EASL 2019 HCV Conference Update April 10 14









![‡ Meta-Analysis Real-World Safety/Effectiveness of GLE/PIB ITT-SVR 12 (Patients [n], Studies [n]) m. ITT-SVR ‡ Meta-Analysis Real-World Safety/Effectiveness of GLE/PIB ITT-SVR 12 (Patients [n], Studies [n]) m. ITT-SVR](https://slidetodoc.com/presentation_image_h2/55c1dd72dcfd390055dcb36f16641a28/image-44.jpg)

- Slides: 54

EASL 2019 HCV Conference Update April 10– 14, 2019 Vienna, Austria

Disclaimer § These non-promotional slides are intended to be used as educational material only in response to an unsolicited question or request § The double-dagger (‡) symbol indicates that these slides may contain information that is not within FDA approved product labeling and has not otherwise been approved by the FDA 2

SOF/VEL

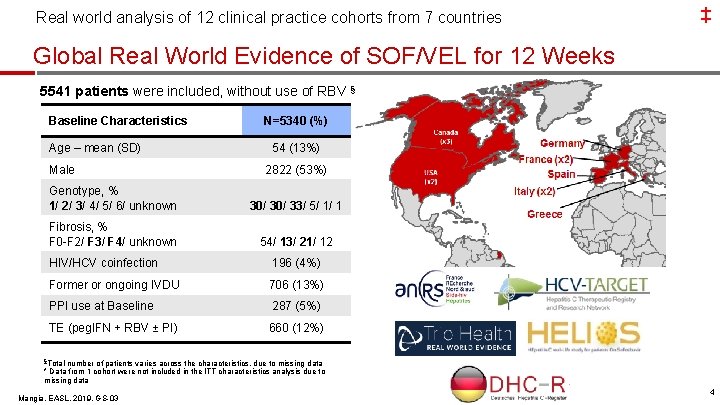
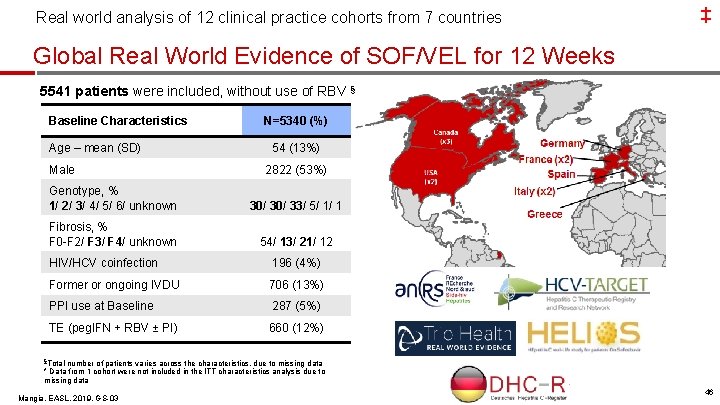
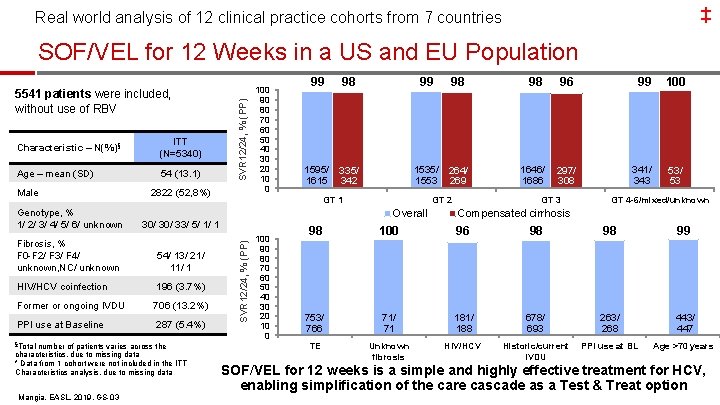
Real world analysis of 12 clinical practice cohorts from 7 countries ‡ Global Real World Evidence of SOF/VEL for 12 Weeks 5541 patients were included, without use of RBV § Baseline Characteristics Age – mean (SD) Male N=5340 (%) 54 (13%) 2822 (53%) Genotype, % 1/ 2/ 3/ 4/ 5/ 6/ unknown 30/ 33/ 5/ 1/ 1 Fibrosis, % F 0 -F 2/ F 3/ F 4/ unknown 54/ 13/ 21/ 12 HIV/HCV coinfection 196 (4%) Former or ongoing IVDU 706 (13%) PPI use at Baseline 287 (5%) TE (peg. IFN + RBV ± PI) 660 (12%) §Total number of patients varies across the characteristics, due to missing data * Data from 1 cohort were not included in the ITT characteristics analysis due to missing data Mangia, EASL, 2019, GS-03 4

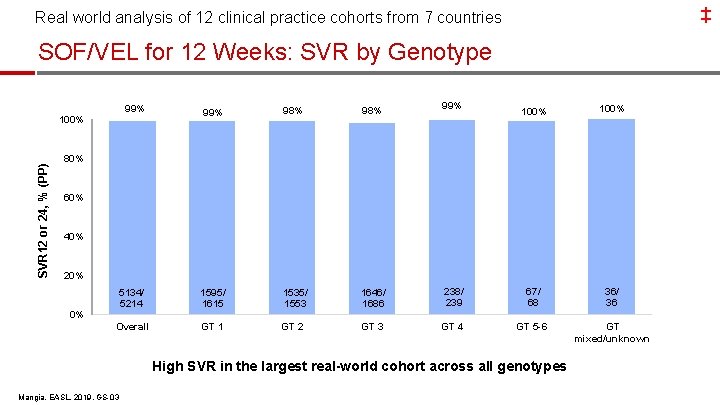
‡ Real world analysis of 12 clinical practice cohorts from 7 countries SOF/VEL for 12 Weeks: SVR by Genotype 99% 98% 5134/ 5214 1595/ 1615 1535/ 1553 1646/ 1686 Overall GT 1 GT 2 GT 3 SVR 12 or 24, % (PP) 100% 99% 100% 238/ 239 67/ 68 36/ 36 GT 4 GT 5 -6 GT mixed/unknown 80% 60% 40% 20% 0% High SVR in the largest real-world cohort across all genotypes Mangia, EASL, 2019, GS-03

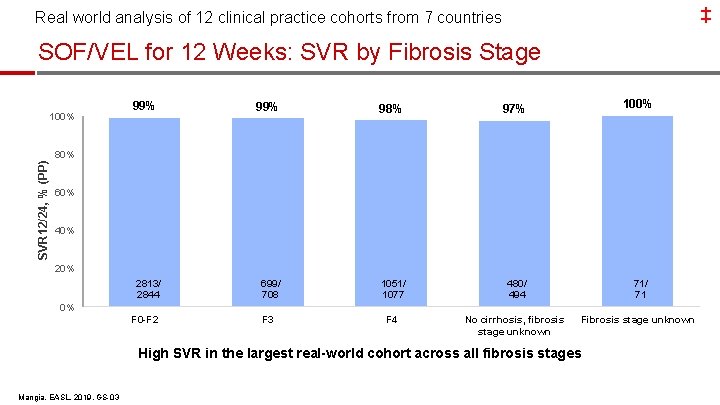
‡ Real world analysis of 12 clinical practice cohorts from 7 countries SOF/VEL for 12 Weeks: SVR by Fibrosis Stage 100% 99% 98% SVR 12/24, % (PP) 80% 100% 97% 264/ 269 60% 40% 2813/ 2844 699/ 708 1051/ 1077 480/ 494 71/ 71 F 3 F 4 No cirrhosis, fibrosis stage unknown Fibrosis stage unknown 0% F 0 -F 2 High SVR in the largest real-world cohort across all fibrosis stages Mangia, EASL, 2019, GS-03

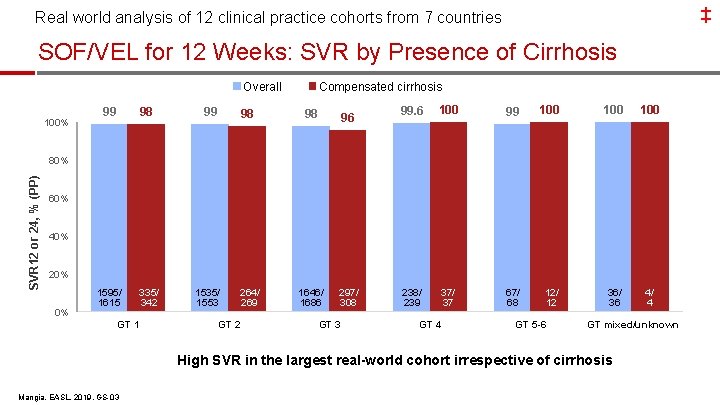
‡ Real world analysis of 12 clinical practice cohorts from 7 countries SOF/VEL for 12 Weeks: SVR by Presence of Cirrhosis Overall 100% Compensated cirrhosis 99 98 98 96 1595/ 1615 335/ 342 1535/ 1553 264/ 269 1646/ 1686 297/ 308 99. 6 100 99 100 100 238/ 239 37/ 37 67/ 68 12/ 12 36/ 36 4/ 4 SVR 12 or 24, % (PP) 80% 60% 40% 20% 0% GT 1 GT 2 GT 3 GT 4 GT 5 -6 GT mixed/unknown High SVR in the largest real-world cohort irrespective of cirrhosis Mangia, EASL, 2019, GS-03

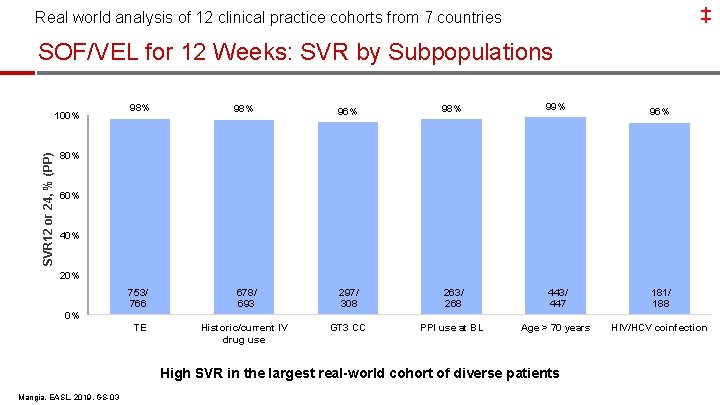
‡ Real world analysis of 12 clinical practice cohorts from 7 countries SOF/VEL for 12 Weeks: SVR by Subpopulations SVR 12 or 24, % (PP) 100% 98% 96% 98% 99% 753/ 766 678/ 693 297/ 308 263/ 268 443/ 447 181/ 188 TE Historic/current IV drug use GT 3 CC PPI use at BL Age > 70 years HIV/HCV coinfection 96% 80% 60% 40% 20% 0% High SVR in the largest real-world cohort of diverse patients Mangia, EASL, 2019, GS-03

Real world analysis of 12 clinical practice cohorts from 7 countries ‡ Conclusion: Largest Real World Cohort With SOF/VEL § High effectiveness of SOF/VEL in diverse patient populations, regardless of: – Genotype – Fibrosis stage – Prior treatment (peg. IFN + RBV ± PI) – Patient characteristics (IV drug use, PPI use, older age, HIV/HCV Co-infection) § Simplification of HCV Care Cascade is possible with SOF/VEL § A Test and Treat strategy with SOF/VEL may further improve HCV care Mangia, EASL, 2019, GS-03 9

