DIELECTRIC PROPERTIES OF ATi O 3 CERAMICS ACa













- Slides: 40

DIELECTRIC PROPERTIES OF ATi. O 3 CERAMICS ( A=Ca, Sr, Ba) SINTERED WITH 5 Mol. % OF Li. F AND Ca. F 2 L. Taïbi - Benziada ; Y. Sedkaoui Algeria AMOMEN ’ 2011, October 27 - 29 2011, Kenitra, MAROCCO

SUMMARY v v INTRODUCTION EXPERIMENTAL PROCEDURES RESULTS AND DISCUSSION CONCLUSION

INTRODUCTION

INTEREST FOR MATERIALS Materials have always represented an essential aspect of Human Society. v Nowadays, the Material became synonymous with Existence for any Industry. v In new Technologies of informations and communications, the Progress and Success are closely linked to the development of Advanced Ceramics with higher and higher performances but also with lower and lower factory cost to be competitive on the huge market of microelectronics. v

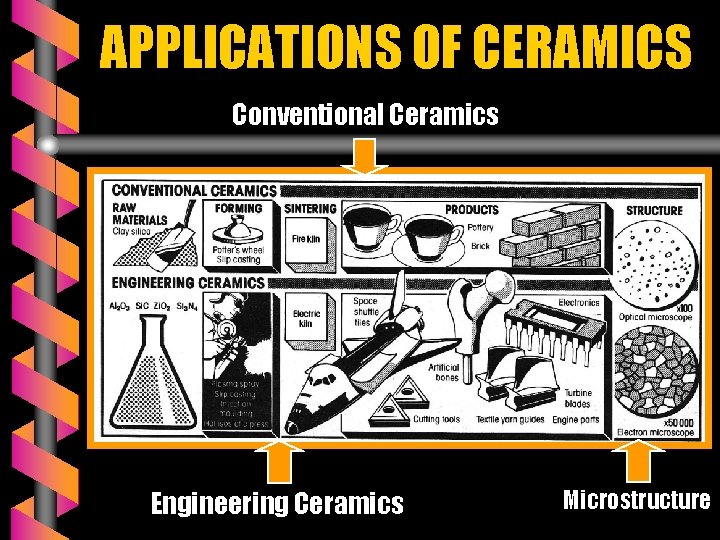
APPLICATIONS OF CERAMICS Conventional Ceramics Engineering Ceramics Microstructure

ABO 3 RELATED MATERIALS Among these new technical ceramics, ABO 3 perovskites and their solid solutions are of great interest for the Microelectronic Industry. v With the devices miniaturization, ATi. O 3 ceramics became the key materials for the devevopment of smart systems with high level of intelligence. v Up to now, the varied PZT have dominated the market of microelectronic components. However, the toxicity of Pb is a serious threat to human health and environment. v

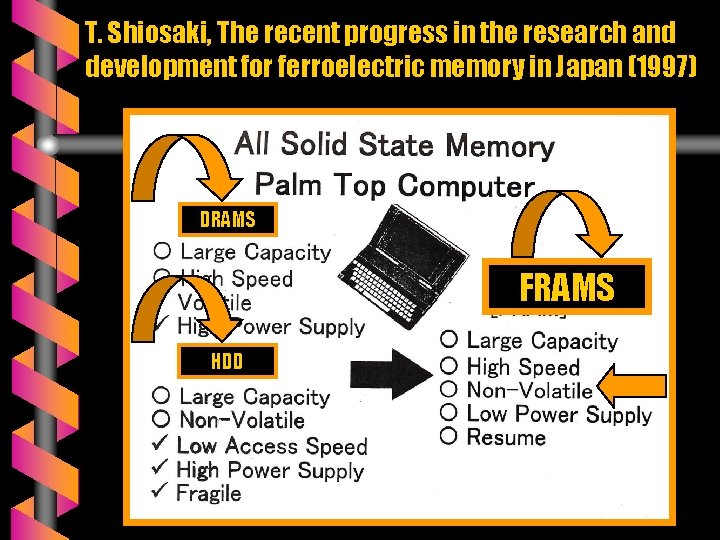
APPLICATIONS OF ABO 3 PEROVSKITES v Capacitors v Sensors v Resonators v Piezoelectric actuators v Pyroelectric infrared detectors v Electro-optical modulators v Computer memories. . . FRAMS

T. Shiosaki, The recent progress in the research and development for ferroelectric memory in Japan (1997) DRAMS FRAMS HDD

OBJECTIVES v The sintering at low temperature of lead free ceramics related to ATi. O 3 ( A = Ca, Sr, Ba ) with the aid of 5 mol. % of Ca. F 2 and Li. F : 0. 95 ATi. O 3 + 0. 05 Ca. F 2 + 0. 05 Li. F v The investigation of the dielectric properties in the obtained samples.

