Chemistry in cosmetics Cosmetics In Greek kosmetik tekhn
















- Slides: 36

Chemistry in cosmetics Cosmetics : In Greek (kosmetikē tekhnē), "technique of dress and ornament" Make-up: Any substances or products used to enhance the appearance. Common cosmetics: Lipstick, mascara, eyeshadow, foundation, rouge, skin cleansers and skin lotions, shampoo, hairstyling products (gel, hair spray, etc. ) and perfume

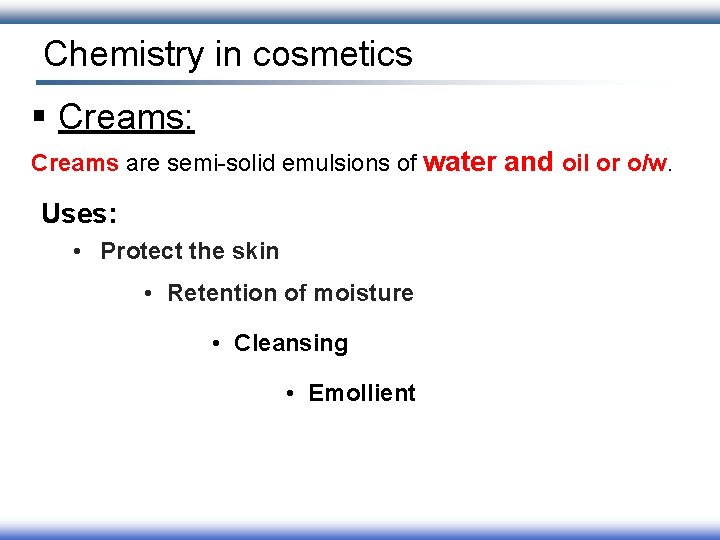
Chemistry in cosmetics § Creams: Creams are semi-solid emulsions of water and oil or o/w. Uses: • Protect the skin • Retention of moisture • Cleansing • Emollient

Chemistry in cosmetics § Creams: • Composition: 1 - Vegetable oil or fat. 2 - Waxes such as bees wax. 3 - Lanolin (from sheep’s wool). 4 - Perfume. 5 - emulsifier. 6 - water.

§ Perfumes: Composition: 1 - Odoriferous substances: organic compounds with characteristic pleasant odors (some is synthetic) 2 - Vehicle: Solvents (usually ethanol and water mixture) 3 - Fixtive: A substance used to reduce the evaporation rate and improve stability. • Benzoin • Glycerol

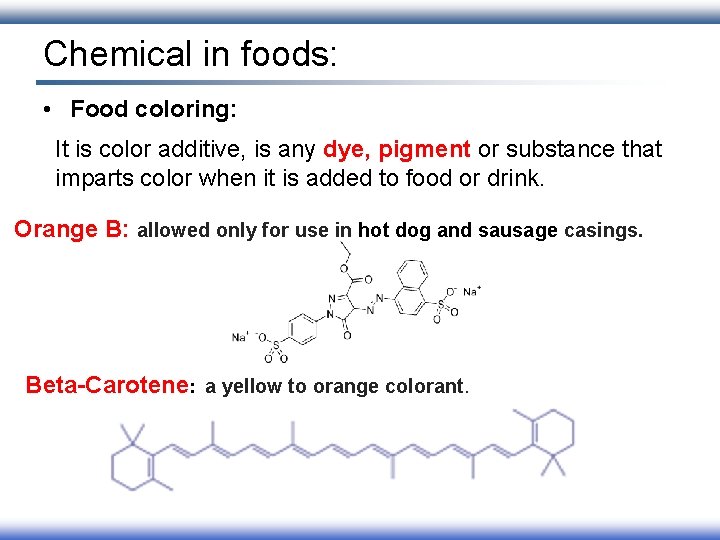
Chemical in foods: • Food coloring: It is color additive, is any dye, pigment or substance that imparts color when it is added to food or drink. Orange B: allowed only for use in hot dog and sausage casings. Beta-Carotene: a yellow to orange colorant.