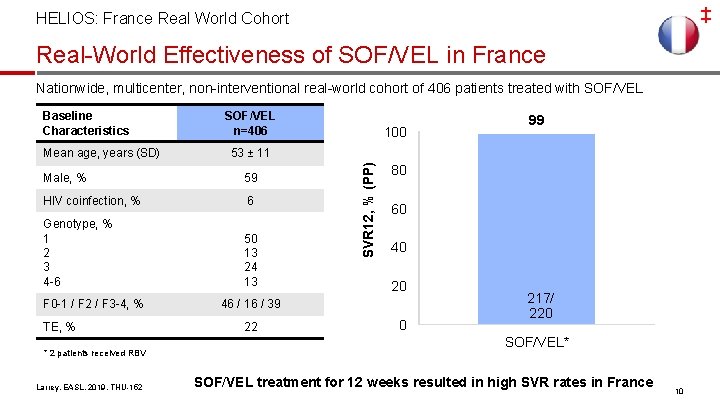
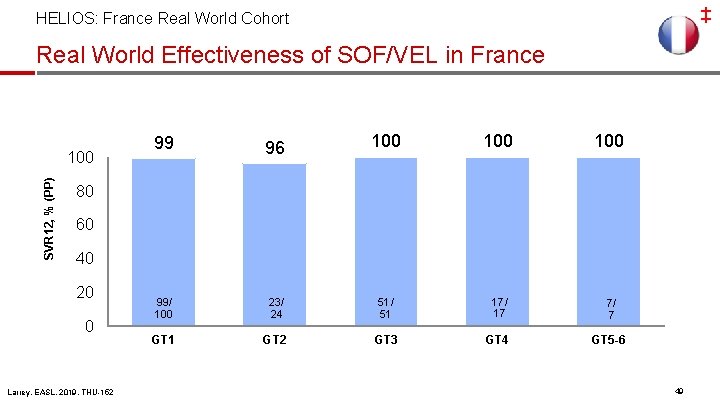
‡ HELIOS: France Real World Cohort Real-World Effectiveness of SOF/VEL in France Nationwide, multicenter, non-interventional real-world cohort of 406 patients treated with SOF/VEL Mean age, years (SD) SOF/VEL n=406 59 HIV coinfection, % 6 Genotype, % 1 2 3 4 -6 50 13 24 13 TE, % * 2 patients received RBV Larrey, EASL, 2019, THU-152 99 53 ± 11 Male, % F 0 -1 / F 2 / F 3 -4, % 100 SVR 12, % (PP) Baseline Characteristics 80 60 40 20 46 / 16 / 39 22 0 217/ 220 SOF/VEL* SOF/VEL treatment for 12 weeks resulted in high SVR rates in France 10

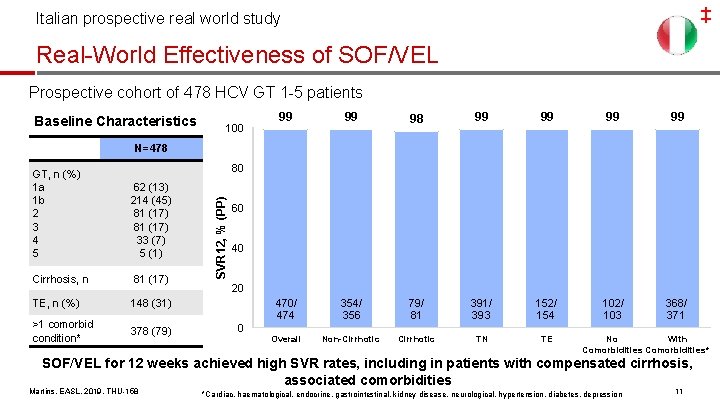
‡ Italian prospective real world study Real-World Effectiveness of SOF/VEL Prospective cohort of 478 HCV GT 1 -5 patients Baseline Characteristics 100 99 99 98 99 99 470/ 474 354/ 356 79/ 81 391/ 393 152/ 154 102/ 103 368/ 371 Overall Non-Cirrhotic TN TE N=478 62 (13) 214 (45) 81 (17) 33 (7) 5 (1) Cirrhosis, n 81 (17) TE, n (%) 148 (31) >1 comorbid condition* 378 (79) SVR 12, % (PP) 80 GT, n (%) 1 a 1 b 2 3 4 5 60 40 20 0 No With Comorbidities* SOF/VEL for 12 weeks achieved high SVR rates, including in patients with compensated cirrhosis, associated comorbidities Martins, EASL, 2019, THU-158 *Cardiac, haematological, endocrine, gastrointestinal, kidney disease, neurological, hypertension, diabetes, depression 11

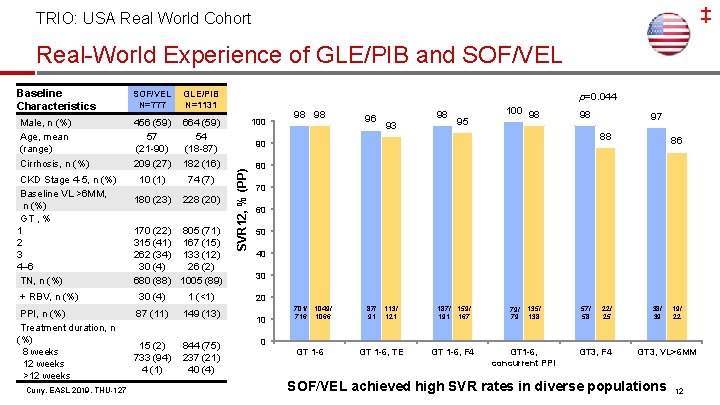
‡ TRIO: USA Real World Cohort Real-World Experience of GLE/PIB and SOF/VEL N=777 GLE/PIB N=1131 Male, n (%) Age, mean (range) Cirrhosis, n (%) 456 (59) 57 (21 -90) 209 (27) 664 (59) 54 (18 -87) 182 (16) 10 (1) 74 (7) 180 (23) 228 (20) CKD Stage 4 -5, n (%) Baseline VL >6 MM, n (%) GT , % 1 2 3 4– 6 TN, n (%) 170 (22) 805 (71) 315 (41) 167 (15) 262 (34) 133 (12) 30 (4) 26 (2) 680 (88) 1005 (89) + RBV, n (%) 30 (4) 1 (<1) PPI, n (%) Treatment duration, n (%) 8 weeks 12 weeks >12 weeks 87 (11) 149 (13) 15 (2) 733 (94) 4 (1) 844 (75) 237 (21) 40 (4) Curry, EASL 2019, THU-127 p=0. 044 100 98 98 96 93 98 95 100 98 98 97 88 90 SVR 12, % (PP) Baseline Characteristics 86 80 70 60 50 40 30 20 10 701/ 1049/ 716 1066 87/ 91 113/ 121 187/ 159/ 191 167 79/ 79 135/ 138 57/ 58 22/ 25 38/ 39 19/ 22 0 GT 1 -6, TE GT 1 -6, F 4 GT 1 -6, concurrent PPI GT 3, F 4 GT 3, VL>6 MM SOF/VEL achieved high SVR rates in diverse populations 12

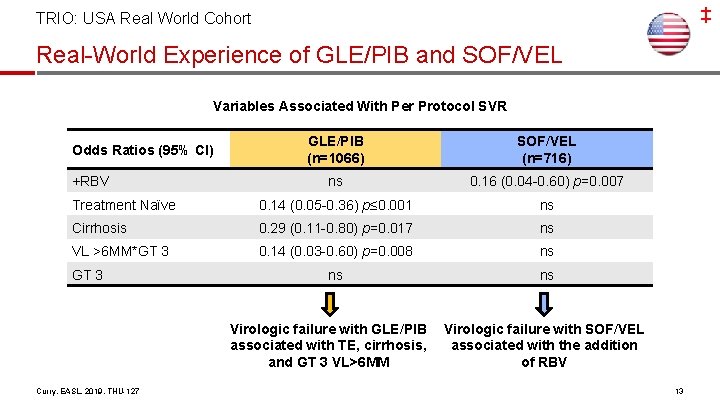
‡ TRIO: USA Real World Cohort Real-World Experience of GLE/PIB and SOF/VEL Variables Associated With Per Protocol SVR GLE/PIB (n=1066) SOF/VEL (n=716) ns 0. 16 (0. 04 -0. 60) p=0. 007 Treatment Naïve 0. 14 (0. 05 -0. 36) p≤ 0. 001 ns Cirrhosis 0. 29 (0. 11 -0. 80) p=0. 017 ns VL >6 MM*GT 3 0. 14 (0. 03 -0. 60) p=0. 008 ns ns ns Odds Ratios (95% CI) +RBV GT 3 Virologic failure with GLE/PIB associated with TE, cirrhosis, and GT 3 VL>6 MM Curry, EASL, 2019, THU-127 Virologic failure with SOF/VEL associated with the addition of RBV 13

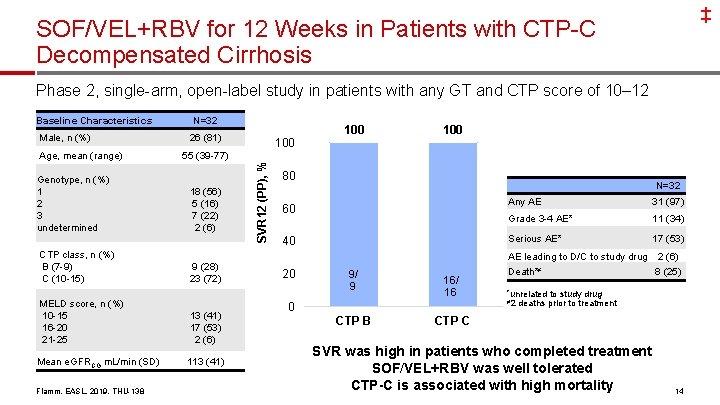
‡ SOF/VEL+RBV for 12 Weeks in Patients with CTP-C Decompensated Cirrhosis Phase 2, single-arm, open-label study in patients with any GT and CTP score of 10– 12 Male, n (%) Age, mean (range) Genotype, n (%) 1 2 3 undetermined CTP class, n (%) B (7 -9) C (10 -15) N=32 26 (81) 55 (39 -77) 18 (56) 5 (16) 7 (22) 2 (6) 9 (28) 23 (72) MELD score, n (%) 10 -15 16 -20 21 -25 13 (41) 17 (53) 2 (6) Mean e. GFRCG m. L/min (SD) 113 (41) Flamm, EASL, 2019, THU-138 100 SVR 12 (PP), % Baseline Characteristics 100 80 N=32 Any AE 60 16/ Grade 3 -4 23 AE* Serious AE* 40 20 9/ 9 16/ 16 0 CTP B 31 (97) 11 (34) 17 (53) AE leading to D/C to study drug 2 (6) Death*≠ 8 (25) *unrelated ≠ 2 to study drug deaths prior to treatment CTP C SVR was high in patients who completed treatment SOF/VEL+RBV was well tolerated CTP-C is associated with high mortality 14