PROPERTIES OF ATi. O 3 CERAMICS ( A=Ca, Sr, Ba)

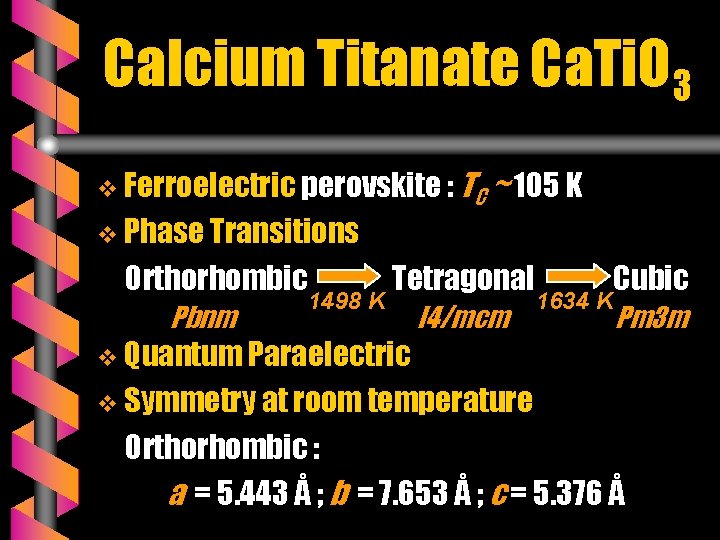
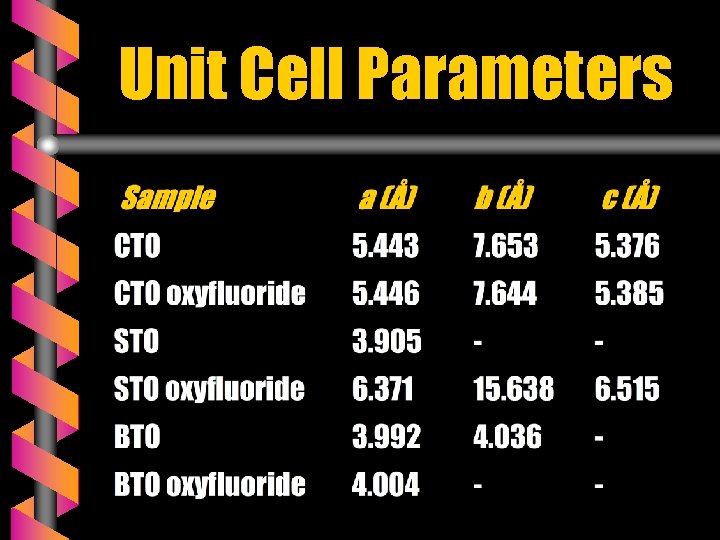
Calcium Titanate Ca. Ti. O 3 v Ferroelectric perovskite : TC ~ 105 K v Phase Transitions Orthorhombic Pbnm 1498 K Tetragonal Cubic I 4/mcm Pm 3 m v Quantum Paraelectric 1634 K v Symmetry at room temperature Orthorhombic : a = 5. 443 Å ; b = 7. 653 Å ; c = 5. 376 Å

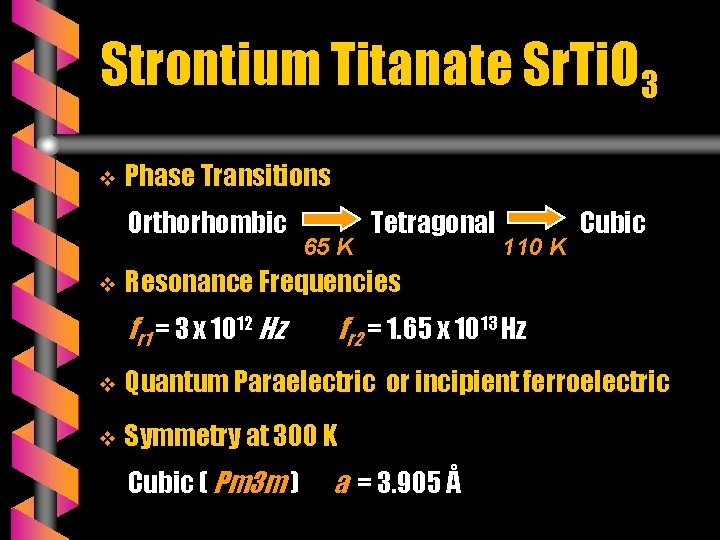
Strontium Titanate Sr. Ti. O 3 v Phase Transitions Orthorhombic v 65 K Tetragonal 110 K Cubic Resonance Frequencies fr 1 = 3 x 1012 Hz fr 2 = 1. 65 x 1013 Hz v Quantum Paraelectric or incipient ferroelectric v Symmetry at 300 K Cubic ( Pm 3 m ) a = 3. 905 Å