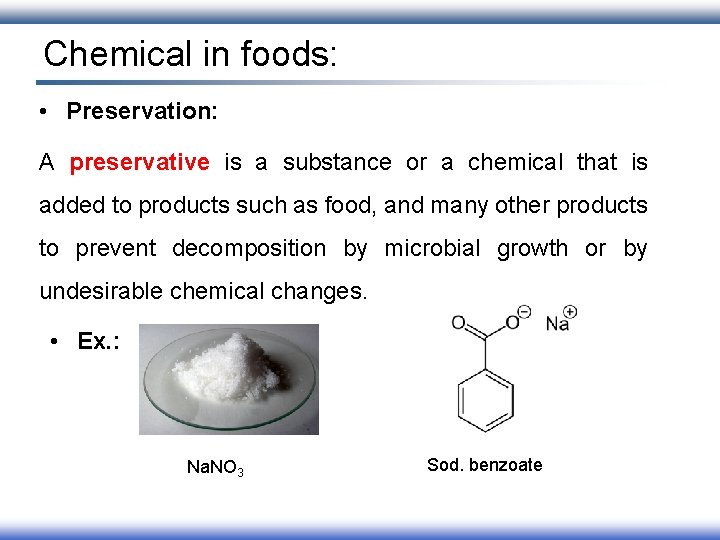
Chemical in foods: • Preservation: A preservative is a substance or a chemical that is added to products such as food, and many other products to prevent decomposition by microbial growth or by undesirable chemical changes. • Ex. : Na. NO 3 Sod. benzoate

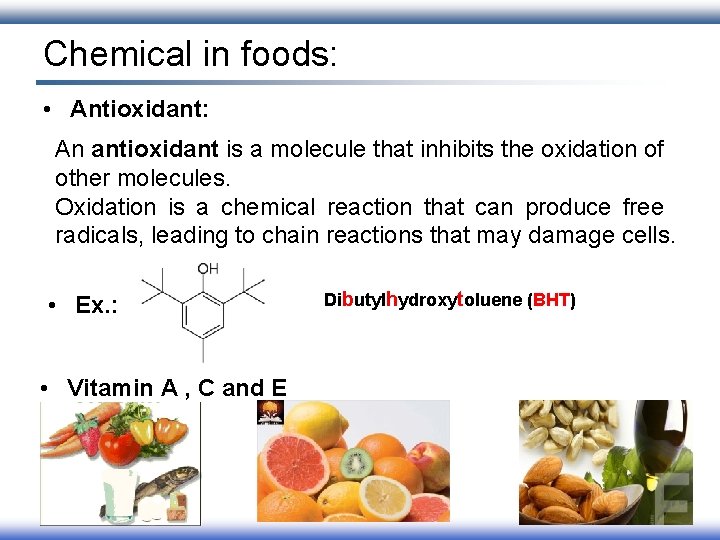
Chemical in foods: • Antioxidant: An antioxidant is a molecule that inhibits the oxidation of other molecules. Oxidation is a chemical reaction that can produce free radicals, leading to chain reactions that may damage cells. • Ex. : • Vitamin A , C and E Dibutylhydroxytoluene (BHT)

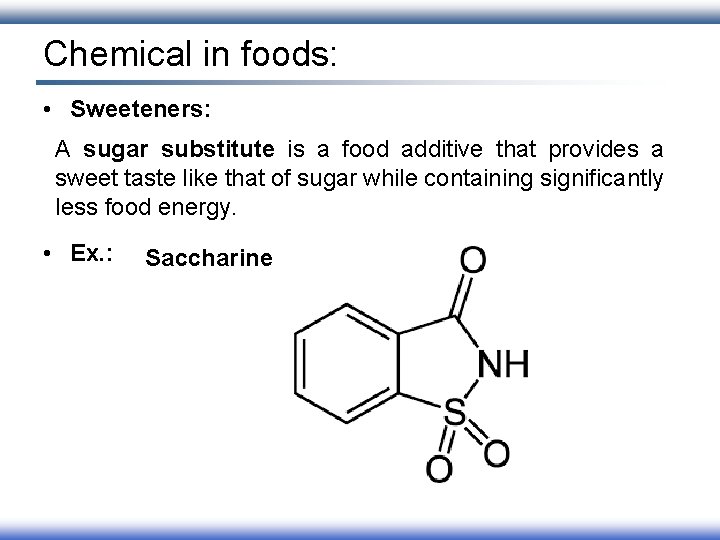
Chemical in foods: • Sweeteners: A sugar substitute is a food additive that provides a sweet taste like that of sugar while containing significantly less food energy. • Ex. : Saccharine