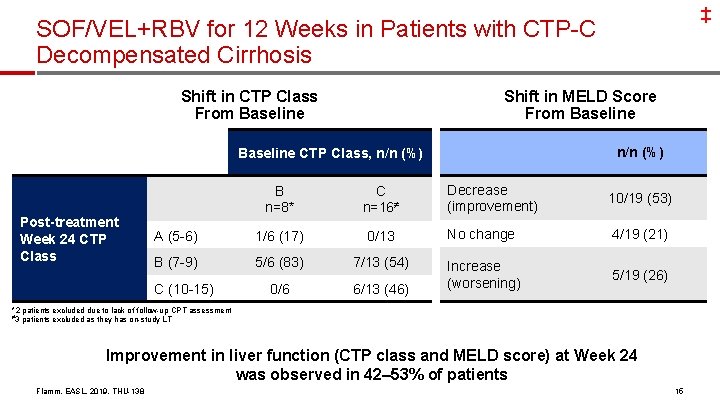
‡ SOF/VEL+RBV for 12 Weeks in Patients with CTP-C Decompensated Cirrhosis Shift in CTP Class From Baseline Shift in MELD Score From Baseline n/n (%) Baseline CTP Class, n/n (%) Post-treatment Week 24 CTP Class Decrease (improvement) 10/19 (53) 0/13 No change 4/19 (21) 5/6 (83) 7/13 (54) 0/6 6/13 (46) Increase (worsening) 5/19 (26) B n=8* C n=16≠ A (5 -6) 1/6 (17) B (7 -9) C (10 -15) *2 patients excluded due to lack of follow-up CPT assessment ≠ 3 patients excluded as they has on-study LT Improvement in liver function (CTP class and MELD score) at Week 24 was observed in 42– 53% of patients Flamm, EASL, 2019, THU-138 15

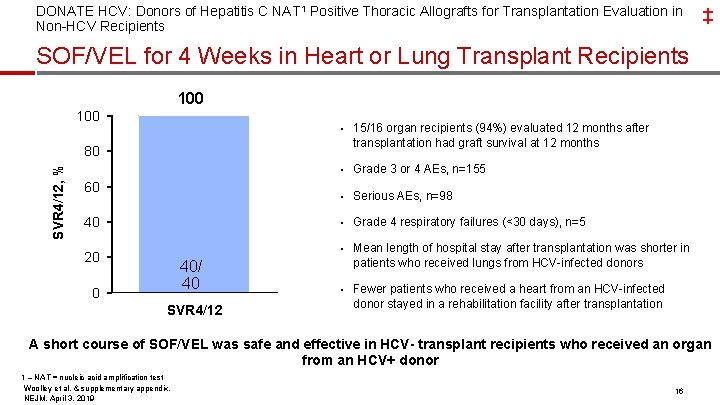
DONATE HCV: Donors of Hepatitis C NAT 1 Positive Thoracic Allografts for Transplantation Evaluation in Non-HCV Recipients ‡ SOF/VEL for 4 Weeks in Heart or Lung Transplant Recipients 100 • 15/16 organ recipients (94%) evaluated 12 months after transplantation had graft survival at 12 months SVR 4/12, % 80 • Grade 3 or 4 AEs, n=155 60 • Serious AEs, n=98 40 • Grade 4 respiratory failures (<30 days), n=5 • Mean length of hospital stay after transplantation was shorter in 20 40/ 40 0 SVR 4/12 patients who received lungs from HCV-infected donors • Fewer patients who received a heart from an HCV-infected donor stayed in a rehabilitation facility after transplantation A short course of SOF/VEL was safe and effective in HCV- transplant recipients who received an organ from an HCV+ donor 1 – NAT = nucleic acid amplification test Woolley et al, & supplementary appendix, NEJM, April 3, 2019 16

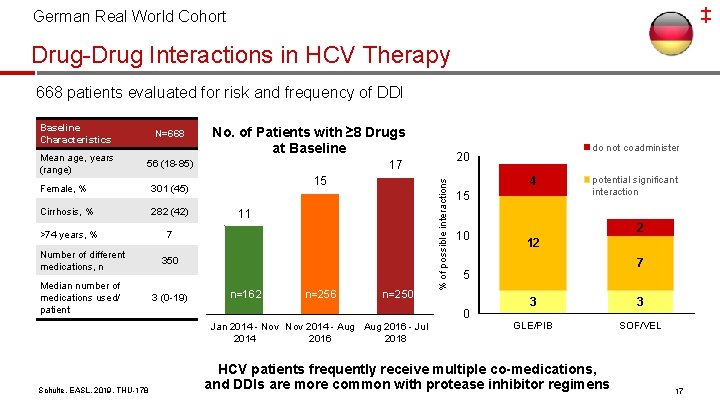
‡ German Real World Cohort Drug-Drug Interactions in HCV Therapy 668 patients evaluated for risk and frequency of DDI Mean age, years (range) N=668 No. of Patients with ≥ 8 Drugs at Baseline 56 (18 -85) Female, % 301 (45) Cirrhosis, % 282 (42) >74 years, % 7 Number of different medications, n 350 Median number of medications used/ patient 3 (0 -19) 17 15 11 n=162 n=256 n=250 Schulte, EASL, 2019, THU-178 4 15 10 potential significant interaction 2 12 7 5 0 Jan 2014 - Nov 2014 - Aug 2016 - Jul 2014 2016 2018 do not coadminister 20 % of possible interactions Baseline Characteristics 3 3 GLE/PIB SOF/VEL HCV patients frequently receive multiple co-medications, and DDIs are more common with protease inhibitor regimens 17

SOF/VEL/VOX

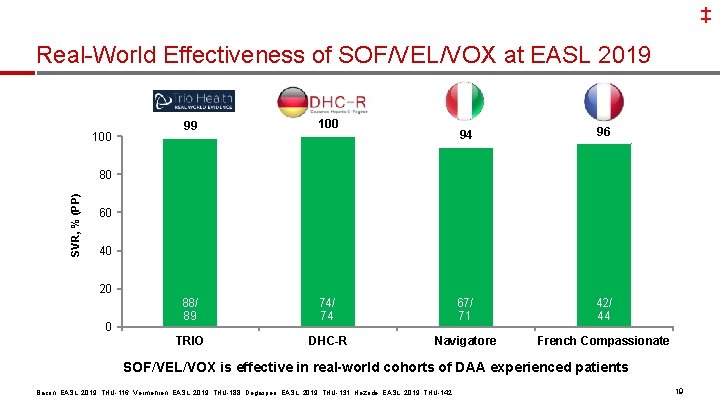
‡ Real-World Effectiveness of SOF/VEL/VOX at EASL 2019 100 99 100 88/ 89 TRIO 94 96 74/ 74 67/ 71 42/ 44 DHC-R Navigatore French Compassionate SVR, % (PP) 80 60 40 20 0 SOF/VEL/VOX is effective in real-world cohorts of DAA experienced patients Bacon, EASL, 2019, THU-116; Vermehren, EASL, 2019, THU-188; Degasperi, EASL, 2019, THU-131; Hezode, EASL, 2019, THU-142 19

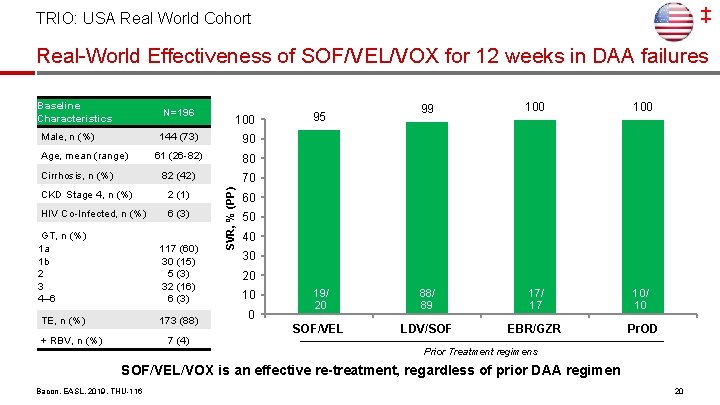
‡ TRIO: USA Real World Cohort Real-World Effectiveness of SOF/VEL/VOX for 12 weeks in DAA failures N=196 Male, n (%) Age, mean (range) Cirrhosis, n (%) 144 (73) 90 61 (26 -82) 80 82 (42) 70 CKD Stage 4, n (%) 2 (1) HIV Co-Infected, n (%) 6 (3) GT, n (%) 1 a 1 b 2 3 4– 6 117 (60) 30 (15) 5 (3) 32 (16) 6 (3) TE, n (%) 173 (88) + RBV, n (%) 100 7 (4) SVR, % (PP) Baseline Characteristics 99 100 19/ 20 88/ 89 17/ 17 10/ 10 SOF/VEL LDV/SOF EBR/GZR Pr. OD 95 60 50 40 30 20 10 0 Prior Treatment regimens SOF/VEL/VOX is an effective re-treatment, regardless of prior DAA regimen Bacon, EASL, 2019, THU-116 20

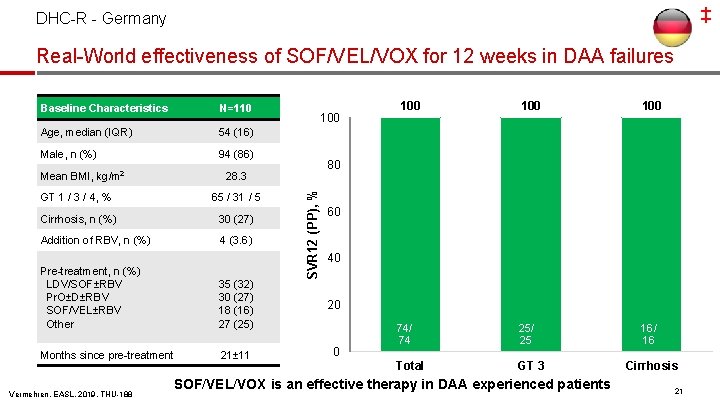
‡ DHC-R - Germany Real-World effectiveness of SOF/VEL/VOX for 12 weeks in DAA failures Baseline Characteristics N=110 Age, median (IQR) 54 (16) Male, n (%) 94 (86) GT 1 / 3 / 4, % 65 / 31 / 5 Cirrhosis, n (%) 30 (27) Addition of RBV, n (%) 4 (3. 6) Pre-treatment, n (%) LDV/SOF±RBV Pr. O±D±RBV SOF/VEL±RBV Other 35 (32) 30 (27) 18 (16) 27 (25) Months since pre-treatment 21± 11 Vermehren, EASL, 2019, THU-188 100 100 74/ 74 25/ 25 16/ 16 Total GT 3 80 28. 3 SVR 12 (PP), % Mean BMI, kg/m 2 100 60 40 20 0 SOF/VEL/VOX is an effective therapy in DAA experienced patients Cirrhosis 21