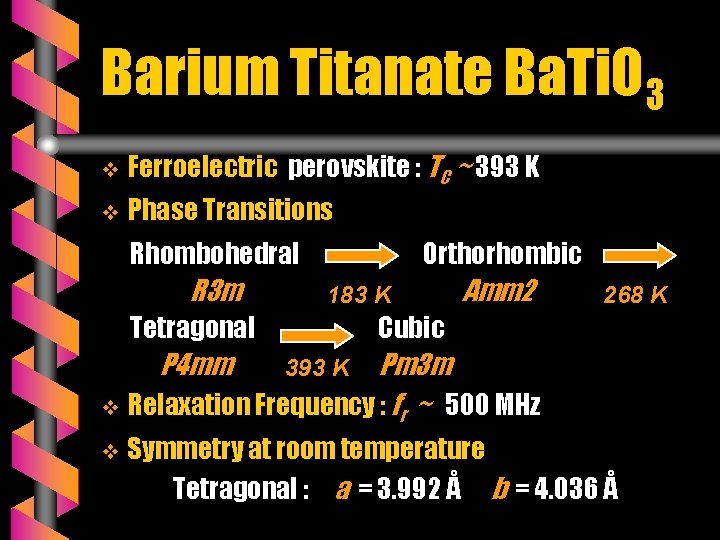
Barium Titanate Ba. Ti. O 3 v Ferroelectric perovskite : TC ~ 393 K v Phase Transitions Rhombohedral R 3 m Orthorhombic 183 K Tetragonal P 4 mm Amm 2 268 K Cubic Pm 3 m v Relaxation Frequency : fr ~ 500 MHz v 393 K Symmetry at room temperature Tetragonal : a = 3. 992 Å b = 4. 036 Å

EXPERIMENTAL PROCEDURES

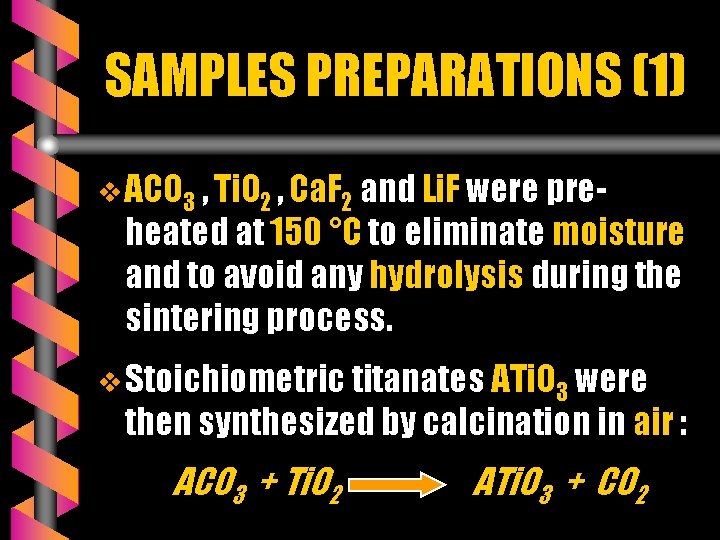
SAMPLES PREPARATIONS (1) v ACO 3 , Ti. O 2 , Ca. F 2 and Li. F were pre- heated at 150 °C to eliminate moisture and to avoid any hydrolysis during the sintering process. v Stoichiometric titanates ATi. O 3 were then synthesized by calcination in air : ACO 3 + Ti. O 2 ATi. O 3 + CO 2

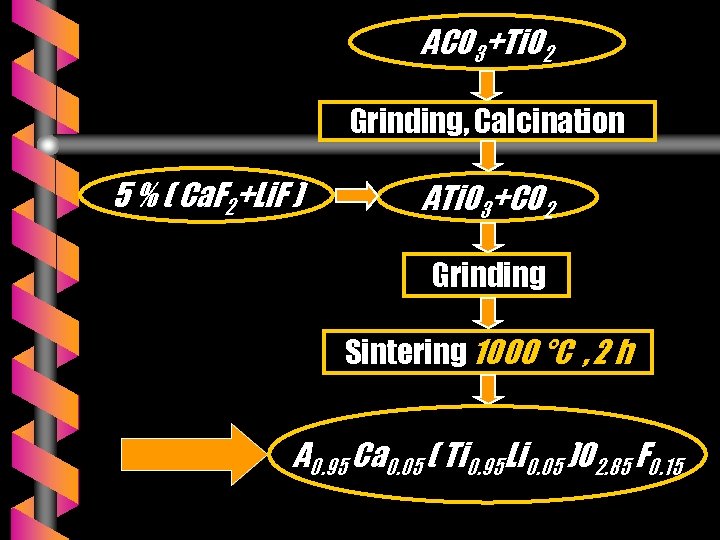
SAMPLES PREPARATIONS (2) v Various chemical compositions were prepared and ground in ethanol : 0. 95 ATi. O 3 + 0. 05 Ca. F 2 + 0. 05 Li. F v The mixtures thus obtained were cold- pressed to pellets with a binder under a pressure of 100 MPa and sintered in air at 1000 °C for 2 h on zircona plates.