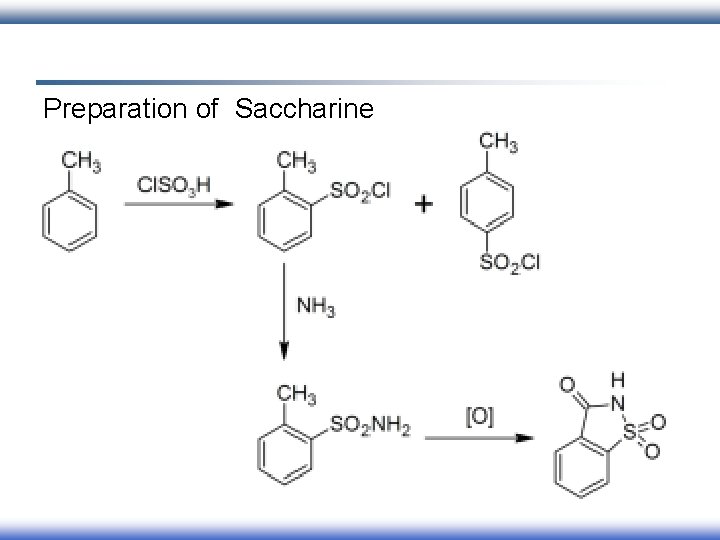
Preparation of Saccharine

Soap and Detergents

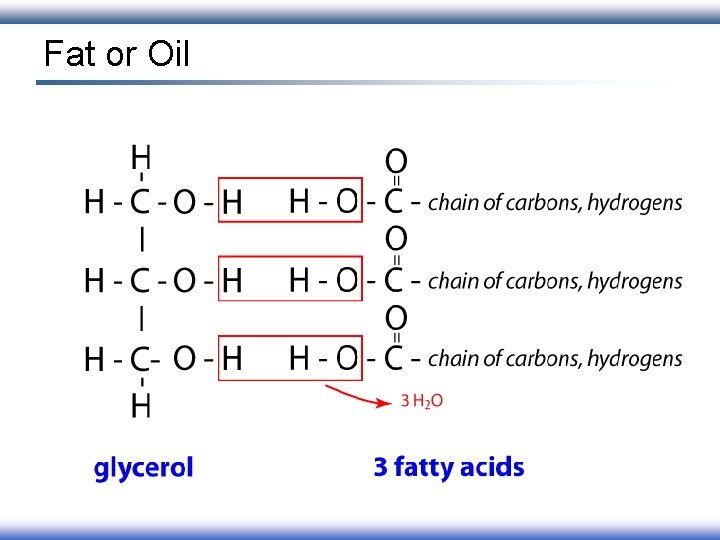
Fat or Oil

Fatty acids

Fat or Oil

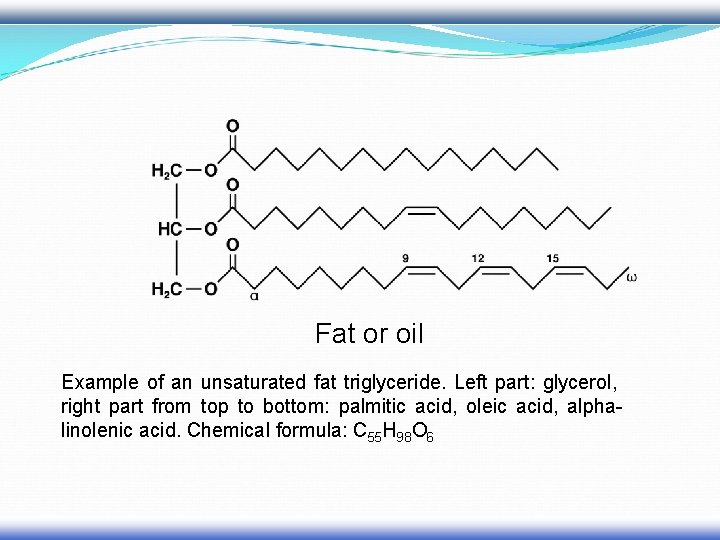
Fat or oil Example of an unsaturated fat triglyceride. Left part: glycerol, right part from top to bottom: palmitic acid, oleic acid, alphalinolenic acid. Chemical formula: C 55 H 98 O 6


Soap is a salt of a fatty acid