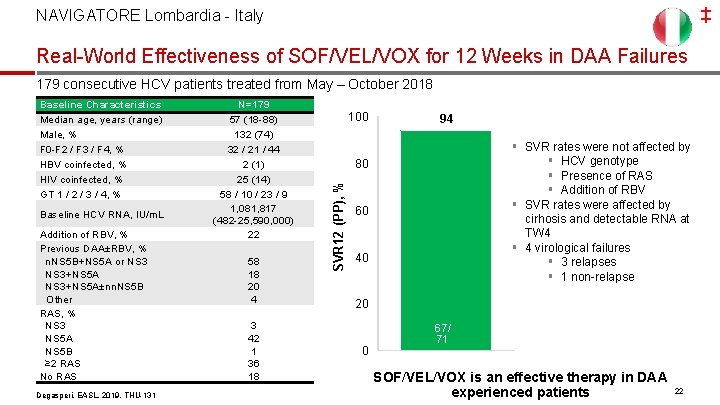
‡ NAVIGATORE Lombardia - Italy Real-World Effectiveness of SOF/VEL/VOX for 12 Weeks in DAA Failures 179 consecutive HCV patients treated from May – October 2018 Baseline HCV RNA, IU/m. L Addition of RBV, % Previous DAA±RBV, % n. NS 5 B+NS 5 A or NS 3+NS 5 A±nn. NS 5 B Other RAS, % NS 3 NS 5 A NS 5 B ≥ 2 RAS No RAS Degasperi, EASL, 2019, THU-131 N=179 57 (18 -88) 132 (74) 32 / 21 / 44 2 (1) 25 (14) 58 / 10 / 23 / 9 1, 081, 817 (482 -25, 590, 000) 22 58 18 20 4 3 42 1 36 18 100 94 § SVR rates were not affected by § HCV genotype § Presence of RAS § Addition of RBV § SVR rates were affected by cirhosis and detectable RNA at TW 4 § 4 virological failures § 3 relapses § 1 non-relapse 80 SVR 12 (PP), % Baseline Characteristics Median age, years (range) Male, % F 0 -F 2 / F 3 / F 4, % HBV coinfected, % HIV coinfected, % GT 1 / 2 / 3 / 4, % 60 40 20 0 67/ 71 SOF/VEL/VOX is an effective therapy in DAA experienced patients 22

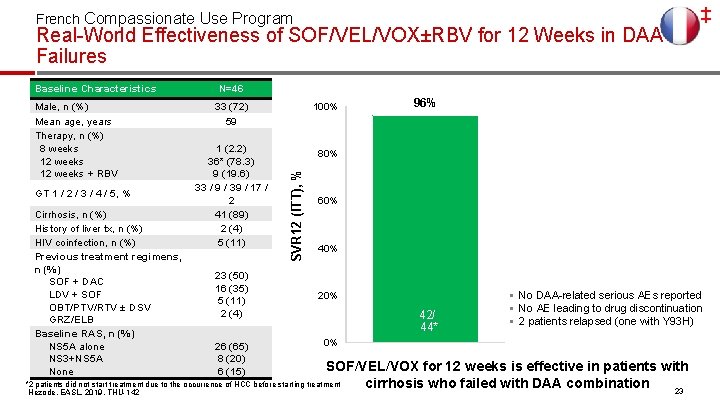
French Compassionate Use Program Real-World Effectiveness of SOF/VEL/VOX±RBV for 12 Weeks in DAA Failures Male, n (%) Mean age, years Therapy, n (%) 8 weeks 12 weeks + RBV GT 1 / 2 / 3 / 4 / 5, % Cirrhosis, n (%) History of liver tx, n (%) HIV coinfection, n (%) Previous treatment regimens, n (%) SOF + DAC LDV + SOF OBT/PTV/RTV ± DSV GRZ/ELB Baseline RAS, n (%) NS 5 A alone NS 3+NS 5 A None N=46 33 (72) 59 1 (2. 2) 36* (78. 3) 9 (19. 6) 33 / 9 / 39 / 17 / 2 41 (89) 2 (4) 5 (11) 23 (50) 16 (35) 5 (11) 2 (4) 26 (65) 8 (20) 6 (15) 100% 96% 80% SVR 12 (ITT), % Baseline Characteristics 60% 40% 20% 42/ 44* § No DAA-related serious AEs reported § No AE leading to drug discontinuation § 2 patients relapsed (one with Y 93 H) 0% SOF/VEL/VOX for 12 weeks is effective in patients with *2 patients did not start treatment due to the occurrence of HCC before starting treatment cirrhosis who failed with DAA combination 23 Hezode, EASL, 2019, THU-142 ‡

Improving the Care Cascade

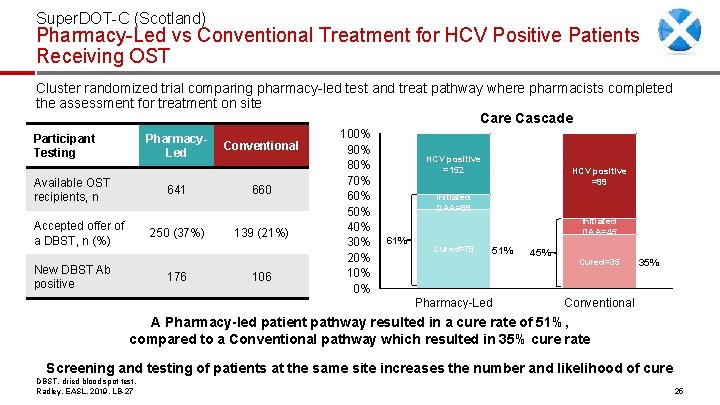
Super. DOT-C (Scotland) Pharmacy-Led vs Conventional Treatment for HCV Positive Patients Receiving OST Cluster randomized trial comparing pharmacy-led test and treat pathway where pharmacists completed the assessment for treatment on site Care Cascade Participant Testing Pharmacy. Led Available OST recipients, n Accepted offer of a DBST, n (%) New DBST Ab positive Conventional 641 660 250 (37%) 139 (21%) 176 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% HCV positive =152 HCV positive =99 Initiated DAA=98 61% Initiated DAA=45 Cured=78 51% Pharmacy-Led 45% Cured=35 35% Conventional A Pharmacy-led patient pathway resulted in a cure rate of 51%, compared to a Conventional pathway which resulted in 35% cure rate Screening and testing of patients at the same site increases the number and likelihood of cure DBST, dried blood spot test. Radley, EASL, 2019, LB-27 25

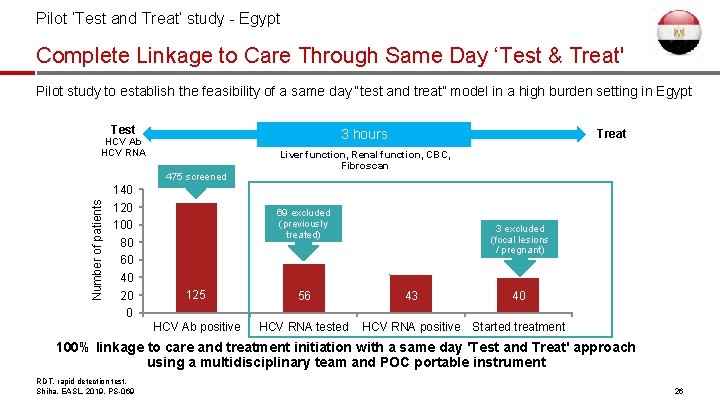
Pilot ‘Test and Treat’ study - Egypt Complete Linkage to Care Through Same Day ‘Test & Treat' Pilot study to establish the feasibility of a same day “test and treat” model in a high burden setting in Egypt Test 3 hours HCV Ab HCV RNA Number of patients 475 screened 140 120 100 80 60 40 20 0 Treat Liver function, Renal function, CBC, Fibroscan 69 excluded (previously treated) 125 56 HCV Ab positive HCV RNA tested 3 excluded (focal lesions / pregnant) 43 40 HCV RNA positive Started treatment 100% linkage to care and treatment initiation with a same day 'Test and Treat' approach using a multidisciplinary team and POC portable instrument RDT, rapid detection test. Shiha, EASL, 2019, PS-069 26

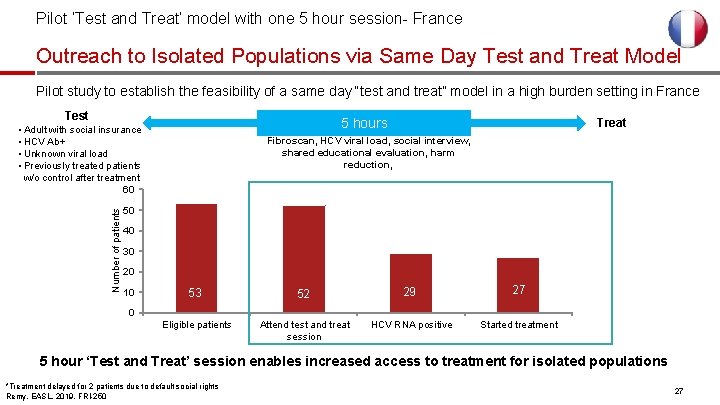
Pilot ‘Test and Treat’ model with one 5 hour session- France Outreach to Isolated Populations via Same Day Test and Treat Model Pilot study to establish the feasibility of a same day “test and treat” model in a high burden setting in France Test 5 hours Number of patients • Adult with social insurance • HCV Ab+ • Unknown viral load • Previously treated patients w/o control after treatment 60 Treat Fibroscan, HCV viral load, social interview, shared educational evaluation, harm reduction, 50 40 30 20 10 53 52 29 27 Eligible patients Attend test and treat session HCV RNA positive Started treatment 0 5 hour ‘Test and Treat’ session enables increased access to treatment for isolated populations *Treatment delayed for 2 patients due to default social rights Remy, EASL, 2019, FRI-250 27

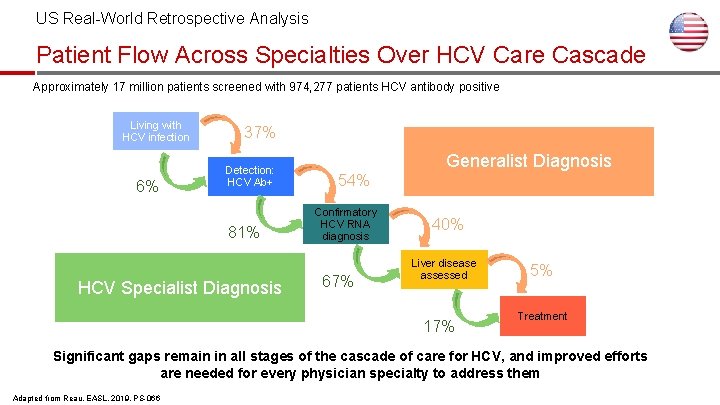
US Real-World Retrospective Analysis Patient Flow Across Specialties Over HCV Care Cascade Approximately 17 million patients screened with 974, 277 patients HCV antibody positive Living with HCV infection 6% 37% Detection: HCV Ab+ 81% HCVSpecialist diagnosis HCV Diagnosis Generalist Diagnosis 54% Confirmatory HCV RNA diagnosis 67% 40% Liver disease assessed 17% 5% Treatment Significant gaps remain in all stages of the cascade of care for HCV, and improved efforts are needed for every physician specialty to address them Adapted from Reau, EASL, 2019, PS-066

