Cosmetics Market and Regulatory of Thailand Cosmetics in









- Slides: 15

Cosmetics Market and Regulatory of Thailand

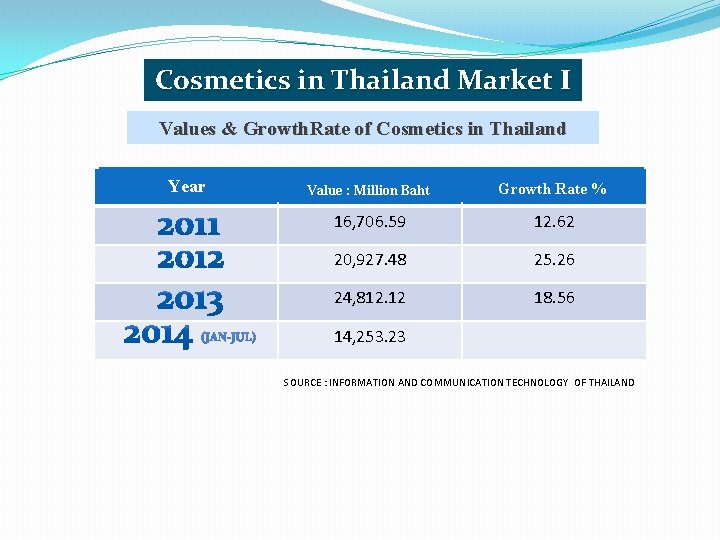
Cosmetics in Thailand Market I Values & Growth. Rate of Cosmetics in Thailand Year Value : Million Baht Growth Rate % 16, 706. 59 12. 62 20, 927. 48 25. 26 24, 812. 12 18. 56 14, 253. 23 SOURCE : INFORMATION AND COMMUNICATION TECHNOLOGY OF THAILAND

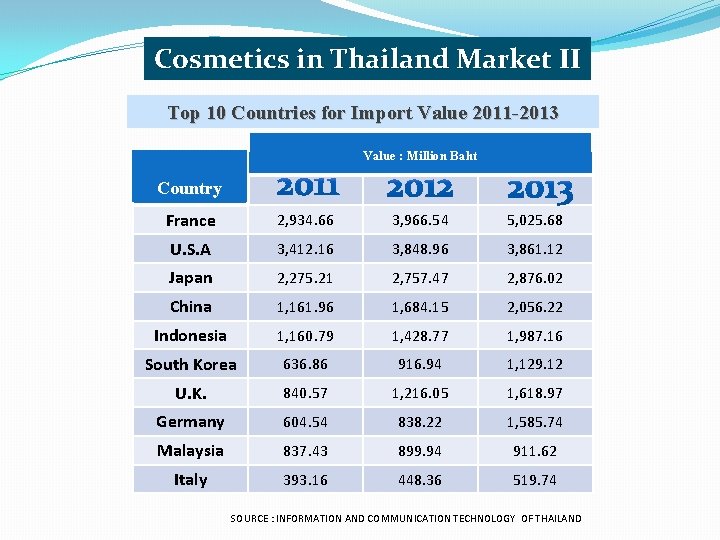
Cosmetics in Thailand Market II Top 10 Countries for Import Value 2011 -2013 Value : Million Baht Country France 2, 934. 66 3, 966. 54 5, 025. 68 U. S. A 3, 412. 16 3, 848. 96 3, 861. 12 Japan 2, 275. 21 2, 757. 47 2, 876. 02 China 1, 161. 96 1, 684. 15 2, 056. 22 Indonesia 1, 160. 79 1, 428. 77 1, 987. 16 South Korea 636. 86 916. 94 1, 129. 12 U. K. 840. 57 1, 216. 05 1, 618. 97 Germany 604. 54 838. 22 1, 585. 74 Malaysia 837. 43 899. 94 911. 62 Italy 393. 16 448. 36 519. 74 SOURCE : INFORMATION AND COMMUNICATION TECHNOLOGY OF THAILAND