Soaps are less effective in hard water, which is water that contains a significant concentration of Mg 2+ and Ca 2+ ions. These ions form precipitates with soap molecules, and this precipitate is often seen as a gray line on a bathtub or sink and is often called “soap scum”.

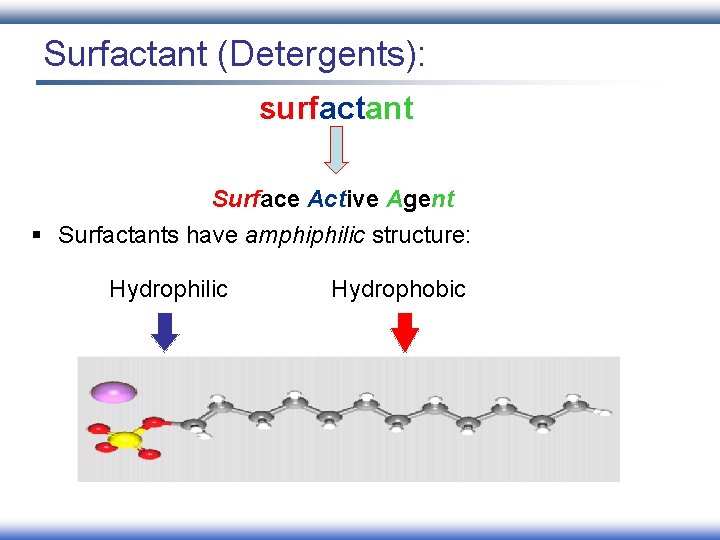
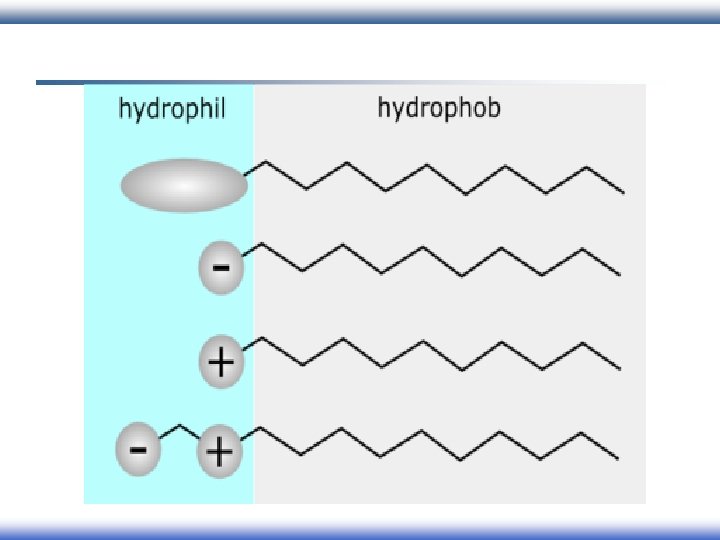
Surfactant (Detergents): surfactant Surface Active Agent § Surfactants have amphiphilic structure: Hydrophilic Hydrophobic