Elimination

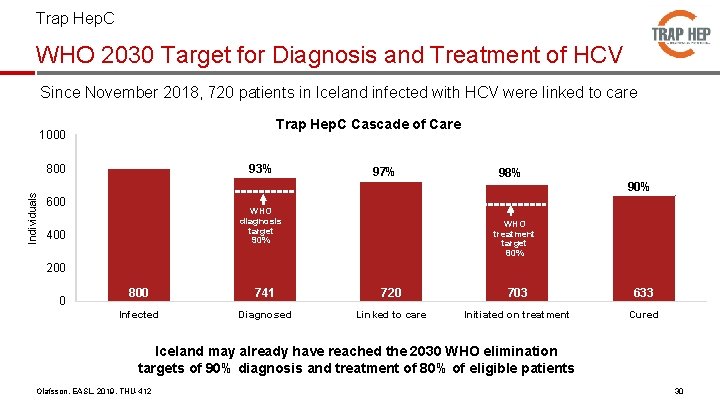
Trap Hep. C WHO 2030 Target for Diagnosis and Treatment of HCV Since November 2018, 720 patients in Iceland infected with HCV were linked to care Trap Hep. C Cascade of Care 1000 Individuals 800 93% 600 97% WHO diagnosis target 90% 400 98% 90% WHO treatment target 80% 200 0 800 741 720 703 633 Infected Diagnosed Linked to care Initiated on treatment Cured Iceland may already have reached the 2030 WHO elimination targets of 90% diagnosis and treatment of 80% of eligible patients Olafsson, EASL, 2019, THU-412 30

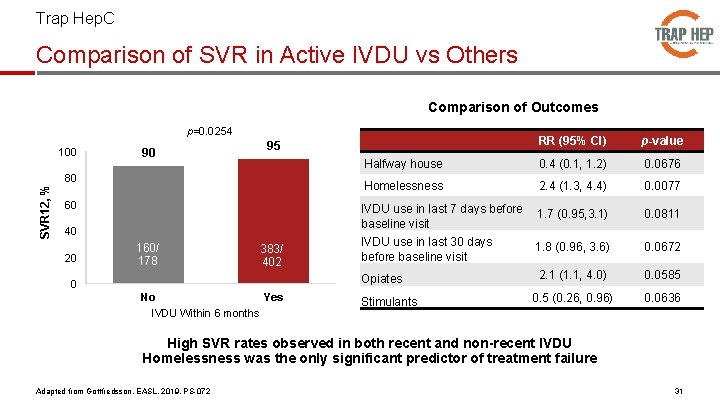
Trap Hep. C Comparison of SVR in Active IVDU vs Others Comparison of Outcomes p=0. 0254 SVR 12, % 100 90 80 60 40 20 160/ 178 RR (95% CI) p-value Halfway house 0. 4 (0. 1, 1. 2) 0. 0676 Homelessness 2. 4 (1. 3, 4. 4) 0. 0077 IVDU use in last 7 days before baseline visit 1. 7 (0. 95, 3. 1) 0. 0811 IVDU use in last 30 days before baseline visit 1. 8 (0. 96, 3. 6) 0. 0672 Opiates 2. 1 (1. 1, 4. 0) 0. 0585 0. 5 (0. 26, 0. 96) 0. 0636 95 383/ 402 0 No Yes IVDU Within 6 months Stimulants High SVR rates observed in both recent and non-recent IVDU Homelessness was the only significant predictor of treatment failure Adapted from Gottfredsson, EASL, 2019, PS-072 31

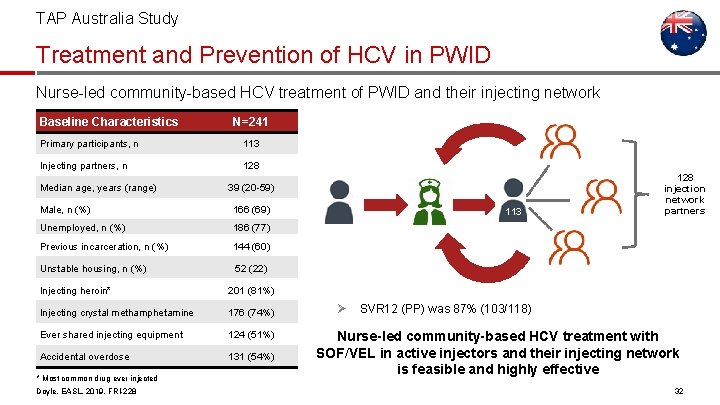
TAP Australia Study Treatment and Prevention of HCV in PWID Nurse-led community-based HCV treatment of PWID and their injecting network Baseline Characteristics N=241 Primary participants, n 113 Injecting partners, n 128 Median age, years (range) 39 (20 -59) Male, n (%) 166 (69) Unemployed, n (%) 186 (77) Previous incarceration, n (%) 144 (60) Unstable housing, n (%) 52 (22) Injecting heroin* 201 (81%) Injecting crystal methamphetamine 176 (74%) Ever shared injecting equipment 124 (51%) Accidental overdose 131 (54%) * Most common drug ever injected Doyle, EASL, 2019, FRI-228 113 Ø 128 injection network partners 103/ 118 was 87% (103/118) SVR 12 (PP) Nurse-led community-based HCV treatment with SOF/VEL in active injectors and their injecting network is feasible and highly effective 32

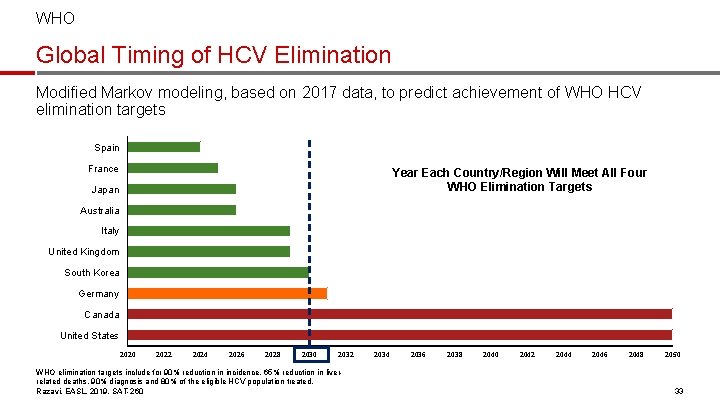
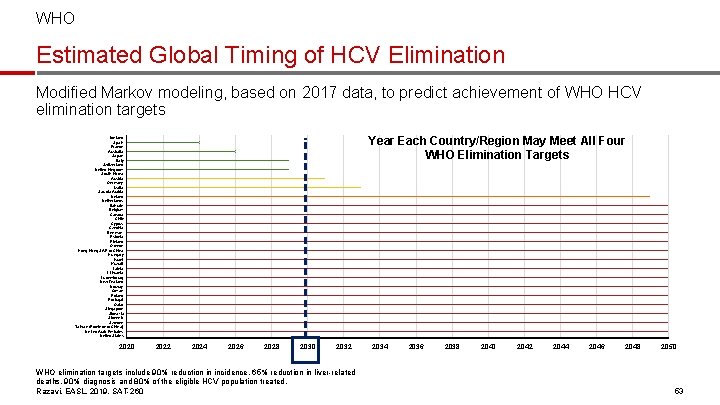
WHO Global Timing of HCV Elimination Modified Markov modeling, based on 2017 data, to predict achievement of WHO HCV elimination targets Spain France Year Each Country/Region Will Meet All Four WHO Elimination Targets Japan Australia Italy United Kingdom South Korea Germany Canada United States 2020 2022 2024 2026 2028 2030 2032 WHO elimination targets include for 90% reduction in incidence, 65% reduction in liverrelated deaths, 90% diagnosis and 80% of the eligible HCV population treated. Razavi, EASL, 2019, SAT-260 2034 2036 2038 2040 2042 2044 2046 2048 2050 33

SOF-based Regimens in Special Populations

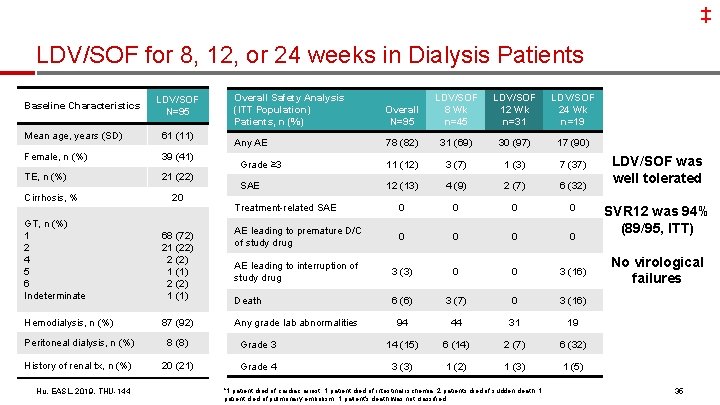
‡ LDV/SOF for 8, 12, or 24 weeks in Dialysis Patients Baseline Characteristics LDV/SOF N=95 Mean age, years (SD) 61 (11) Female, n (%) 39 (41) TE, n (%) 21 (22) Cirrhosis, % GT, n (%) 1 2 4 5 6 Indeterminate Hemodialysis, n (%) 20 68 (72) 21 (22) 2 (2) 1 (1) 87 (92) Overall Safety Analysis (ITT Population) Patients, n (%) Overall N=95 LDV/SOF 8 Wk n=45 LDV/SOF 12 Wk n=31 LDV/SOF 24 Wk n=19 Any AE 78 (82) 31 (69) 30 (97) 17 (90) Grade ≥ 3 11 (12) 3 (7) 1 (3) 7 (37) SAE 12 (13) 4 (9) 2 (7) 6 (32) Treatment-related SAE 0 0 AE leading to premature D/C of study drug 0 0 AE leading to interruption of 95 study drug 3 (3) 0 0 3 (16) Death 6 (6) 342/ (7) 0 94 44 31 31/3 (16) 31 89/ Any grade lab abnormalities 45 8 (8) Grade 3 14 (15) 6 (14) 2 (7) 6 (32) History of renal tx, n (%) 20 (21) Grade 4 3 (3) 1 (2) 1 (3) 1 (5) *1 patient died of cardiac arrest, 1 patient died of intestinal ischemia, 2 patients died of sudden death, 1 patient died of pulmonary embolism, 1 patient's death was not classified SVR 12 was 94% (89/95, ITT) No virological failures 19 Peritoneal dialysis, n (%) Hu, EASL, 2019, THU-144 LDV/SOF was well tolerated 35