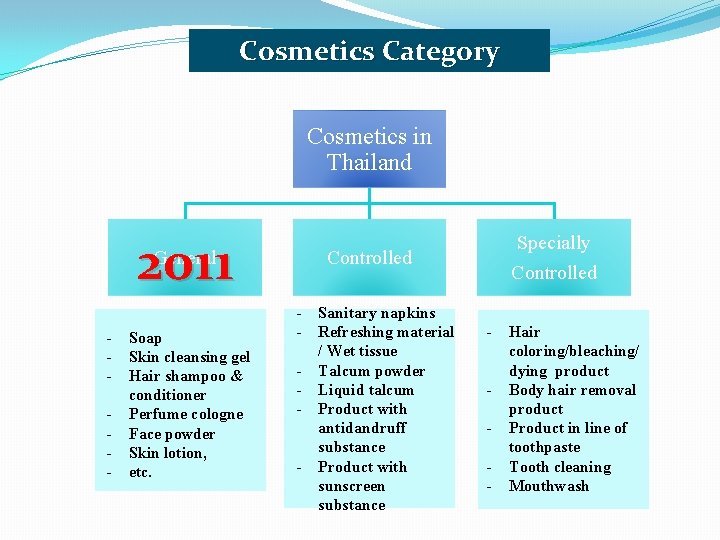
Cosmetics Category Cosmetics in Thailand 2011 General - Soap Skin cleansing gel Hair shampoo & conditioner Perfume cologne Face powder Skin lotion, etc. Specially Controlled - Sanitary napkins Refreshing material / Wet tissue Talcum powder Liquid talcum Product with antidandruff substance Product with sunscreen substance - Hair coloring/bleaching/ dying product Body hair removal product Product in line of toothpaste Tooth cleaning Mouthwash

Cosmetics Pre-Marketing Control Notification This is applicable to the controlled cosmetics classified in the ministerial notification. Either type of products or ingredients used classify the controlled cosmetics. For instance, products such as talcum powder and liquid talcum are classified as controlled cosmetics as well as those products containing ingredients such as zinc pyrithione or oxybenzone, etc. Registration As part of the notion supporting the enactment of the Cosmetic Act of B. E. 2535 (1992), its purpose is to strengthen the standard of manufacture; and the registration of cosmetic products clearly reflects this through the registration requirements. The registration procedure focuses mainly on the manufacturing processes such as production and quality control.

Requirement for Pre-Marketing Control Requirements for Notification Requirements for Registration 1. Application form and attachments 2. Master formula certified by authorized person 3. Certificate of free sale (for importation of products) duly notarized by the Thai Embassy 4. Corporate registration issued by the Ministry of products) duly notarized by the Thai Embassy 4. Commerce of Thailand 5. Labeling information Corporation registration issued by the Ministry of Commerce of Thailand 5. Labeling information 6. Analysis method approved by the Medical Sciences Department of the Thai Ministry of Public Health 7. Storage direction 8. Photocopy of sample permit 9. Batch process 10. Sample products 11. Photocopy of draft label 12. Documents indicating evidence for supporting claims

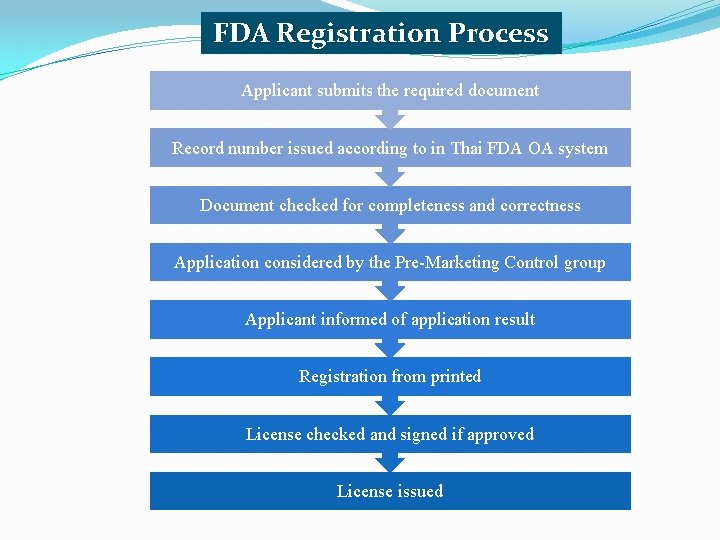
FDA Registration Process Applicant submits the required document Record number issued according to in Thai FDA OA system Document checked for completeness and correctness Application considered by the Pre-Marketing Control group Applicant informed of application result Registration from printed License checked and signed if approved License issued

