Drugs and Cosmetics Act 1940 and Rules 1945




























































- Slides: 70

Drugs and Cosmetics Act 1940 and Rules 1945 Mr. B. Brahmaiah, M. Pharm, (Ph. D) Assistant Professor Department of Pharmaceutical Regulatory Affairs Hindu College of Pharmacy, Guntur. 1

Contents �History and Objectives �Definitions �Administration of the act and rules �Provisions related to Import �Provisions related to Manufacture �Provisions related to Sale �Labeling and Packaging �Schedules to the act and rules �Recent amendment act, 2008 �List of forms 2

History �British misrule-Providing poor healthcare system to Indian citizens �Observations made by-Drugs Enquiry Committee, Indian Medical Association �Reports in- Indian Medical Gazette during 1920 -30 � 1940 – Drugs and Cosmetics Act � 1945 – Rules under the Act Extended to whole of India…… 3

Objectives �To regulate the import, manufacture, distribution and sale of drugs & cosmetics through licensing. �Manufacture, distribution and sale of drugs and cosmetics by qualified persons only. �To prevent substandard in drugs. �To regulate the manufacture and sale of Ayurvedic, Siddha and Unani drugs. �To establish Drugs Technical Advisory Board(DTAB) and Drugs Consultative Committees(DCC) for Allopathic and allied drugs and cosmetics. 4

Definitions �Drugs : All medicines for internal or external use of human beings or animals and all substances intended to be used for or in the diagnosis, treatment, mitigation or prevention of any disease or disorder in human beings or animals, including preparations applied on human body for the purpose of repelling insects like mosquitoes. 5

�Cosmetic : Any article intended to be rubbed, poured, sprinkled or sprayed on, or introduced into, or otherwise applied to, the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance, and includes any article intended for use as a component of cosmetic. 6

�Misbranded drugs : (a) if it is so coloured, coated, powdered or polished that damage is concealed or if it is made to appear of better or greater therapeutic value than it really is; or (b) if it is not labelled in the prescribed manner. 7

�Adulterated drug : (a) if it consists, in whole or in part, of any filthy, putrid or decomposed substance; or (b) if it has been prepared, packed or stored under insanitary conditions whereby it may have been contaminated with filth or whereby it may have been rendered injurious to health; or (c) if its container is composed in whole or in part, of any poisonous or deleterious substance which may render the contents 8 injurious to health.

�Spurious drugs : (a) if it is imported under a name which belongs to another drug; or (b) if it is an imitation of, or a substitute for, another drug or resembles another drug in a manner likely to deceive or bears upon it or upon its label or container the name of another drug 9

�Manufacture : In relation to any drug or cosmetic, it includes any process or part of a process for making, altering, ornamenting, finishing, packing, labelling, breaking up or otherwise treating or adopting any drug or cosmetic with a view to its sale or distribution but does not include the compounding or dispensing of any drug, or the packing of any drug or cosmetic, in the ordinary course of retail business. 10

�Patent or Proprietary medicine : A drug which is a remedy or prescription presented in a form ready for internal or external administration of human beings or animals and which is not included in the edition of the Indian Pharmacopoeia for the time being or any other Pharmacopoeia authorized in this behalf by the Central Government. 11

Administration of the act and rules A) Advisory : 1)Drugs Technical Advisory Board-DTAB 2)Drugs Consultative Committee-D. C. C. B) Analytical : 1)Central Drugs Laboratory - CDL 2)Drug Control Laboratory in states 3)Government Analysts C) Executives : 1)Licensing authorities 2)Controlling authorities 3)Drug Inspectors 12

Drugs Technical Advisory Board(DTAB) �Ex-Officio: (i) Director General of Health Services (Chairman) (ii) Drugs Controller, India (iii)Director of the Central Drugs Laboratory, Calcutta (iv) Director of the Central Research Institute, Kasauli (v)Director of Indian Veterinary Research Institute, Izatnagar (vi) President of Medical Council of India (vii) President of the Pharmacy Council of India (viii)Director of Central Drug Research Institute, Lucknow 13

�Nominated: 1) Two persons by the Central Government. 2) One person by the Central Government from the pharmaceutical industry 3) Two persons holding the appointment of Government Analyst under this Act, 14

�Elected: 1)one person, to be elected by the Executive Committee of the Pharmacy Council of India, 2)one person, to be elected by the Executive Committee of the Medical Council of India, 3)one pharmacologist to be elected by the Governing Body of the Indian Council of Medical Research; 4)one person to be elected by the Central Council of the Indian Medical Association; 5)one person to be elected by the Council of the Indian Pharmaceutical Association; 15

�Functions: To advise the Central Government and the State Governments on technical matters. To carry out the other functions assigned to it by this Act. 16

Drugs Consultative Committee(DCC) �It is also an advisory body constituted by central government. �Constitution: Two representatives of the Central Government One representative of each State Government 17

�Functions: § To advise the Central Government, the State Governments and the Drugs Technical Advisory Board on any other matter tending to secure uniformity throughout India in the administration of this Act. § The Drugs Consultative Committee shall meet when required § Has power to regulate its own procedure. 18