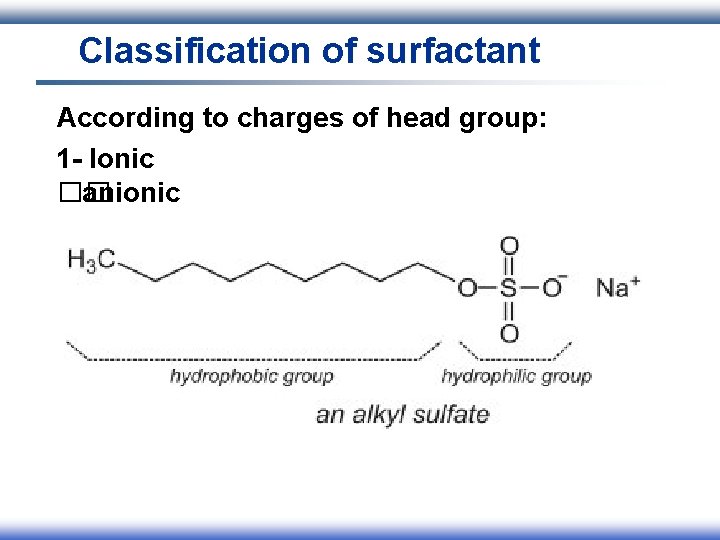
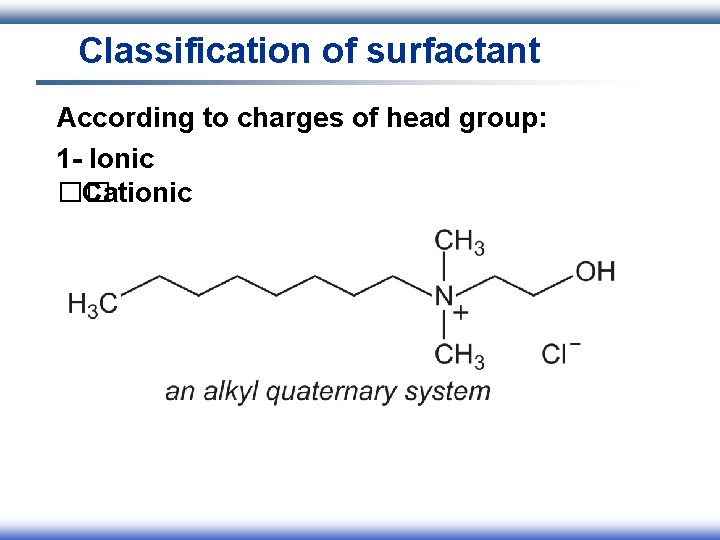
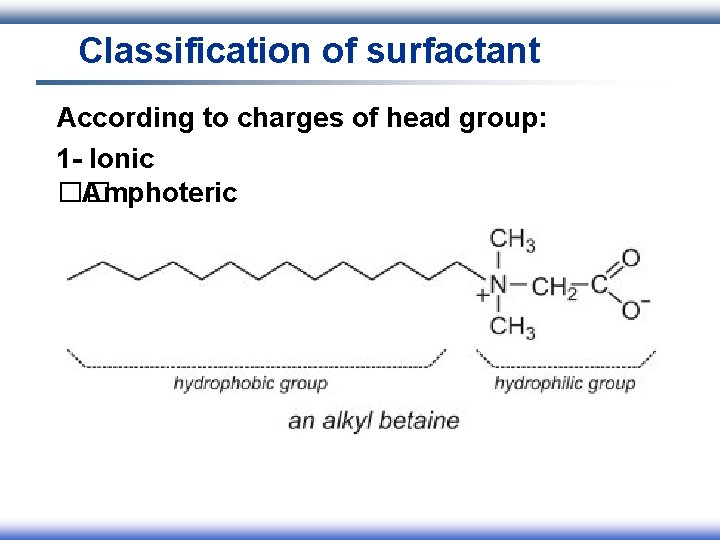
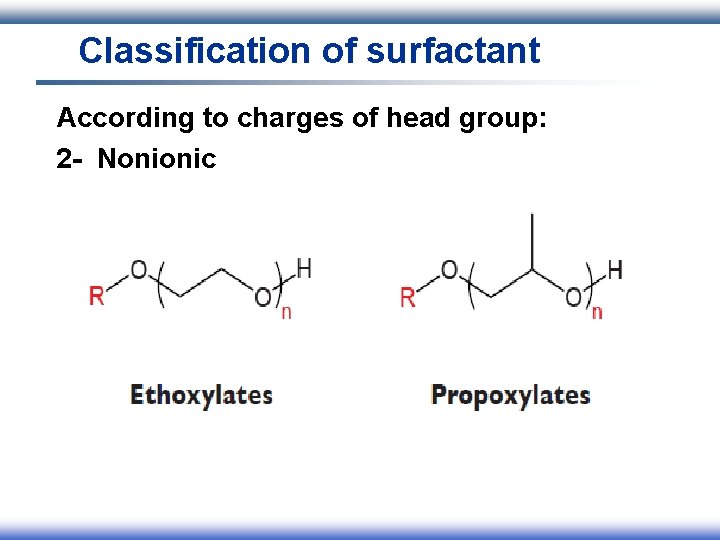
Classification of surfactant According to charges of head group: 1 - Ionic I. Anionic II. Cationic III. Amphoteric 2 - Nonionic