Labeling means any figure, relief, or epithet pertaining to the cosmetics displayed on the cosmetic container, or package, or accompanying the cosmetic or inserted in the container or package, including the document or handbook provided with the cosmetic ; The Labeling Requirements Product name Type of product The words “Controlled/Specially Controlled Cosmetics" Registration number Name and quantity of Specially Controlled and Active Ingredients Name and address of manufacturer (importer including country of origin in case of imported products) Batch number Manufacturing date Instructions for use Net contents Statutory warning All information shall be imprinted on the pamphlets or leaflets delivered with products except that items 1, 4, and 10 shall be imprinted on the label if the label is smaller than 20 cm 2

Cosmetics Post-Marketing Control Inspections are performed periodically throughout the year. The activities, including routine inspection and product sampling, are carried out in accordance with the annual plan and in response to complaints or reports of adverse reactions or the results of surveillance. Surveillance Post – Marketing surveillance is performed all year round and includes the inspection of product labeling and the sampling on of marketed product at the locations under inspection. All printed claims on the labels of cosmetic products must have the supporting evidence readily available. Advertisement Inspection Advertisements for cosmetics must indicate the product's benefits relating to cosmetic capacity and purposes. Claims that the products have any pharmaceutical characteristics or capability to affect or alter body functions or structure are not permitted. Additionally, no advertisement must claim or suggest that the products have any capability which, in fact, does not exist or that may lead to misunderstanding of their quality. Development & Strengthening of manufacturing Process

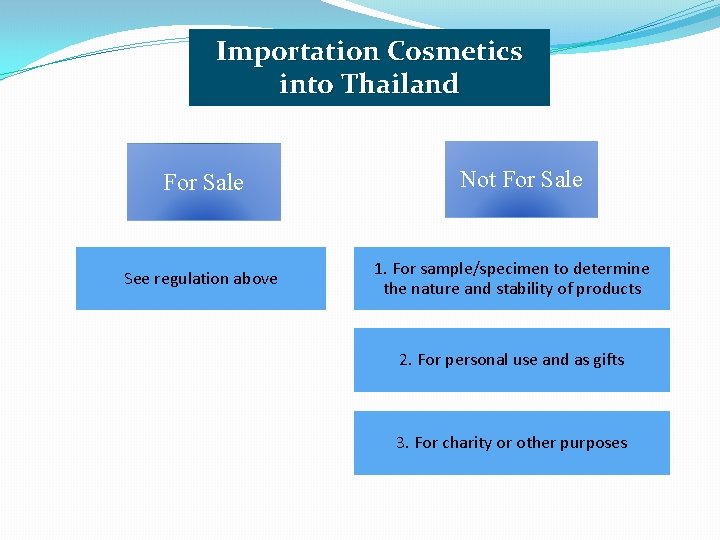
Importation Cosmetics into Thailand For Sale See regulation above Not For Sale 1. For sample/specimen to determine the nature and stability of products 2. For personal use and as gifts 3. For charity or other purposes