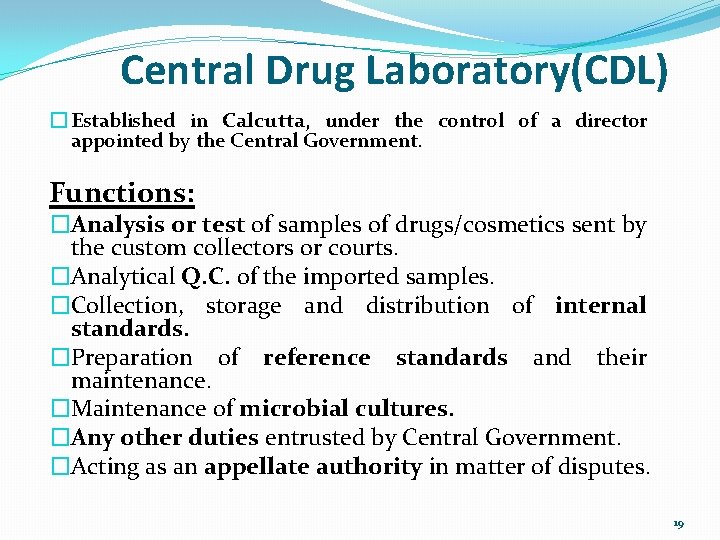
Central Drug Laboratory(CDL) � Established in Calcutta, under the control of a director appointed by the Central Government. Functions: �Analysis or test of samples of drugs/cosmetics sent by the custom collectors or courts. �Analytical Q. C. of the imported samples. �Collection, storage and distribution of internal standards. �Preparation of reference standards and their maintenance. �Maintenance of microbial cultures. �Any other duties entrusted by Central Government. �Acting as an appellate authority in matter of disputes. 19

Drug control laboratories in state In gujarat three laboratories established which collect, analysed and report the various sample of the drugs and food. 1) Baroda: Established in 1959. 2) Bhuj: Established in 1979. 3) Rajkot: Established in 1983 The laboratory has the following devision: �Pharmaceutical Chemistry Division �Immunology Division �Pharmacognocy Division �Food Division �Ayurvedic Division 20

Function: �Testing of drug sample �Analysis of food sample �Analysis of exicse sample 21

Government analyst �These officers are appointed by the central or state government and perform the duties. Qualification of government analysist 1 Persons having qualification for appointment as government as govermental Analysis for allopathic drugs ; 2 having a degree in medicine, ayurved, sidha or unani system and not less than three year post graduate experience in the analysis of drugs in a laboratory under control of a government analyst. 22

Duties: � 1) The Government Analyst shall cause to be analysed or tested such samples or drugs and cosmetics as may be sent to him by Inspectors. � 2)A Government Analyst shall from time to time forward reports to the Government giving the result of analytical work and research with a view to their publication. 23

Licensing authority Qualification: (i) Graduate in Pharmacy on Pharmaceutical Chemistry or in Medicine with specialization in clinical pharmacology or microbiology from a University established in India by law; and (ii)Experience in the manufacture or testing of drugs a minimum period of five years, Provided that the requirements as to the academic qualification shall not apply to those inspectors. 24

Duties: (1) to inspect all establishments licensed for the sale of drugs within the area assigned to him; (2) to satisfy himself that the conditions of the licences are being observed; (3) to procure and send for test or analysis, if necessary, imported packages. (4) to investigate any complaint. 25

(5) to maintain a record of all inspections made and action taken by him in the performance of his duties, (6) to make such enquiries and inspections as may be necessary to detect the sale of drugs in contravention to the Act; 26

Controlling authority DRUG INSPECTOR Qualification: � graduate in Pharmacy or Pharmaceutical Chemistry or in Medicine with specialization in clinical Pharmacology or microbiology from a University established in India by law and �experience in the manufacture or testing of drugs or enforcement of the provisions of the Act for a minimum period of five years: 27

Drug Inspector Qualification 1 Persons having qualification for appointment as government as govermental Analysis for allopathic drugs ; or 2 having a degree in ayurved, sidha or unani system and not less than three year post graduate experience in the analysis of drugs in a laboratory under control of (a) a government analyst, or (b) a chemical examinar, or (c) head of an institution specially approved for this purpose. 3 Degree in Pharmacy 0 r Pharmaceutical Sciences 28

Power: Duties of Drug Inspector a) Inspect, -- (i) any premises where in any drug or cosmetic is being manufactured. (ii) any premises where in any drug or cosmetic is being sold, or stocked or exhibited or offered for sale, or distributed ; (b) Take samples of any drug or cosmetic, -- (i) which is being manufactured or being sold or is stocked or exhibited or offered for sale, or is being distributed; (ii) from any person who is in the course of conveying, delivering or preparing to deliver such drug or cosmetic to a purchaser or a consignee. C) Other Duties �Search in places, Persons, vechicles, etc �Verification of stock registers �Seizing of medical shops any offences under this act 29