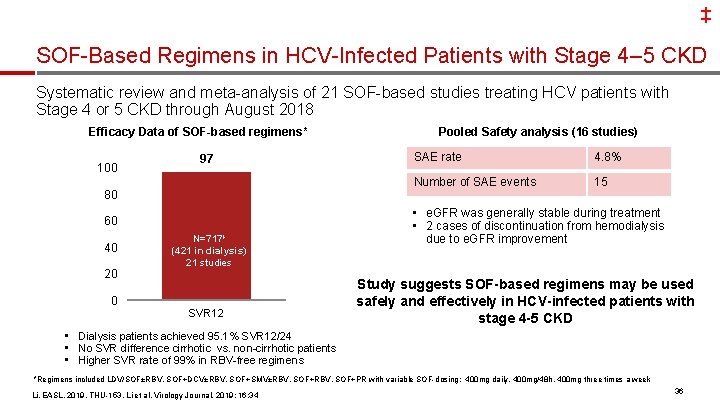
‡ SOF-Based Regimens in HCV-Infected Patients with Stage 4– 5 CKD Systematic review and meta-analysis of 21 SOF-based studies treating HCV patients with Stage 4 or 5 CKD through August 2018 Efficacy Data of SOF-based regimens* 100 97 80 60 40 20 N=717ⱡ (421 in dialysis) 21 studies 0 SVR 12 Pooled Safety analysis (16 studies) SAE rate 4. 8% Number of SAE events 15 • e. GFR was generally stable during treatment • 2 cases of discontinuation from hemodialysis due to e. GFR improvement Study suggests SOF-based regimens may be used safely and effectively in HCV-infected patients with stage 4 -5 CKD • Dialysis patients achieved 95. 1% SVR 12/24 • No SVR difference cirrhotic vs. non-cirrhotic patients • Higher SVR rate of 99% in RBV-free regimens *Regimens included LDV/SOF±RBV, SOF+DCV±RBV, SOF+SMV±RBV, SOF+PR with variable SOF-dosing: 400 mg daily, 400 mg/48 h, 400 mg three times a week Li, EASL, 2019, THU-153. Li et al, Virology Journal, 2019; 16: 34 36

GLE/PIB Data

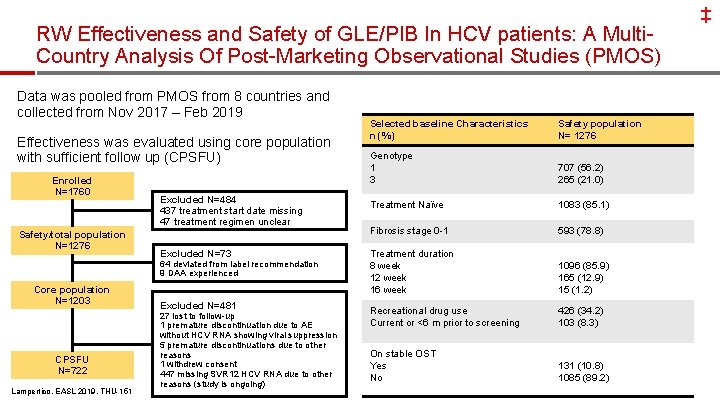
‡ RW Effectiveness and Safety of GLE/PIB In HCV patients: A Multi. Country Analysis Of Post-Marketing Observational Studies (PMOS) Data was pooled from PMOS from 8 countries and collected from Nov 2017 – Feb 2019 Selected baseline Characteristics n (%) Safety population N= 1276 Genotype 1 3 707 (56. 2) 265 (21. 0) Excluded N=484 437 treatment start date missing 47 treatment regimen unclear Treatment Naïve 1083 (85. 1) Fibrosis stage 0 -1 593 (78. 8) Excluded N=73 Treatment duration 8 week 12 week 16 week 1096 (85. 9) 165 (12. 9) 15 (1. 2) Recreational drug use Current or <6 m prior to screening 426 (34. 2) 103 (8. 3) On stable OST Yes No 131 (10. 8) 1085 (89. 2) Effectiveness was evaluated using core population with sufficient follow up (CPSFU) Enrolled N=1760 Safety/total population N=1276 64 deviated from label recommendation 9 DAA experienced Core population N=1203 CPSFU N=722 Lampertico, EASL 2019, THU-151 Excluded N=481 27 lost to follow-up 1 premature discontinuation due to AE without HCV RNA showing viral suppression 5 premature discontinuations due to other reasons 1 withdrew consent 447 missing SVR 12 HCV RNA due to other reasons (study is ongoing) 38

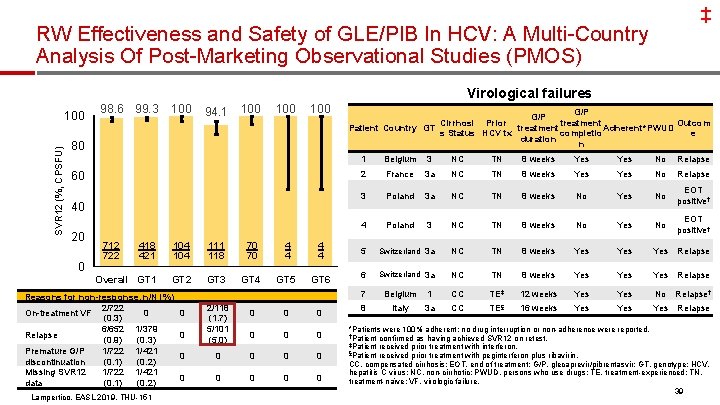
‡ RW Effectiveness and Safety of GLE/PIB In HCV: A Multi-Country Analysis Of Post-Marketing Observational Studies (PMOS) Virological failures SVR 12 (%, CPSFU) 100 98. 6 99. 3 100 94. 1 100 100 80 60 40 20 0 712 722 G/P treatment Cirrhosi Prior Outcom Patient Country GT treatment Adherent*PWUD completio s Status HCV tx e duration n 1 Belgium 3 NC TN 8 weeks Yes No Relapse 2 France 3 a NC TN 8 weeks Yes No Relapse 3 Poland 3 a NC TN 8 weeks No Yes No EOT positive† 4 Poland 3 NC TN 8 weeks No Yes No EOT positive† 418 421 104 111 118 70 70 4 4 5 Switzerland 3 a NC TN 8 weeks Yes Yes Relapse Overall GT 1 GT 2 GT 3 GT 4 GT 5 GT 6 6 Switzerland 3 a NC TN 8 weeks Yes Yes Relapse Reasons for non-response, n/N (%) 2/722 On-treatment VF 0 (0. 3) 6/652 1/379 Relapse (0. 9) (0. 3) Premature G/P 1/722 1/421 discontinuation (0. 1) (0. 2) Missing SVR 12 1/722 1/421 data (0. 1) (0. 2) Lampertico, EASL 2019, THU-151 0 0 2/118 (1. 7) 5/101 (5. 0) 0 0 0 0 7 Belgium 1 CC TE‡ 12 weeks Yes No Relapse† 8 Italy 3 a CC TE§ 16 weeks Yes Yes Relapse *Patients were 100% adherent; no drug interruption or non-adherence were reported. †Patient confirmed as having achieved SVR 12 on retest. ‡Patient received prior treatment with interferon. §Patient received prior treatment with peginterferon plus ribavirin. CC, compensated cirrhosis; EOT, end of treatment; G/P, glecaprevir/pibrentasvir; GT, genotype; HCV, hepatitis C virus; NC, non-cirrhotic; PWUD, persons who use drugs; TE, treatment-experienced; TN, treatment-naïve; VF, virologic failure. 39

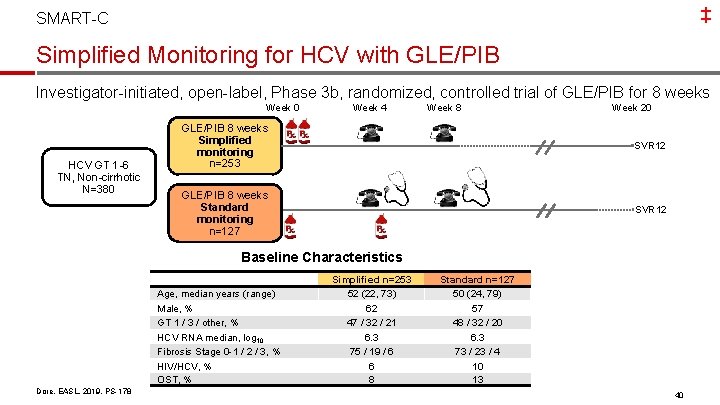
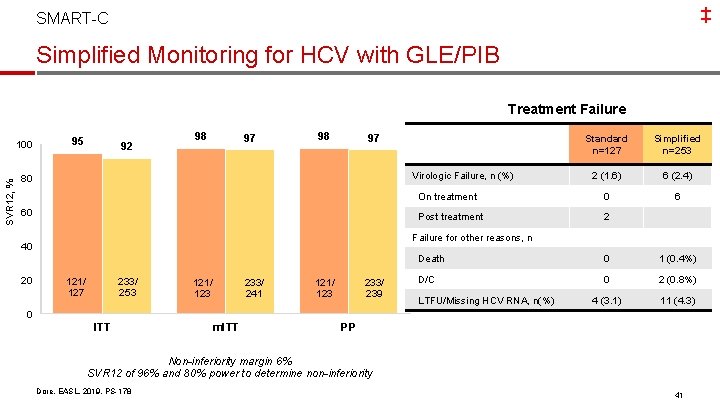
‡ SMART-C Simplified Monitoring for HCV with GLE/PIB Investigator-initiated, open-label, Phase 3 b, randomized, controlled trial of GLE/PIB for 8 weeks Week 0 HCV GT 1 -6 TN, Non-cirrhotic N=380 Week 4 Week 8 Week 20 GLE/PIB 8 weeks Simplified monitoring n=253 SVR 12 GLE/PIB 8 weeks Standard monitoring n=127 SVR 12 Baseline Characteristics Age, median years (range) Male, % GT 1 / 3 / other, % HCV RNA median, log 10 Fibrosis Stage 0 -1 / 2 / 3, % HIV/HCV, % OST, % Dore, EASL, 2019, PS-178 Simplified n=253 52 (22, 73) 62 47 / 32 / 21 6. 3 75 / 19 / 6 6 8 Standard n=127 50 (24, 79) 57 48 / 32 / 20 6. 3 73 / 23 / 4 10 13 40

‡ SMART-C Simplified Monitoring for HCV with GLE/PIB Treatment Failure SVR 12, % 100 95 92 98 97 Simplified n=253 2 (1. 6) 6 (2. 4) On treatment 0 6 Post treatment 2 Virologic Failure, n (%) 80 60 Failure for other reasons, n 40 20 Standard n=127 233/ 253 121/ 127 233/ 241 121/ 123 233/ 239 Death 0 1 (0. 4%) D/C 0 2 (0. 8%) 4 (3. 1) 11 (4. 3) LTFU/Missing HCV RNA, n(%) 0 ITT m. ITT PP Non-inferiority margin 6% SVR 12 of 96% and 80% power to determine non-inferiority Dore, EASL, 2019, PS-178 41