Classification of surfactant According to charges of head group: 1 - Ionic �� anionic

Classification of surfactant According to charges of head group: 1 - Ionic �� Cationic

Classification of surfactant According to charges of head group: 1 - Ionic �� Amphoteric

Classification of surfactant According to charges of head group: 2 - Nonionic




Behavior of surfactants: § When a molecule with amphiphilic structure is dissolved in aqueous medium, the hydrophobic group distorts the structure of the water. § As a result of this distortion, some of the surfactant molecules are expelled to the surfaces of the system with their hydrophobic groups oriented to minimize contact with the water molecules. Nonpolar tail Polar head

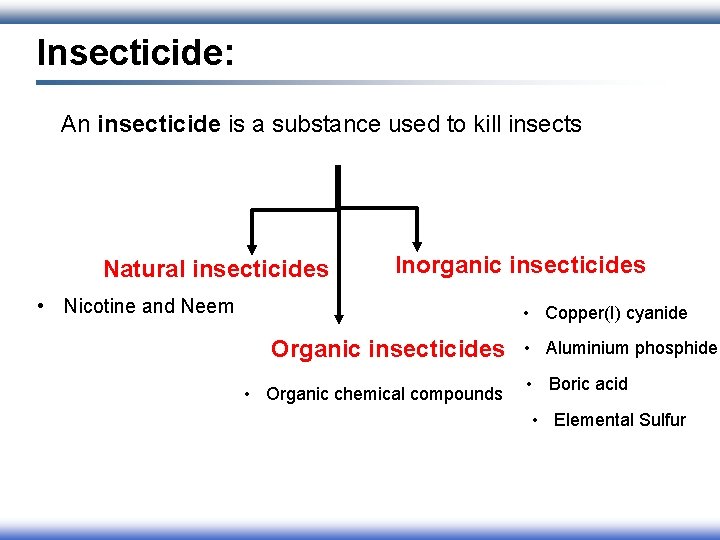
Insecticide: An insecticide is a substance used to kill insects Natural insecticides Inorganic insecticides • Nicotine and Neem • Copper(I) cyanide Organic insecticides • Organic chemical compounds • Aluminium phosphide • Boric acid • Elemental Sulfur