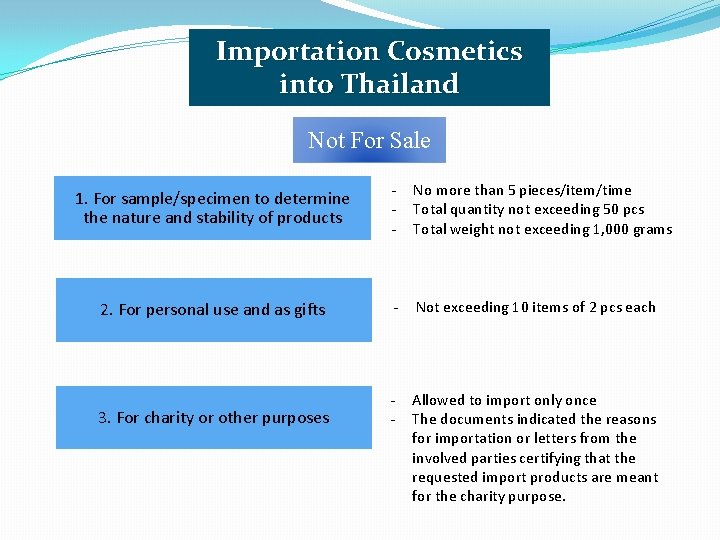
Importation Cosmetics into Thailand Not For Sale 1. For sample/specimen to determine the nature and stability of products - No more than 5 pieces/item/time Total quantity not exceeding 50 pcs Total weight not exceeding 1, 000 grams 2. For personal use and as gifts - Not exceeding 10 items of 2 pcs each 3. For charity or other purposes - Allowed to import only once The documents indicated the reasons for importation or letters from the involved parties certifying that the requested import products are meant for the charity purpose.

Prohibition of Manufacture or Sales I 1. The cosmetics are not safe for use, as described under section 33 (1), (2) and (3) of Cosmetics Act B. E. 2535 (1992) (1) Cosmetics containing ingredients that may be harmful to users (2) Cosmetics containing forbidden ingredients pursuant to Section 5 (4) of Cosmetics Act B. E. 2535 (1992) - name of substances forbidden to be used as admixtures in the manufacture of cosmetics 3) Cosmetics manufactured improperly or contained in nonhygienic container 2. The cosmetics have a name that is pretensions or impolite or deceptive 3. The cosmetics have a name that is inconsistent with Thai culture or language. 4. The place of manufacture or instruments and tools used in manufacture for sale are not correct according to the ministerial regulation

Prohibition of Manufacture or Sales II 5. The Cosmetics are considered as a counterfeit, as described under Section 34 of Cosmetics Act. B. E 2535 (1992) (1) Cosmetics with any ingredient imitated for the active ingredient of such cosmetics or without the active ingredient as notified to the competent official; (2) Cosmetics claimed to have been duly registered, which is false; (3) Cosmetics with a label designating their manufacturer or origin, which is false (4) Cosmetics with the active ingredients short of or surpassing the amount registered or notified to the competent official or shown in the label by more than twenty percent. 6. The cosmetics at variance with the standard pursuant to Section 35 of Cosmetics Act. B. E 2535 (1992) Cosmetics with active ingredients in the amount less than or exceeding that registered or notified to the competent official or shown in the label by a value exceeding the deviation value set and announced in the Royal Government Gazette by the Minister but not exceeding that pursuant to Section 34(4) shall be considered cosmetics at variance with the standards.


End of Presentation Thank you
 Mac cosmetics target market
Mac cosmetics target market Targeting and positioning
Targeting and positioning Regulatory framework of money market in india
Regulatory framework of money market in india Market leader market challenger market follower
Market leader market challenger market follower Milady chapter 24 review questions answers
Milady chapter 24 review questions answers Blood bank regulation under drugs and cosmetics act
Blood bank regulation under drugs and cosmetics act Music of thailand grade 8
Music of thailand grade 8 Desert daughter cosmetics
Desert daughter cosmetics Mac cosmetics business plan
Mac cosmetics business plan Vcosmetics
Vcosmetics Bsc cosmetics
Bsc cosmetics Alquimia cosmetics
Alquimia cosmetics Nabi cosmetics wikipedia
Nabi cosmetics wikipedia Example of oily binder in compact
Example of oily binder in compact Anytime cosmetics
Anytime cosmetics Stackstorage
Stackstorage