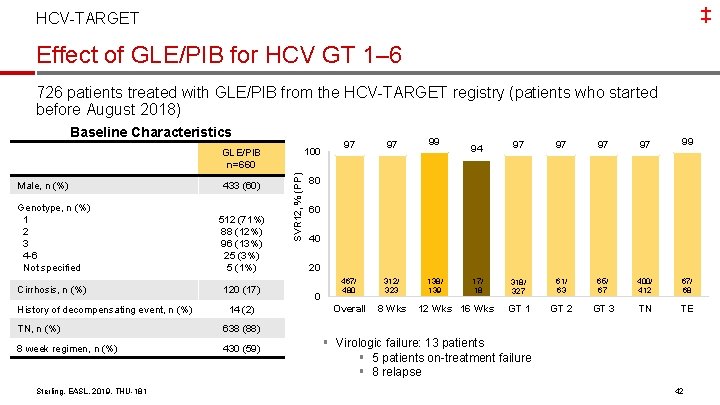
‡ HCV-TARGET Effect of GLE/PIB for HCV GT 1– 6 726 patients treated with GLE/PIB from the HCV-TARGET registry (patients who started before August 2018) Baseline Characteristics 100 Male, n (%) 433 (60) Genotype, n (%) 1 2 3 4 -6 Not specified 512 (71%) 88 (12%) 96 (13%) 25 (3%) 5 (1%) Cirrhosis, n (%) 120 (17) History of decompensating event, n (%) 14 (2) TN, n (%) 638 (88) 8 week regimen, n (%) 430 (59) Sterling, EASL, 2019, THU-181 SVR 12, % (PP) GLE/PIB n=660 97 97 99 467/ 480 312/ 323 138/ 139 Overall 8 Wks 94 97 97 99 318/ 327 61/ 63 65/ 67 400/ 412 67/ 68 GT 1 GT 2 GT 3 TN TE 80 60 40 20 0 17/ 18 12 Wks 16 Wks § Virologic failure: 13 patients § 5 patients on-treatment failure § 8 relapse 42

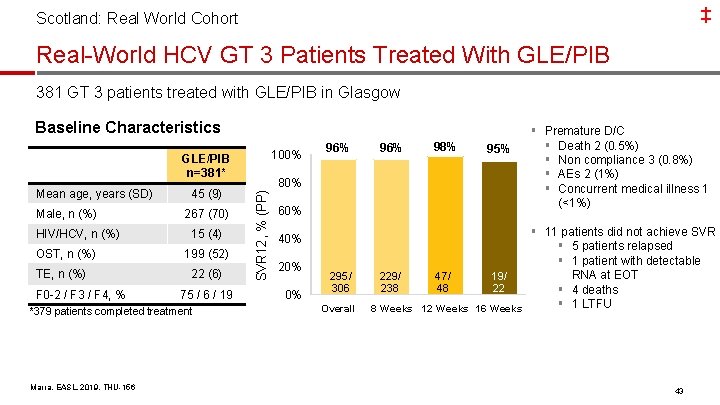
‡ Scotland: Real World Cohort Real-World HCV GT 3 Patients Treated With GLE/PIB 381 GT 3 patients treated with GLE/PIB in Glasgow Baseline Characteristics 100% GLE/PIB n=381* Male, n (%) HIV/HCV, n (%) OST, n (%) TE, n (%) F 0 -2 / F 3 / F 4, % 45 (9) 267 (70) 15 (4) 199 (52) 22 (6) 75 / 6 / 19 *379 patients completed treatment Marra, EASL, 2019, THU-156 80% SVR 12, % (PP) Mean age, years (SD) 96% 60% 40% 20% 0% 295/ 306 Overall 96% 98% 95% § Premature D/C § Death 2 (0. 5%) § Non compliance 3 (0. 8%) § AEs 2 (1%) § Concurrent medical illness 1 (<1%) § 11 patients did not achieve SVR § 5 patients relapsed § 1 patient with detectable RNA at EOT 229/ 47/ 19/ 238 48 22 § 4 deaths § 1 LTFU 8 Weeks 12 Weeks 16 Weeks 43
![MetaAnalysis RealWorld SafetyEffectiveness of GLEPIB ITTSVR 12 Patients n Studies n m ITTSVR ‡ Meta-Analysis Real-World Safety/Effectiveness of GLE/PIB ITT-SVR 12 (Patients [n], Studies [n]) m. ITT-SVR](https://slidetodoc.com/presentation_image_h2/55c1dd72dcfd390055dcb36f16641a28/image-44.jpg)
‡ Meta-Analysis Real-World Safety/Effectiveness of GLE/PIB ITT-SVR 12 (Patients [n], Studies [n]) m. ITT-SVR 12 (Patients [n], Studies [n]) Overall 96. 6 (8279, 14) 98. 1 (5995, 11) HCV GT 1 95. 5 (1685, 6) 97. 5 (2468, 4) HCV GT 2 96. 4 (375, 6) 97. 1 (383, 4) HCV GT 3 95. 2 (1084, 6) 95. 8 (700, 6) HCV GT 4 99. 0 (214, 4) 98. 5 (165, 2) 97. 8 (629, 6) / 96. 9 (3862, 5) 97. 7 (688 , 7) / 97. 7 (4685, 6) NA 97. 3 (3893, 4) HCV TE (PRS or DAA) 97. 7 (181, 5) 96. 5 (643, 5) Treatment duration 8 W 96. 2 (1781, 4) 98. 1 (3514, 6) Treatment duration 12 W 95. 7 (603, 4) 95. 7 (593, 3) Treatment duration 16 W NA NA Efficacy Cirrhosis (Y/N) HCV TN Cornberg, EASL, 2019, PS-184 SVR 12 (ITT), % Systematic review and meta-analysis of RWE from 10, 048 adults from 16 studies (prospective/retrospective) 97 96 97 95 100 98 96 TE 8 wk 80 60 40 20 0 Overall GT 1 GT 2 Safety AEs GT 3 % (Patients [n], Studies [n]) 12. 7% (724/5685, 6) - Pruritus 4. 7% (126/2698) - Fatigue 4. 4% (146/3305) - Headache 2. 7% (102/3759) Treatment discontinuations due to AE 0. 5% (24/4508, 5) 44

Back up

Real world analysis of 12 clinical practice cohorts from 7 countries ‡ Global Real World Evidence of SOF/VEL for 12 Weeks 5541 patients were included, without use of RBV § Baseline Characteristics Age – mean (SD) Male N=5340 (%) 54 (13%) 2822 (53%) Genotype, % 1/ 2/ 3/ 4/ 5/ 6/ unknown 30/ 33/ 5/ 1/ 1 Fibrosis, % F 0 -F 2/ F 3/ F 4/ unknown 54/ 13/ 21/ 12 HIV/HCV coinfection 196 (4%) Former or ongoing IVDU 706 (13%) PPI use at Baseline 287 (5%) TE (peg. IFN + RBV ± PI) 660 (12%) §Total number of patients varies across the characteristics, due to missing data * Data from 1 cohort were not included in the ITT characteristics analysis due to missing data Mangia, EASL, 2019, GS-03 46

‡ Real world analysis of 12 clinical practice cohorts from 7 countries 5541 patients were included, without use of RBV Characteristic – N(%)§ ITT (N=5340) Age – mean (SD) 54 (13. 1) Genotype, % 1/ 2/ 3/ 4/ 5/ 6/ unknown 2822 (52, 8%) 54/ 13/ 21/ 1 HIV/HCV coinfection 196 (3. 7%) Former or ongoing IVDU 706 (13. 2%) PPI use at Baseline 287 (5. 4%) number of patients varies across the characteristics, due to missing data * Data from 1 cohort were not included in the ITT Characteristics analysis, due to missing data Mangia, EASL, 2019, GS-03 99 98 1595/ 1615 335/ 342 99 100 90 80 70 60 50 40 30 20 10 0 98 1535/ 264/ 1553 269 GT 2 Overall 30/ 33/ 5/ 1/ 1 Fibrosis, % F 0 -F 2/ F 3/ F 4/ unknown, NC/ unknown §Total 100 90 80 70 60 50 40 30 20 10 0 GT 1 SVR 12/24, % (PP) Male SVR 12/24, % (PP) SOF/VEL for 12 Weeks in a US and EU Population 98 96 99 100 1646/ 1686 297/ 308 341/ 343 53/ 53 GT 4 -6/mixed/unknown Compensated cirrhosis 98 100 96 98 98 99 753/ 766 71/ 71 181/ 188 678/ 693 263/ 268 443/ 447 TE Unknown fibrosis HIV/HCV Historic/current IVDU PPI use at BL Age >70 years SOF/VEL for 12 weeks is a simple and highly effective treatment for HCV, enabling simplification of the care cascade as a Test & Treat option

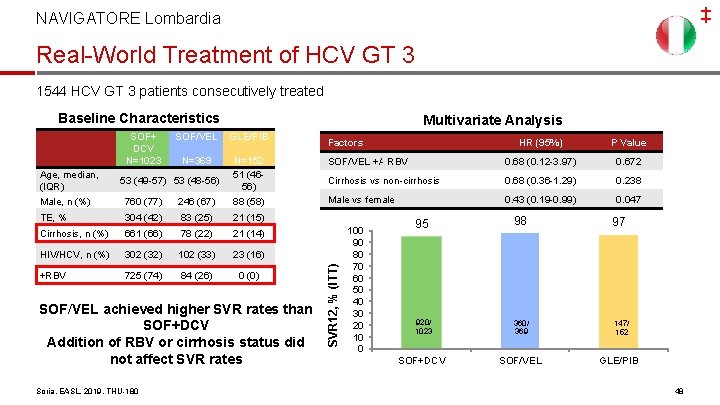
‡ NAVIGATORE Lombardia Real-World Treatment of HCV GT 3 1544 HCV GT 3 patients consecutively treated Baseline Characteristics SOF/VEL GLE/PIB N=369 760 (77) 246 (67) N=152 51 (4656) 88 (58) TE, % 304 (42) 83 (25) 21 (15) Cirrhosis, n (%) 661 (66) 78 (22) 21 (14) HIV/HCV, n (%) 302 (32) 102 (33) 23 (16) +RBV 725 (74) 84 (26) 0 (0) Age, median, (IQR) Male, n (%) 53 (49 -57) 53 (48 -56) SOF/VEL achieved higher SVR rates than SOF+DCV Addition of RBV or cirrhosis status did not affect SVR rates Soria, EASL, 2019, THU-180 Factors HR (95%) P Value SOF/VEL +/- RBV 0. 68 (0. 12 -3. 97) 0. 672 Cirrhosis vs non-cirrhosis 0. 68 (0. 36 -1. 29) 0. 238 0. 43 (0. 19 -0. 99) 147/ 0. 047 Male vs female SVR 12, % (ITT) SOF+ DCV N=1023 Multivariate Analysis 100 90 80 70 1202/ 60 50 1264 40 30 20 10 0 95 360/ 369 98 152 97 768/ 817 920/ 1023 360/ 369 SOF+DCV SOF/VEL 147/ 152 GLE/PIB 48

‡ HELIOS: France Real World Cohort Real World Effectiveness of SOF/VEL in France SVR 12, % (PP) 100 99 96 99/ 100 399/ 405 23/ 24 GT 1 GT 2 100 100 51/ 51 17/ 17 7/ 7 GT 3 GT 4 GT 5 -6 80 60 40 20 0 Larrey, EASL, 2019, THU-152 49

