e Health Technologies and the FDA William A



















- Slides: 21

e. Health Technologies and the FDA William A. Herman Office of Science and Technology Center for Devices and Radiological Health, FDA

Emerging Technology Trends CDRH TECHNOLOGY FORECAST • • • Computer-related Technology Molecular Medicine Home- and Self-care Minimally Invasive Procedures Device/Drug Products Organ Replacements and Assists http: //www. fda. gov/cdrh/ost/trends/toc. html

Survey Participants Robert Abodeely James Allen Clement Bezold Stephen P. Bruttig Joseph F. Coates and Jarrett Gilbert B. Devey NSF Henry S. Eden Don Giddens Harry Handlesman Peter Katona Ian H. Leverton Kaiser-Permanente Barbara Mc. Neil Harvard University Anthony J. Montagnolo Robert M. Nerem Charles H. Swanson Pfizer - MTG U. S. Army NIH Whitaker Foundation ECRI AMA Ga. Inst. Of Tech. Institute for Alternative Futures AHCPR Medtronic

Medical Device Technology Drivers • Demographic trends • Economic trends • Technology trends

Computer-related device trends • Substantial new product development 5 -10 yrs • Integrated patient medical information systems • Smart cards • Computer-aided medical decisionmaking • Smart artificial organ implants • Miniaturized biosensors & sensor “fusion” • Customized microprocessor devices

Homecare technology trends • Substantial new product development 5 -10 yrs • Limited expectations for advanced technologies • Monitoring -- blood, urine, drug concentrations • Simplified drug delivery systems • Telemedicine • Smart devices

Home Care Technologies for the 21 st Century • NSF-FDA Workshop - 150 participants - Industry - Academia - Government - Clinicians - Providers

21 st Century Home Care Technology • Prevention-oriented devices • Consumer health model • Noninvasive sensors • Smart devices • Customized products, flexible configurations • Data analysis tools for medical decisions • Electronic patient records • Wearable products • Wireless net-linked systems

US Food & Drug Administration • • Center for Drugs Center for Food Safety and Applied Nutrition Center for Biologics Center for Veterinary Medicine Center for Devices and Radiological Health National Center for Toxicological Research Office of Regional Affairs

CDRH Focus Ensuring the safety and effectiveness of medical devices

FDA’s Mandate for Regulation • Medical Device Amendments (1976) • Regulations implementing FD&C Act - Title 21 Code of Federal Regulations (21 CFR) Parts 800 – 1299 • Safe Medical Devices Act (1990) • FDA Modernization Act (1997)

What is a medical device? • Diagnosis, cure, mitigation, treatment or prevention of disease or condition • Affects the structure and function of the body • Does not achieve intended use through chemical reaction • Is not metabolized

Device Classification • 1700 generic types of devices • Three Classes - Class III • intended use; intended user • Classification determines extent of regulatory control

Device Classification Risk Class I - General Controls Low - General Controls and Special Controls Medium Class III - General Controls and Premarket Approval High

Premarket approval – 510 k • Marketing for first time, or significant change to existing device • Demonstration of Substantial Equivalence (SE) to legally marketed device in U. S. • SE means “As safe and as effective” - engineering - clinical outcome

Premarket approval – PMA • Only applies to Class III devices - New device - Device found not substantially equivalent • Proof of safety and effectiveness with clinical data • Investigational Device Exemption (IDE) may be desired or required

Investigational Device Exemption • • Used for clinical trials Significant risk devices Protection of human subjects Allows sponsor to recoup R&D costs

FDA’s Framework -- The traditional strategy • Regulatory gatekeeper • Unilateral responsibilities • Reactive orientation

FDA’s Framework -- The emerging strategy • Multifaceted, information-based strategy • Collaborative multi-party harmonization • Anticipatory orientation

e. Health Technology Issues • What’s a Device? • Labeling • “Smart Devices” • Tele-health • Interacting Systems of Devices • Architectural Considerations • Environmental Factors

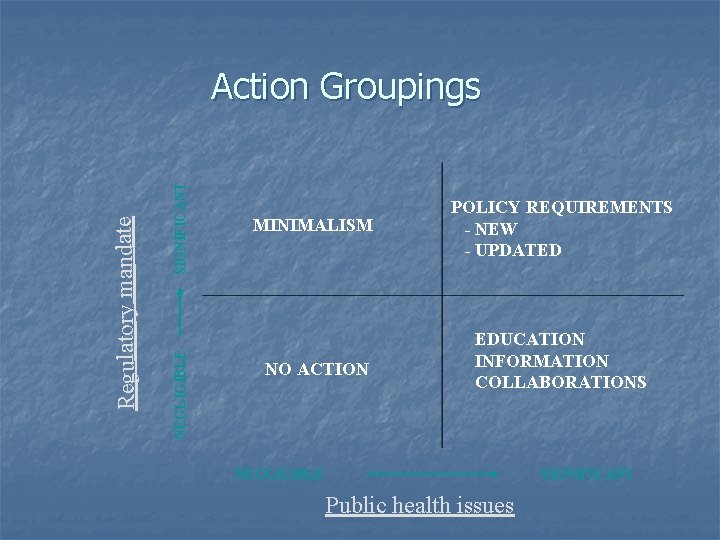
SIGNIFICANT NEGLIGIBLE Regulatory mandate Action Groupings MINIMALISM POLICY REQUIREMENTS - NEW - UPDATED NO ACTION EDUCATION INFORMATION COLLABORATIONS NEGLIGIBLE SIGNIFICANT Public health issues
 Global health technologies coalition
Global health technologies coalition Fda v brown and williamson
Fda v brown and williamson Health and social care component 3
Health and social care component 3 Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống









