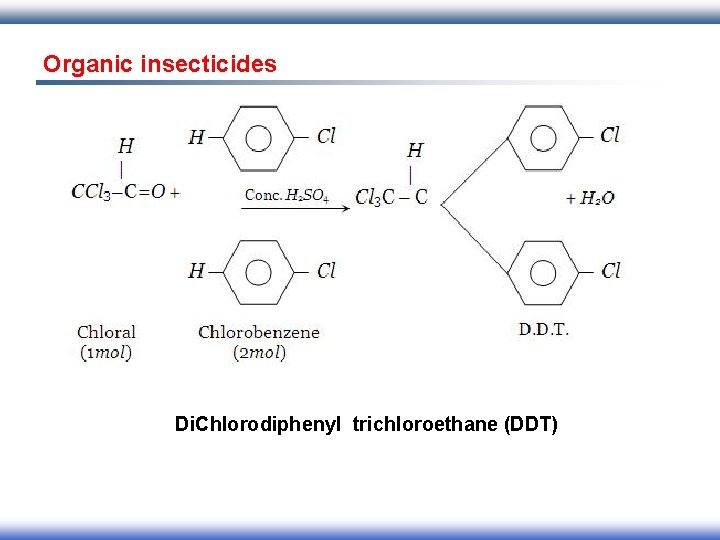
Organic insecticides Di. Chlorodiphenyl trichloroethane (DDT)

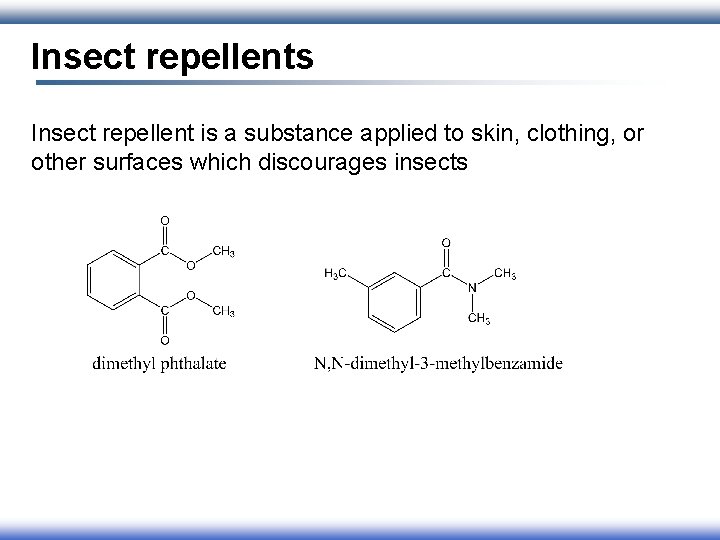
Insect repellents Insect repellent is a substance applied to skin, clothing, or other surfaces which discourages insects

Drugs 1. Analgesics and antipyretics • Analgesic drugs relieve pain. • Antipyretics are substances that reduce fever.

Aspirin (acetylsalicylic acid) • Used for more than 100 years • Treats mild to moderate pain • Antipyretic effect

Side Effects • Gastrointestinal irritation and bleeding • Increases bleeding time • Tinnitus • Children: Reye’s syndrome

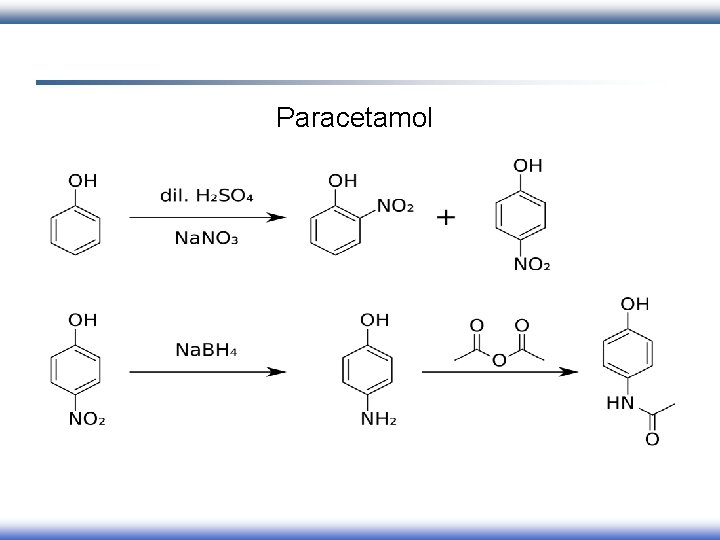
Paracetamol

Sulpha drugs Sulfonamide (also called sulphonamide or sulpha drugs) areanti bacterial that contain the sulfonamide group Sulphadiazine sluphaguanidine