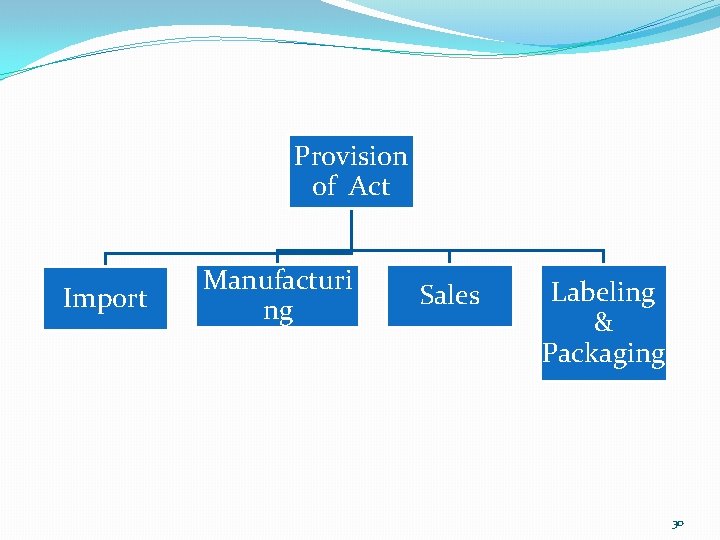
Provision of Act Import Manufacturi ng Sales Labeling & Packaging 30

IMPORT 31

IMPORT of drugs �Classes of drugs prohibited to import �Import of drug under license 1)Specified in Schedule-C/C 1 2)Specified in Schedule-X 3)Imported for Test/Analysis 4)Imported for personal use 5)Any new drugs �Drugs exempted from provisions of import �Offences and Penalties 32

Classes of drugs prohibited to import �Misbranded drugs �Drugs of substandard quality �Drugs claiming to cure diseases specified in Sch-J �Adulterated drugs �Spurious drugs �Drugs whose manufacture, sale/distribution are prohibited in original country, except for the purpose of test, examination and analysis. �Patent/Proprietary medicines whose true formula is not disclosed. 33

Import of the biological drugs(C/C 1) Conditions to be fulfilled: �Licensee must have adequate facility for the storage. �Licensee must maintain a record of the sale. �Licensee must allow an inspector to inspect premises and to check the records. �Licensee must furnish the sample to the authority. �Licensee must not sell drugs from which sample is withdrawn and he is advised not to sale, and recall the batch from the market. 34

Import of the Schedule-X drugs (Narcotic & Psychotropic drugs) Conditions to be fulfilled: �Licensee must have adequate storage facility. �Applicant must be reputable in the occupation, trade or business. �The license granted even before should not be suspended or cancelled. �The licensee has not been convicted any offence under the Drugs and Cosmetics Act or Narcotic and Psychotropic Substances Act. 35

Drugs imported for personal use Conditions to be fulfilled: �Up to 100 average doses may be imported without any permit, provided it is part of passenger’s luggage. �More than 100 doses imported with license. Apply on form no. -12 -A, 12 -B �Drugs must be bonafide personal use. �Drugs must be declared to the custom collectors if so directed. 36

Drugs Imported for examination, test or analysis Conditions to be fulfilled: �License is necessary under form-11 �Must use imported drugs only for said purpose and at the place specified in the license. �Must keep the record with respect to quantities, name of the manufacturer and date of import. �Must allow an inspector to inspect the premises and check the records. 37

Import of drugs without license �Substances not used for medicinal pupose �Drugs in Sch-C 1 required for manufacturing and not for medicinal use. �Substances which are both drugs and foods such as: Condensed/Powdered Milk Malt Lactose Farex/Cereal Oats �Predigested foods �Ginger, Pepper, Cumin, Cinnamon 38

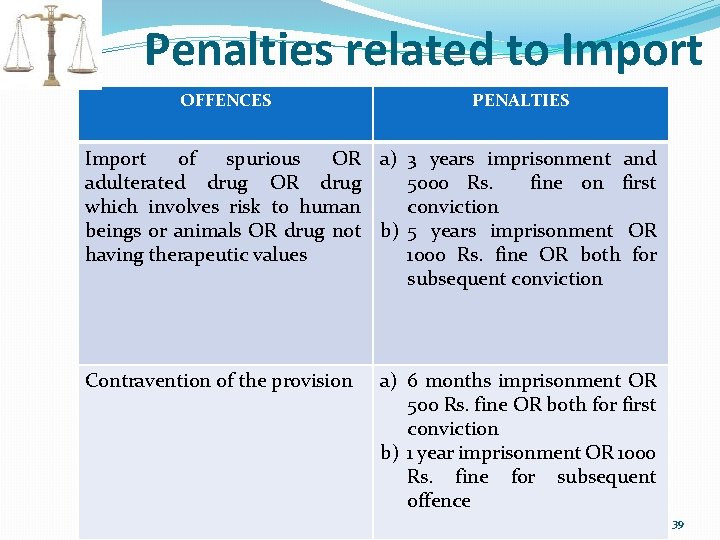
Penalties related to Import OFFENCES PENALTIES Import of spurious OR a) 3 years imprisonment and adulterated drug OR drug 5000 Rs. fine on first which involves risk to human conviction beings or animals OR drug not b) 5 years imprisonment OR having therapeutic values 1000 Rs. fine OR both for subsequent conviction Contravention of the provision a) 6 months imprisonment OR 500 Rs. fine OR both for first conviction b) 1 year imprisonment OR 1000 Rs. fine for subsequent offence 39

Cosmetics prohibited to import �Misbranded cosmetics �Spurious cosmetics �Cosmetic containing harmful ingredients �Cosmetics not of standard quality �which contains more than-2 ppm Arsenic, 20 ppm lead, 100 ppm heavy metals 40

