Oxidative Phosphorylation and the Electron Transport Chain Glycolysis
- Slides: 6

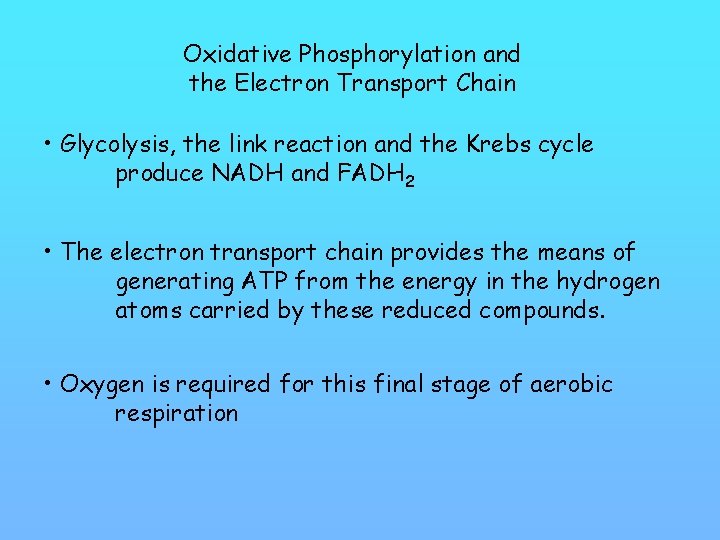
Oxidative Phosphorylation and the Electron Transport Chain • Glycolysis, the link reaction and the Krebs cycle produce NADH and FADH 2 • The electron transport chain provides the means of generating ATP from the energy in the hydrogen atoms carried by these reduced compounds. • Oxygen is required for this final stage of aerobic respiration

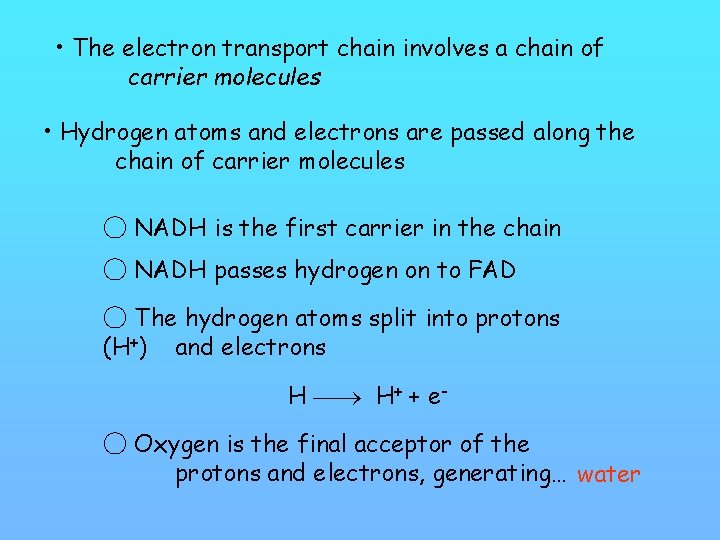
• The electron transport chain involves a chain of carrier molecules • Hydrogen atoms and electrons are passed along the chain of carrier molecules ◯ NADH is the first carrier in the chain ◯ NADH passes hydrogen on to FAD ◯ The hydrogen atoms split into protons (H+) and electrons H H+ + e◯ Oxygen is the final acceptor of the protons and electrons, generating… water

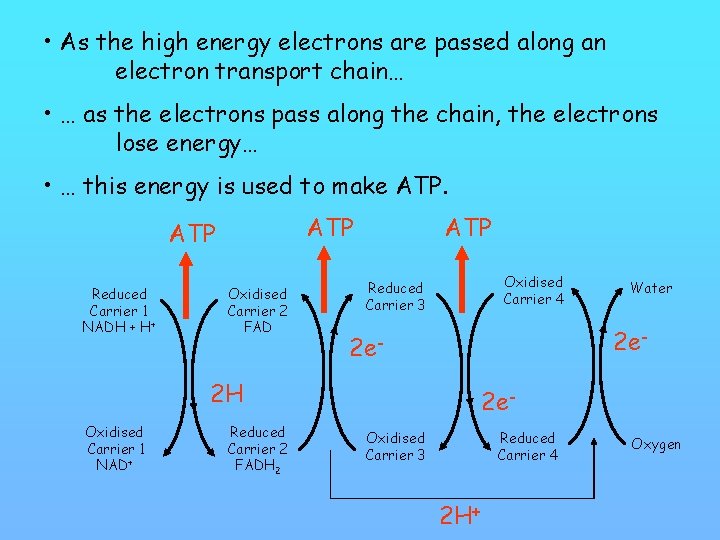
• As the high energy electrons are passed along an electron transport chain… • … as the electrons pass along the chain, the electrons lose energy… • … this energy is used to make ATP Reduced Carrier 1 NADH + H+ Oxidised Carrier 2 FAD ATP Oxidised Carrier 4 Reduced Carrier 3 2 e- 2 H Oxidised Carrier 1 NAD+ Reduced Carrier 2 FADH 2 Water 2 e. Oxidised Carrier 3 Reduced Carrier 4 2 H+ Oxygen