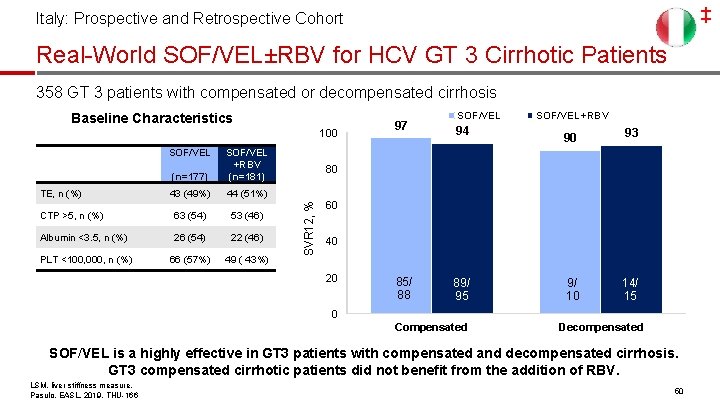
‡ Italy: Prospective and Retrospective Cohort Real-World SOF/VEL±RBV for HCV GT 3 Cirrhotic Patients 358 GT 3 patients with compensated or decompensated cirrhosis Baseline Characteristics 100 (n=177) SOF/VEL +RBV (n=181) 43 (49%) 44 (51%) CTP >5, n (%) 63 (54) 53 (46) Albumin <3. 5, n (%) 26 (54) 22 (46) PLT <100, 000, n (%) 66 (57%) 49 ( 43%) TE, n (%) 94 85/ 88 89/ 95 95 SOF/VEL+RBV 90 93 9/ 10 14/ 15 15 80 SVR 12, % SOF/VEL 97 60 40 20 0 Compensated Decompensated SOF/VEL is a highly effective in GT 3 patients with compensated and decompensated cirrhosis. GT 3 compensated cirrhotic patients did not benefit from the addition of RBV. LSM, liver stiffness measure. Pasulo, EASL, 2019, THU-166 50

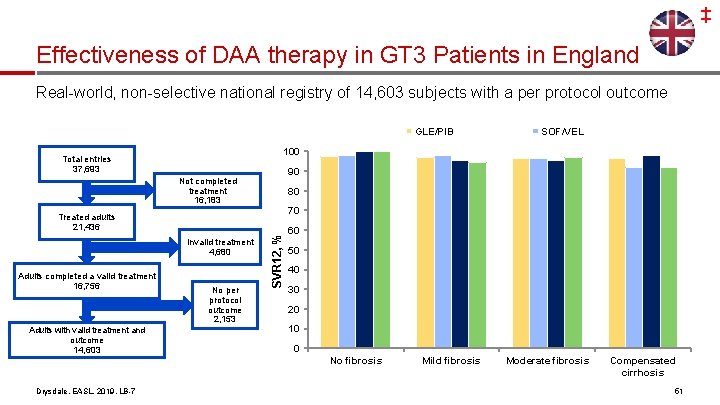
‡ Effectiveness of DAA therapy in GT 3 Patients in England Real-world, non-selective national registry of 14, 603 subjects with a per protocol outcome GLE/PIB 100 Total entries 37, 693 90 Not completed treatment 16, 183 80 70 Invalid treatment 4, 680 13, 959/ 14, 603 Adults with valid treatment and outcome 14, 603 No per protocol outcome 2, 153 SVR 12, % Treated adults 21, 436 Adults completed a valid treatment 16, 756 60 50 40 30 20 10 0 No fibrosis Drysdale, EASL, 2019, LB-7 SOF/VEL Mild fibrosis Moderate fibrosis Compensated cirrhosis 51

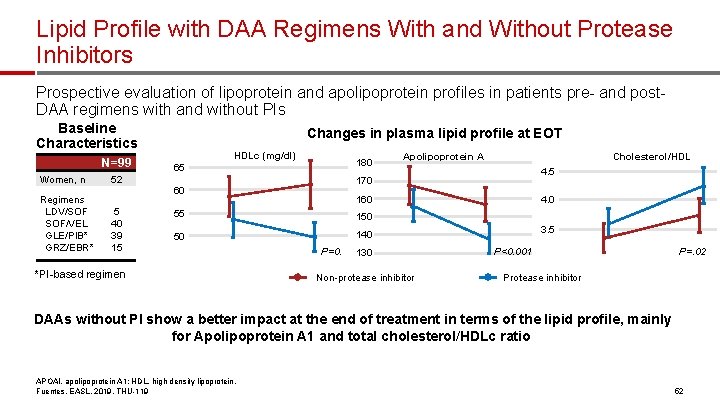
Lipid Profile with DAA Regimens With and Without Protease Inhibitors Prospective evaluation of lipoprotein and apolipoprotein profiles in patients pre- and post. DAA regimens with and without PIs N=99 Regimens LDV/SOF SOF/VEL GLE/PIB* GRZ/EBR* Changes in plasma. APOAI lipid profile at EOT HDLc (mg/dl) 65 p=0. 001 180 52 170 150 60 5 40 39 15 55 50 *PI-based regimen P=0. APOAI, mg/dl Women, n HDL Apolipoprotein A 140 p<0. 001 160 Cholesterol/HDL 4. 08 160 150 100 6 4. 2 3. 5 140 P<0. 001 130 50 Non-protease inhibitor 4 p=0. 02 3. 7 P=. 02 2 Protease inhibitor 0 PI-based Non-PI-based regimen treatment in terms of the 0 DAAs without PI show a better impact at the end of lipid for Apolipoprotein A 1 and total cholesterol/HDLc ratio APOAI, apolipoprotein A 1; HDL, high density lipoprotein. Fuentes, EASL, 2019, THU-119 Total Cholesterol/ HDL Ratio 10 4. 5 TC/HDL ratio Baseline Characteristics PI-based Non-PI-based regimen mainly regimen profile, 52

WHO Estimated Global Timing of HCV Elimination Modified Markov modeling, based on 2017 data, to predict achievement of WHO HCV elimination targets Year Each Country/Region May Meet All Four WHO Elimination Targets Iceland Spain France Australia Japan Italy Switzerland United Kingdom South Korea Austria Germany Malta Saudia Arabia Ireland Netherlands Bahrain Belgium Canada Chile Cyprus Czechia Denmark Estonia Finland Greece Hong Kong SAR of China Hungary Israel Kuwait Latvia Lithuania Luxembourg New Zealand Norway Oman Poland Portugal Qatar Singapore Slovakia Slovenia Sweden Taiwan (Province of China) United Arab Emirates United States 2020 2022 2024 2026 2028 2030 2032 WHO elimination targets include 90% reduction in incidence, 65% reduction in liver-related deaths, 90% diagnosis and 80% of the eligible HCV population treated. Razavi, EASL, 2019, SAT-260 2034 2036 2038 2040 2042 2044 2046 2048 2050 53

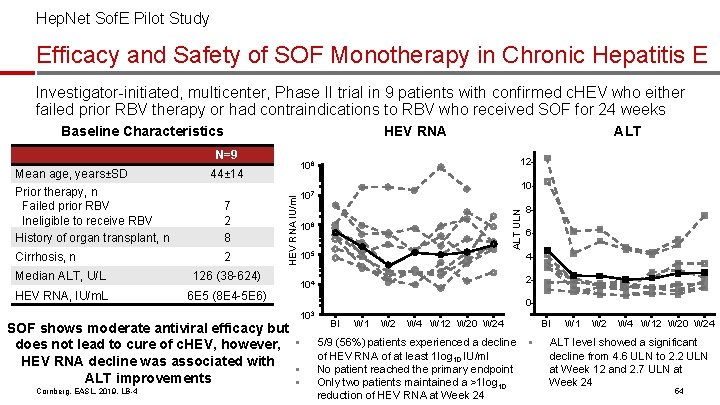
Hep. Net Sof. E Pilot Study Efficacy and Safety of SOF Monotherapy in Chronic Hepatitis E Investigator-initiated, multicenter, Phase II trial in 9 patients with confirmed c. HEV who either failed prior RBV therapy or had contraindications to RBV who received SOF for 24 weeks HEV RNA Baseline Characteristics N=9 Cirrhosis, n 2 Median ALT, U/L 126 (38 -624) HEV RNA, IU/m. L 6 E 5 (8 E 4 -5 E 6) 106 105 8642 - 104 0103 SOF shows moderate antiviral efficacy but does not lead to cure of c. HEV, however, § HEV RNA decline was associated with § ALT improvements § Cornberg, EASL, 2019, LB-4 10 - 107 ALT ULN 7 2 8 12 - 108 44± 14 HEV RNA IU/ml Mean age, years±SD Prior therapy, n Failed prior RBV Ineligible to receive RBV History of organ transplant, n ALT BI W 1 W 2 W 4 W 12 W 20 W 24 5/9 (56%) patients experienced a decline of HEV RNA of at least 1 log 10 IU/ml No patient reached the primary endpoint Only two patients maintained a >1 log 10 reduction of HEV RNA at Week 24 BI § W 1 W 2 W 4 W 12 W 20 W 24 ALT level showed a significant decline from 4. 6 ULN to 2. 2 ULN at Week 12 and 2. 7 ULN at Week 24 54
 What is an alternative of log based recovery
What is an alternative of log based recovery Otto hoffman's by product oven
Otto hoffman's by product oven Douglas t dietrich
Douglas t dietrich Hcv treatment
Hcv treatment Hiv test window period
Hiv test window period Hcv treatment
Hcv treatment Hep c symptoms female
Hep c symptoms female Easl slide deck
Easl slide deck Nafld summit
Nafld summit Easl wilson disease
Easl wilson disease Easl guidelines psc
Easl guidelines psc Easl 2018 decompensated cirrhosis
Easl 2018 decompensated cirrhosis Ba conference 2019
Ba conference 2019 Cdha benefits
Cdha benefits Third party risk management conference 2019 new york
Third party risk management conference 2019 new york Wasfaa conference 2019
Wasfaa conference 2019 Mil-std-889d
Mil-std-889d Nordic navigation
Nordic navigation Appa eo conference 2019
Appa eo conference 2019 Executive assistant conference 2019
Executive assistant conference 2019 Vasfaa conference 2019
Vasfaa conference 2019 Png investment conference 2019
Png investment conference 2019 Esop conference 2019 las vegas
Esop conference 2019 las vegas Fasfaa conference
Fasfaa conference Kca deutag investor relations
Kca deutag investor relations 2019 dod allied nations technical corrosion conference
2019 dod allied nations technical corrosion conference Wind energy science conference
Wind energy science conference Nmls resource center
Nmls resource center The microcap conference 2019
The microcap conference 2019 Ppl members
Ppl members Grundfos go
Grundfos go Gmod
Gmod Sab. update. com
Sab. update. com Smart view update
Smart view update Multiplicative update
Multiplicative update Power bi february 2018 update
Power bi february 2018 update Www.gov.ukdbs
Www.gov.ukdbs Delphi sql update
Delphi sql update Bankhead primary school rutherglen
Bankhead primary school rutherglen Database recovery techniques
Database recovery techniques Dhl global forwarding market update
Dhl global forwarding market update Update cmp
Update cmp Deferred update
Deferred update Position update formula
Position update formula Inf update
Inf update Atalhos teclado windows 7
Atalhos teclado windows 7 Klasifikasi failure berdasarkan storage:
Klasifikasi failure berdasarkan storage: What are these
What are these Multiplicative weights update algorithm
Multiplicative weights update algorithm 508 refresh
508 refresh Windws update
Windws update Sql queries for insert update and delete
Sql queries for insert update and delete Non-updating function module called for updating
Non-updating function module called for updating Rational clear quest
Rational clear quest Iso legislation update
Iso legislation update