MANUFACTURE 41

Manufacture �Prohibition of manufacture �Manufacture of other than in Sch-C/C 1 �Manufacture of those in Sch-C/C 1 �Manufacture of Sch-X drugs �Loan license �Repackaging license �Offences & Penalties 42

Prohibition of manufacture �Drug not of standard quality or misbranded, adulterated or spurious. �Patent or Proprietary medicine �Drugs in Sch-J �Risky to human beings or animals �Drugs without therapeutic value �Preparation containing cyclamates 43

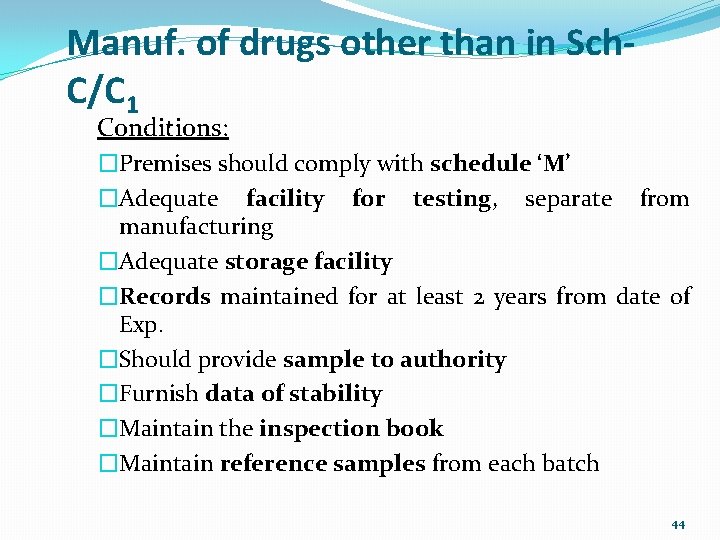
Manuf. of drugs other than in Sch. C/C 1 Conditions: �Premises should comply with schedule ‘M’ �Adequate facility for testing, separate from manufacturing �Adequate storage facility �Records maintained for at least 2 years from date of Exp. �Should provide sample to authority �Furnish data of stability �Maintain the inspection book �Maintain reference samples from each batch 44

Manuf. of drugs those in Schedule. C/C 1(Biological) Conditions: �Drugs must be issued in previously sterilized sealed glass or suitable container �Containers should comply with Sch-F �Some classes tested for aerobic & anaerobic microorganism. eg. Sera , Insulin, Pituitary hormones. �Serum tested for abnormal toxicity �Parentral in doses of 10 ml or more should be tested for freedom from Pyrogens �Separate lab. for culture & manipulation of spore bearing Pathogens �Test for sterility should be carried out. 45

Manufacture Of Sch-X drugs Conditions: �Accounts of all transactions regarding manuf. should be maintained in serially. (Preserved for 5 years) �Have to sent invoice of sale to licensing authority every 3 months �Store drugs in direct custody of responsible person. �Preparation must be labeled with XRx �Marketed in packings not exceeding � 100 unit dose –Tablets/Capsules � 300 ml- Oral liquid � 5 ml - Injection 46

Loan License �Definition: A person(applicant) who does not have his own arrangements(factory) for manufacture but who wish to manufacturing facilities owned by another licensee. Such licenses are called Loan licenses are issued for: 1) Drugs other than specified in C/C 1 & X. 2) Drugs specified in Schedule-C/C 1 47

Repackaging license �Definition: Process of breaking up any drug from a bulk container into small packages and labeling with a view to their sale and distribution. Repackaging of drugs is granted of drugs other than Schdule-C/C 1 and X. 48

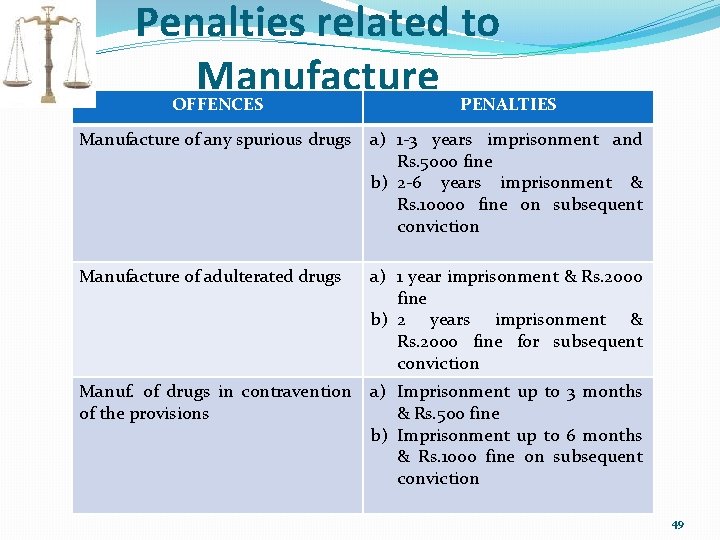
Penalties related to Manufacture OFFENCES PENALTIES Manufacture of any spurious drugs a) 1 -3 years imprisonment and Rs. 5000 fine b) 2 -6 years imprisonment & Rs. 10000 fine on subsequent conviction Manufacture of adulterated drugs a) 1 year imprisonment & Rs. 2000 fine b) 2 years imprisonment & Rs. 2000 fine for subsequent conviction Manuf. of drugs in contravention of the provisions a) Imprisonment up to 3 months & Rs. 500 fine b) Imprisonment up to 6 months & Rs. 1000 fine on subsequent conviction 49