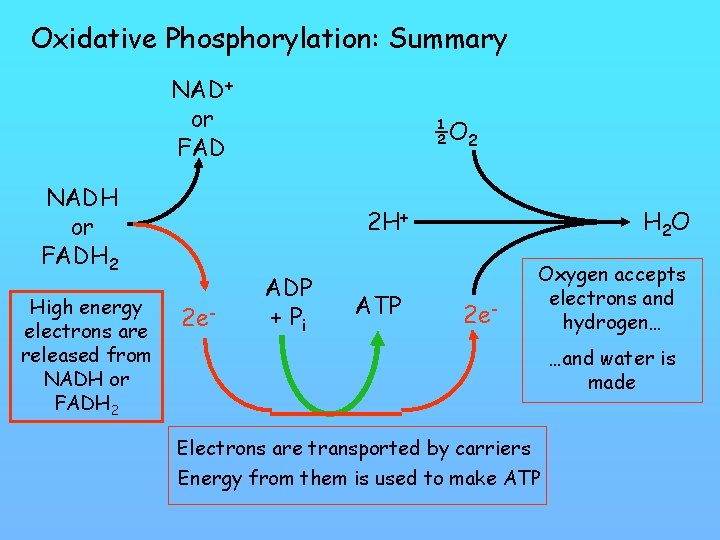
Oxidative Phosphorylation: Summary NAD+ or FAD NADH or FADH 2 High energy electrons are released from NADH or FADH 2 ½O 2 2 H+ 2 e- ADP + Pi ATP H 2 O 2 e- Oxygen accepts electrons and hydrogen… …and water is made Electrons are transported by carriers Energy from them is used to make ATP

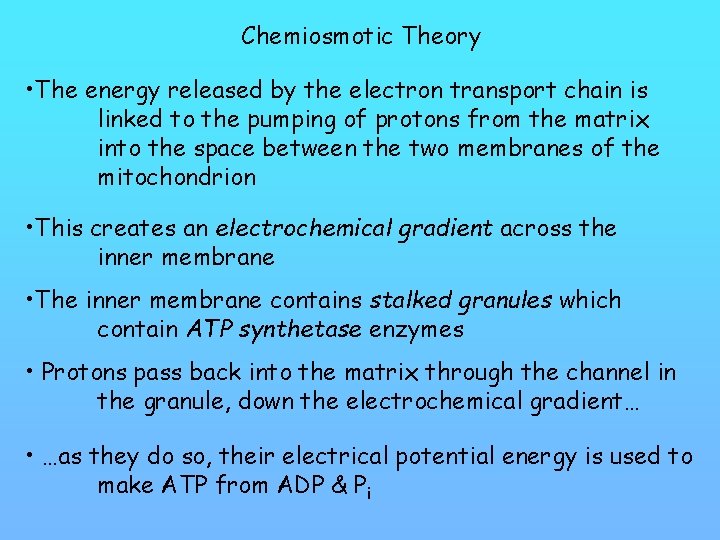
Chemiosmotic Theory • The energy released by the electron transport chain is linked to the pumping of protons from the matrix into the space between the two membranes of the mitochondrion • This creates an electrochemical gradient across the inner membrane • The inner membrane contains stalked granules which contain ATP synthetase enzymes • Protons pass back into the matrix through the channel in the granule, down the electrochemical gradient… • …as they do so, their electrical potential energy is used to make ATP from ADP & Pi

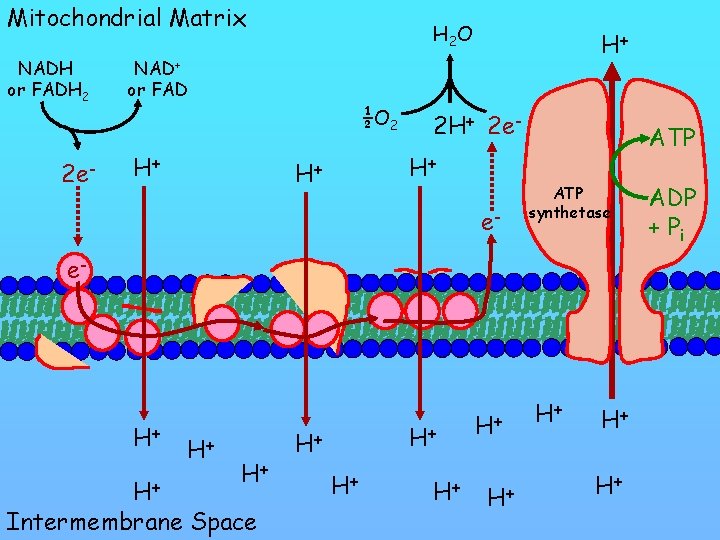
Mitochondrial Matrix NADH or FADH 2 2 e- H 2 O H+ NAD+ or FAD ½O 2 H+ 2 e. H+ H+ e- ATP synthetase e- H+ H+ Intermembrane Space H+ H+ H+ ADP + Pi