 14. JPG

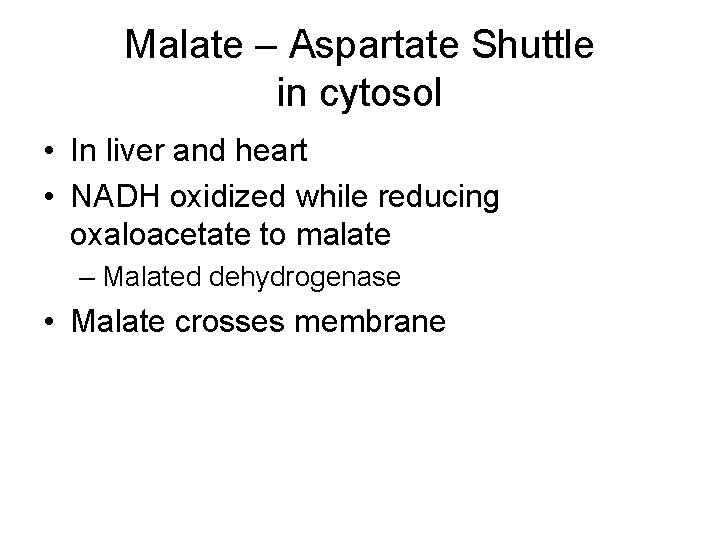
Malate – Aspartate Shuttle in cytosol • In liver and heart • NADH oxidized while reducing oxaloacetate to malate – Malated dehydrogenase • Malate crosses membrane

Malate – Aspartate Shuttle in matrix • Malate reoxidized to oxaloacetate – Malate dehydrogenase – NAD+ reduced to NADH • NADH via electron transport yields 2. 5 ATP

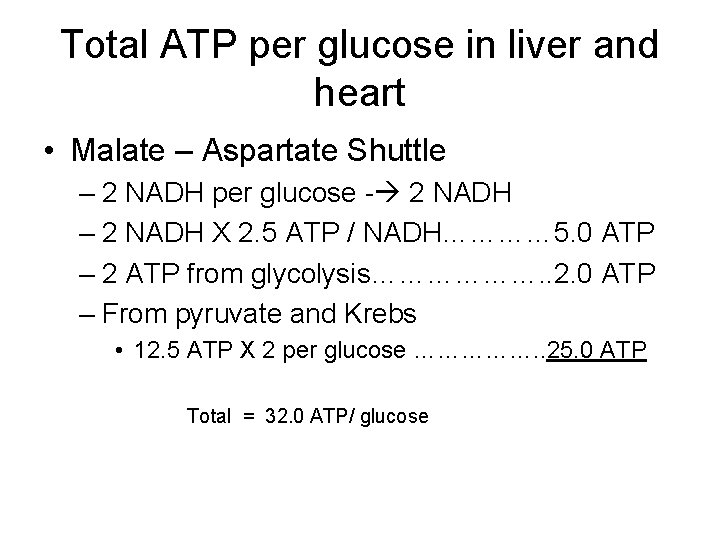
Total ATP per glucose in liver and heart • Malate – Aspartate Shuttle – 2 NADH per glucose - 2 NADH – 2 NADH X 2. 5 ATP / NADH………… 5. 0 ATP – 2 ATP from glycolysis………………. . 2. 0 ATP – From pyruvate and Krebs • 12. 5 ATP X 2 per glucose ……………. . 25. 0 ATP Total = 32. 0 ATP/ glucose

Summary • Total ATP / glucose – Muscle and brain 30. 0 ATP • Uses glycerol phosphate shuttle – Heart and liver • Uses malate aspartate shuttle 32. 0 ATP