Manufacture of cosmetics Prohibited for the following classes of drug: �Misbranded or spurious cosmetics and of substandard quality �Cosmetics containing hexachlorophene or mercury compounds �Cosmetics containing color which contain more than- 2 ppm of arsenic - 20 ppm of lead - 100 ppm of heavy metals �Eye preparations containing coal-tar color 50

SALE 51

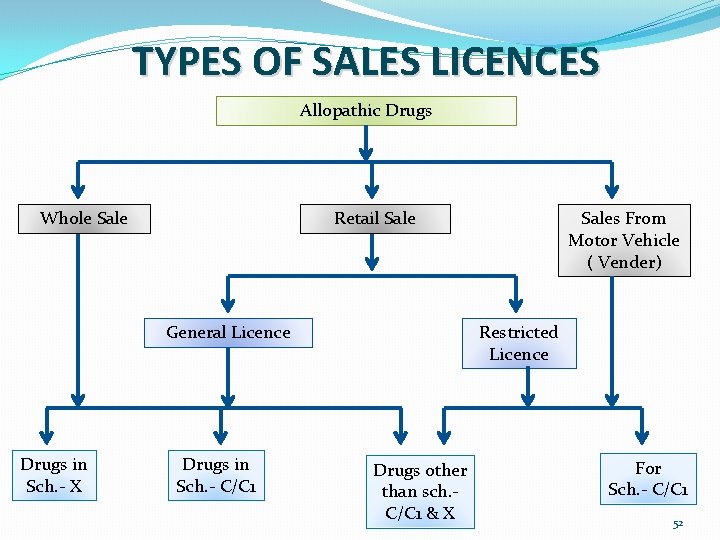
TYPES OF SALES LICENCES Allopathic Drugs Whole Sale Retail Sale General Licence Drugs in Sch. - X Drugs in Sch. - C/C 1 Sales From Motor Vehicle ( Vender) Restricted Licence Drugs other than sch. C/C 1 & X For Sch. - C/C 1 52

Sale of Drugs �Classes of drugs prohibited to be sold �Wholesale of biological (C/C 1) �Wholesale of other than those specified in C/C 1 and X 53

Class of drug prohibited to sale �Misbranded, spurious, adulterated and drugs not of standard quality �Patent/Proprietary drugs with undisclosed formula �Sch-J drugs �Expired drugs. �Drugs used for consumption by government schemes such as, Armed force. �Physician’s samples 54

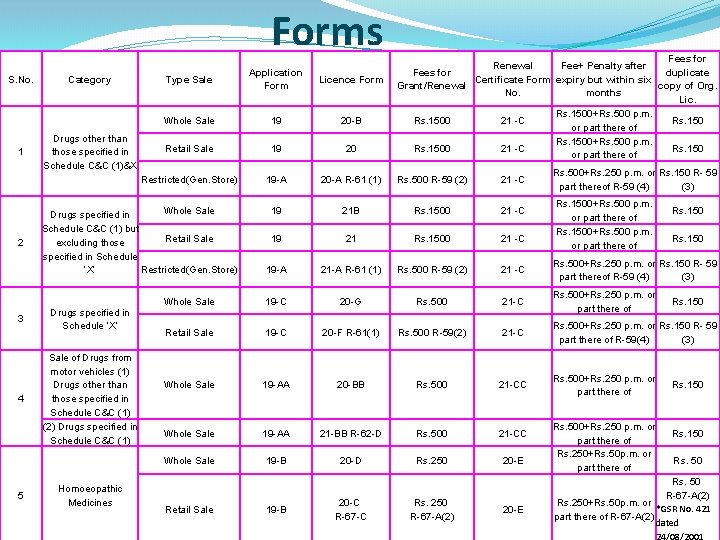
Forms S. No. 1 2 3 4 5 Category Drugs other than those specified in Schedule C&C (1)&X Type Sale Licence Form Whole Sale 19 20 -B Retail Sale 19 20 Restricted(Gen. Store) 19 -A 20 -A R-61 (1) Rs. 500 R-59 (2) 21 -C 19 21 B Rs. 1500 21 -C 19 21 Rs. 1500 21 -C 19 -A 21 -A R-61 (1) Rs. 500 R-59 (2) 21 -C Rs. 500+Rs. 250 p. m. or Rs. 150 R- 59 part thereof R-59 (4) (3) Whole Sale 19 -C 20 -G Rs. 500 21 -C Rs. 500+Rs. 250 p. m. or part there of Retail Sale 19 -C 20 -F R-61(1) Rs. 500 R-59(2) 21 -C Rs. 500+Rs. 250 p. m. or Rs. 150 R- 59 part there of R-59(4) (3) Whole Sale 19 -AA 20 -BB Rs. 500 21 -CC Whole Sale 19 -AA 21 -BB R-62 -D Rs. 500 21 -CC Whole Sale 19 -B 20 -D Rs. 250 20 -E Whole Sale Drugs specified in Schedule C&C (1) but Retail Sale excluding those specified in Schedule Restricted(Gen. Store) ‘X’ Drugs specified in Schedule ‘X’ Sale of Drugs from motor vehicles (1) Drugs other than those specified in Schedule C&C (1) (2) Drugs specified in Schedule C&C (1) Homoeopathic Medicines 4/2/2012 Fees for Renewal Fee+ Penalty after Fees for duplicate Certificate Form expiry but within six Grant/Renewal copy of Org. No. months Lic. Rs. 1500+Rs. 500 p. m. Rs. 1500 21 -C Rs. 150 or part there of Application Form Retail Sale 19 -B 20 -C R-67 -C Rs. 250 R-67 -A(2) 20 -E Rs. 500+Rs. 250 p. m. or Rs. 150 R- 59 part thereof R-59 (4) (3) Rs. 1500+Rs. 500 p. m. or part there of Rs. 500+Rs. 250 p. m. or part there of Rs. 250+Rs. 50 p. m. or part there of Rs. 150 Rs. 50 R-67 -A(2) Rs. 250+Rs. 50 p. m. or *GSR No. 421 part there of R-67 -A(2) dated 55 24/08/2001