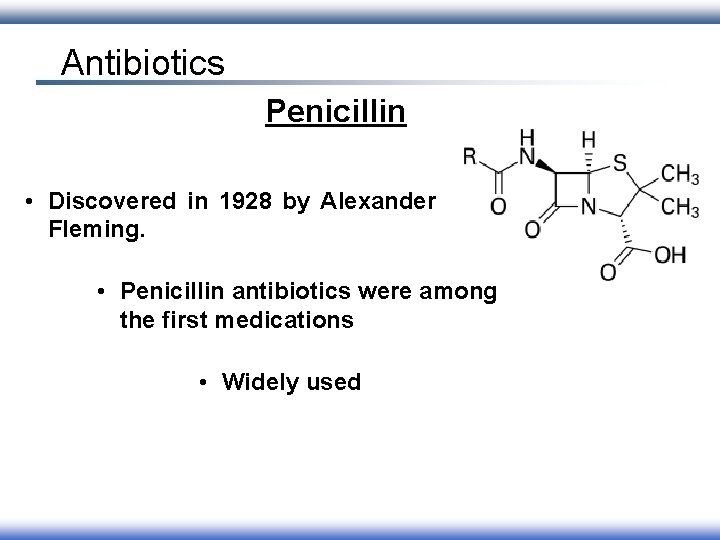
Antibiotics Penicillin • Discovered in 1928 by Alexander Fleming. • Penicillin antibiotics were among the first medications • Widely used
 Katalog kosmetik
Katalog kosmetik Kosmetiklovgivningen
Kosmetiklovgivningen Peraturan kawalan dadah dan kosmetik 1984
Peraturan kawalan dadah dan kosmetik 1984 Anytime cosmetics
Anytime cosmetics Chapter 24 review questions milady
Chapter 24 review questions milady Blood bank regulation under drugs and cosmetics act
Blood bank regulation under drugs and cosmetics act Nabi cosmetics wikipedia
Nabi cosmetics wikipedia Stackstorage
Stackstorage Cosmetics are substances that are used to enhance……..
Cosmetics are substances that are used to enhance…….. Mary kay cosmetics mission statement
Mary kay cosmetics mission statement Mac cosmetics target market
Mac cosmetics target market Desert daughter
Desert daughter Liquid crystal cosmetics
Liquid crystal cosmetics Behavioure
Behavioure Bsc cosmetics
Bsc cosmetics Beneficence examples
Beneficence examples Example of oily binder in compact
Example of oily binder in compact Cosmetics business proposal
Cosmetics business proposal Wonder products roorkee
Wonder products roorkee Alquimia essential oil
Alquimia essential oil Phthalates in cosmetics
Phthalates in cosmetics Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Ancient greek chemistry
Ancient greek chemistry What is the greek miracle in greek mythology
What is the greek miracle in greek mythology Example of oxidizing agent
Example of oxidizing agent Chapter 1 introduction to chemistry
Chapter 1 introduction to chemistry How to calculate molality
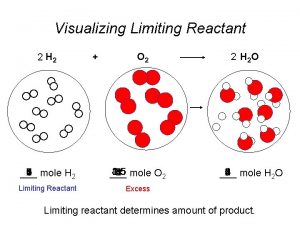
How to calculate molality Limiting reagent problems
Limiting reagent problems How to separate salt and water
How to separate salt and water Rodrigo andrade chemistry
Rodrigo andrade chemistry Hmpa in chemistry
Hmpa in chemistry Chemistry
Chemistry How to calculate density in chemistry
How to calculate density in chemistry Three characteristics of metals
Three characteristics of metals General chemistry
General chemistry Ap chemistry big idea 2 review answers
Ap chemistry big idea 2 review answers