FORM 19 -C {See Rule 59(2)} Application for grant or renewal of a {licence to sell, stock, exhibit or offer for sale, or distribute} drugs specified in Schedule X 1. I/We ……………. . of …………. . hereby apply for a licence to sell by Wholesale/retail drugs specified in Schedule-X to the Drugs and Cosmetics Rules, 1945. We operate a pharmacy on the premises, situated at…………. 2. The sale and dispensing of drugs will be made under the personal supervision of the qualified persons mentioned below: Name…………………. (Qualification) 1. Name of drugs to be sold. 2. Particulars of storage accommodation. 3. A fee of rupees…………. . has been credited to Government account under the head of account…………………. Date……………. Signature………… 56

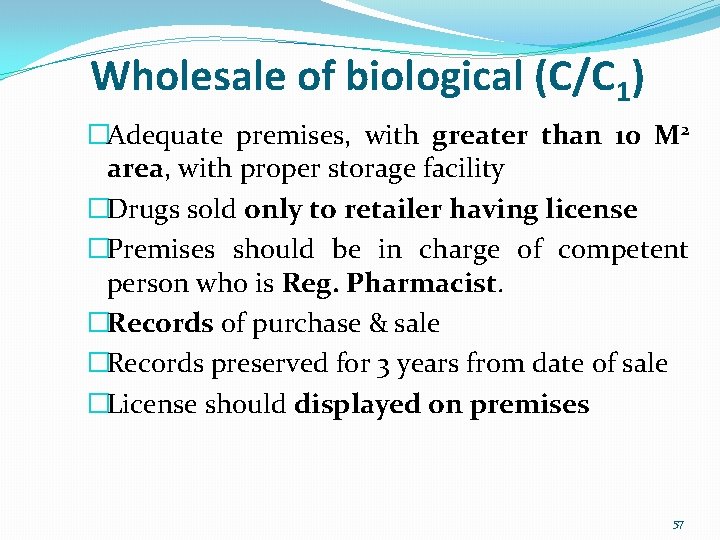
Wholesale of biological (C/C 1) �Adequate premises, with greater than 10 M 2 area, with proper storage facility �Drugs sold only to retailer having license �Premises should be in charge of competent person who is Reg. Pharmacist. �Records of purchase & sale �Records preserved for 3 years from date of sale �License should displayed on premises 57

whole sale from other than specified in c/c 1 and x �All the conditions as discussed in for biological. �Compounding is made by or under the direct and personal supervision of a qualified person. 58

Retail sale �For retail sale, two types of licenses are issued: i) General licenses ii) Restricted licenses Restricted license: Granted to those dealers who do not engage the services of a qualified person and only deal with such classes of drugs whose sales can be effected without qualified person and vendors who do not have fixed premises. 59

FORM 19 -A {(See Rule 59(2)} Application for the grant or renewal of a restricted licence to sell, stock or exhibit {or offer} for sale or distribute drugs by retail by dealers Who do not engage the service of a qualified person. 1. I/We ………………. . of ……………. . hereby apply for a licence to sell by retail (i){Drugs other than those specified in Schedule C, C(1) and X on the premises situated at ……………. or (ii) Drugs specified in {Schedule C(1) on the premises situated drugs specified in {Schedule C(1) as vendor in the at…………. . are………………. . 2. Sales shall be restricted to such drugs as can be sold without the supervision of a qualified person under the Drugs and Cosmetics Rules. 3. Names or classes of drugs proposed to be sold…………………. . 4. Particulars of the storage accommodation for the storage of {Schedule C(1) drugs on the premises referred to above. 5. The drugs for sale will be purchased from the following dealers and such other dealers as may be endorsed on the licence by the licensing authority from time to time. 6. A fee of rupees _____ has been credited to Government under the head of account …………… Date…………. . Signature……………. 60

Labeling & Packaging �All the general and specific labeling and packaging specified to all classes of drugs and cosmetics should be as per the provisions made under the act. 61

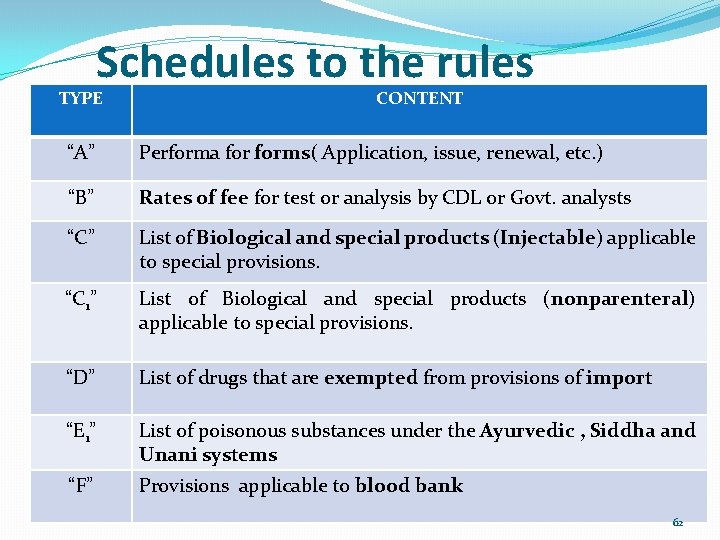
Schedules to the rules TYPE CONTENT “A” Performa forms( Application, issue, renewal, etc. ) “B” Rates of fee for test or analysis by CDL or Govt. analysts “C” List of Biological and special products (Injectable) applicable to special provisions. “C 1” List of Biological and special products (nonparenteral) applicable to special provisions. “D” List of drugs that are exempted from provisions of import “E 1” List of poisonous substances under the Ayurvedic , Siddha and Unani systems “F” Provisions applicable to blood bank 62

Schedules to the rules TYPE CONTENT “F 1” Special provision applicable to biological and special products, eg. Bacterial and viral vaccines, sera from living animals, bacterial origin diagnostic agents “F 2” Standards for surgical dressings “F 3” Standards for umbilical tapes “FF” Standards for ophthalmic preparations “G” List of substances required to be used supervision and labelled accordingly “H” List of substances (prescription) that should be sold by retail only on prescriptions of R. M. P. under medical 63

Schedules to the rules TYPE CONTENT “J” List of diseases and ailments that drug should not claim to cure “K” List of drugs that are exempted from certain provisions regarding manufacture “M” Requirements of manufacturing premises, GMP requirements of factory premises, plants and equipments “M 1” Requirements of factory premises for manufacture of Homeopathic medicines “M 2” Requirements of factory premises for manufacture of cosmetics “M 3” Requirements of factory premises for manufacture of medical devices “N” List of equipment to run a Pharmacy “O” Standards for disinfectant fluids 64

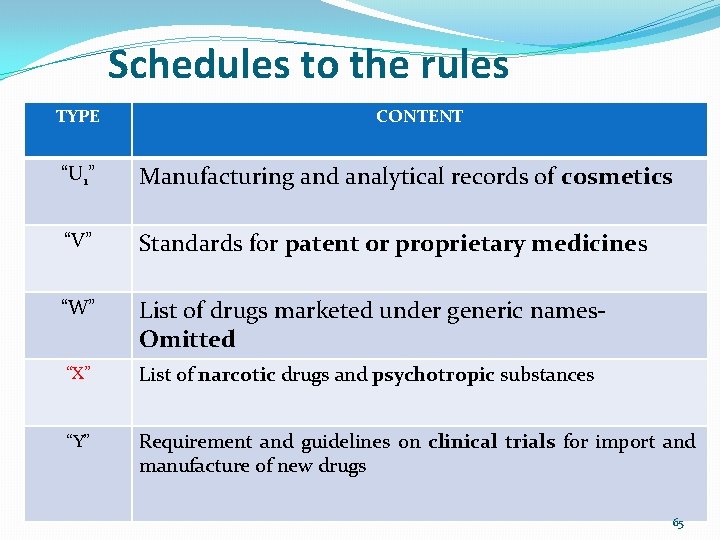
Schedules to the rules TYPE CONTENT “U 1” Manufacturing and analytical records of cosmetics “V” Standards for patent or proprietary medicines “W” List of drugs marketed under generic names. Omitted “X” List of narcotic drugs and psychotropic substances “Y” Requirement and guidelines on clinical trials for import and manufacture of new drugs 65

Schedules to the rules TYPE CONTENT “P” Life period(expiry) of drugs “Q” Coal tar colors permitted to be used in cosmetics “R” Standards for mechanical contraceptives “R 1” Standards for medical devices “S” Standards for cosmetics “T” Requirements (GMP) of factory premises for Ayurvedic, Siddha, Unani drugs “U” Manufacturing and analytical records of drugs 66

Drugs and Cosmetics (Amendment) Act, 2008 Salient features of the Act: Ø Substantial enhancement in punishment Ø Life imprisonment for offenders involved in manufacture, sale and distribution of spurious and adulterated drug likely to cause grievous hurt Ø Minimum punishment of seven years which may extend to life imprisonment Ø Provision for compensation to affected person 67

QUESTIONS �Describe the functions of Central Drug Laboratory. � Sale of drugs according to Drugs and Cosmetic Act. �Describe the administration of Drug and cosmetics act �Manufacture of drugs according to Drugs and Cosmetics Act. �Describe the Administration of DTAB �Define the fallowing Terms �A) Misbranded Drugs �B) Adulterated Drugs �C)Spurious Drugs 68

References �www. cdsco. nic. in �“Pharmaceutical Jurisprudence”, Jani GK, Atul prakashan; Fifth edition(2005 -06); 28. �“Forensic Pharmacy”, Kokate CK and Gokhle SB, Pharma Book Syndicate; 152 �“Laboratories” http: //www. gujhealth. gov. in/fdclaboratory. htm �A text book of Forensic Pharmacy, B. M. Mithal 69

 Blood bank regulation under drugs and cosmetics act
Blood bank regulation under drugs and cosmetics act Commonwealth act no. 578
Commonwealth act no. 578 List 8 types of facial cosmetics and how they are used
List 8 types of facial cosmetics and how they are used Anabis massage
Anabis massage Mac cosmetics business plan
Mac cosmetics business plan Cosmetics are substances that are used to enhance
Cosmetics are substances that are used to enhance Bsc cosmetics
Bsc cosmetics Alquimia essential oil
Alquimia essential oil Xrf analyser wiki
Xrf analyser wiki Mac cosmetics target market
Mac cosmetics target market Example of oily binder in compact
Example of oily binder in compact Anytime cosmetics
Anytime cosmetics Stackstorage
Stackstorage Noha beauty
Noha beauty Liquid crystal cosmetics
Liquid crystal cosmetics Wonder products roorkee
Wonder products roorkee Mary kay philosophy
Mary kay philosophy Beneficence in medical ethics
Beneficence in medical ethics Phthalates in cosmetics
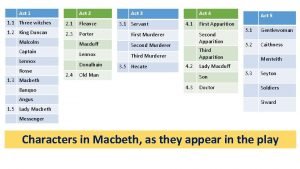
Phthalates in cosmetics Macbeth summary
Macbeth summary Self portrait with monkey
Self portrait with monkey Computador de 1940
Computador de 1940 Qarardad lahore in urdu
Qarardad lahore in urdu Eniac full form in computer
Eniac full form in computer Japanese empire 1940
Japanese empire 1940 Tiub hampagas
Tiub hampagas Baltic states 1940
Baltic states 1940 Kamplyrikk
Kamplyrikk Regla de erwin chargaff 1940
Regla de erwin chargaff 1940 Fortius fide
Fortius fide Edhint
Edhint Komputer 1940
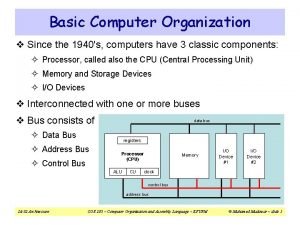
Komputer 1940 Organization of basic computer
Organization of basic computer 1925-1940
1925-1940 Fashion 1930 to 1940
Fashion 1930 to 1940 Glass spinning top
Glass spinning top Tungetaledebatten modernisme
Tungetaledebatten modernisme Nursing research definition
Nursing research definition 1940 moda
1940 moda Self portrait with monkey 1940
Self portrait with monkey 1940 Camaro 1940
Camaro 1940 Kallocain analys
Kallocain analys 50 luvun tyyli
50 luvun tyyli Elbocsátás 1940
Elbocsátás 1940 Isu-isu pokok etika komputer
Isu-isu pokok etika komputer Sumbangan mohammad eunos abdullah
Sumbangan mohammad eunos abdullah Section 17 misuse of drugs act
Section 17 misuse of drugs act Anticholinergic drugs mechanism of action
Anticholinergic drugs mechanism of action Davis and moore 1945
Davis and moore 1945 Yalta conference cartoon
Yalta conference cartoon Slovenska drama po 1945
Slovenska drama po 1945 Penyimpangan uud 1945 orde lama
Penyimpangan uud 1945 orde lama Pasal 30 ayat (1) uud 1945
Pasal 30 ayat (1) uud 1945 4 pokok pikiran pembukaan uud 1945
4 pokok pikiran pembukaan uud 1945 The modernist period (1910 to 1945)
The modernist period (1910 to 1945) Sidang pertama ppki 18 agustus 1945
Sidang pertama ppki 18 agustus 1945 Kulturradikale og kulturkonservative
Kulturradikale og kulturkonservative Sistematika konstitusi ris
Sistematika konstitusi ris Eesti laulja
Eesti laulja Cultuur en mentaliteit na 1945
Cultuur en mentaliteit na 1945 Mega giga tera peta
Mega giga tera peta 4 pokok pikiran pembukaan uud 1945
4 pokok pikiran pembukaan uud 1945 Brlem
Brlem Baionako hitzarmena
Baionako hitzarmena 1945 world war ii
1945 world war ii 1990-1945
1990-1945 Tehron konferensiyasi
Tehron konferensiyasi Nilai nilai dan moral dalam konstitusi
Nilai nilai dan moral dalam konstitusi History of manchester united f.c. (1945–1969)
History of manchester united f.c. (1945–1969) Bagaimana kedudukan pembukaan dalam uud nri tahun 1945
Bagaimana kedudukan pembukaan dalam uud nri tahun 1945 Bildlich gesprochen text
Bildlich gesprochen text