ACO 3+Ti. O 2 Grinding, Calcination 5 % ( Ca. F 2+Li. F ) ATi. O 3+CO 2 Grinding Sintering 1000 °C , 2 h A 0. 95 Ca 0. 05 ( Ti 0. 95 Li 0. 05 )O 2. 85 F 0. 15

METHODS OF INVESTIGATIONS v The purity and the symmetry were checked by XRay diffraction on crushed ceramics at 300 K. v The ceramic’s microstructures were observed by Scanning Electron Microscopy on fractured samples. v Dielectric measurements were carried out as a function of temperature ( 100 K - 550 K ) and frequency ( 102 Hz - 4 x 106 Hz ).

RESULTS AND DISCUSSION

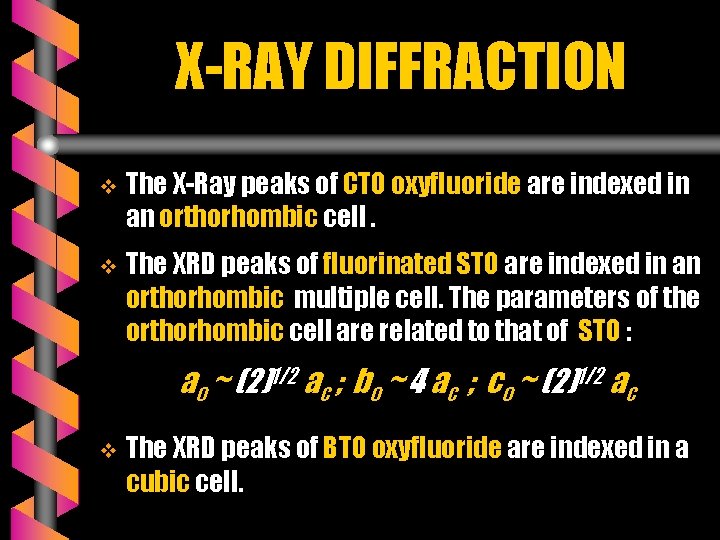
X-RAY DIFFRACTION v The X-Ray peaks of CTO oxyfluoride are indexed in an orthorhombic cell. v The XRD peaks of fluorinated STO are indexed in an orthorhombic multiple cell. The parameters of the orthorhombic cell are related to that of STO : ao ~ (2)1/2 ac ; bo ~ 4 ac ; co ~ (2)1/2 ac v The XRD peaks of BTO oxyfluoride are indexed in a cubic cell.

Unit Cell Parameters

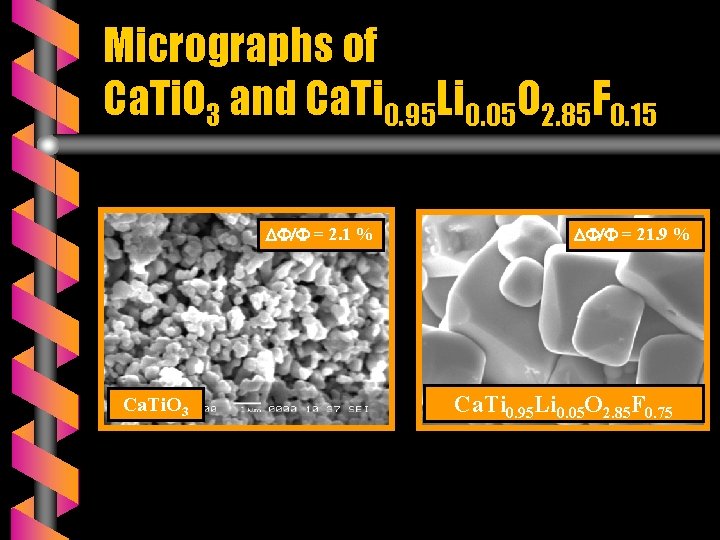
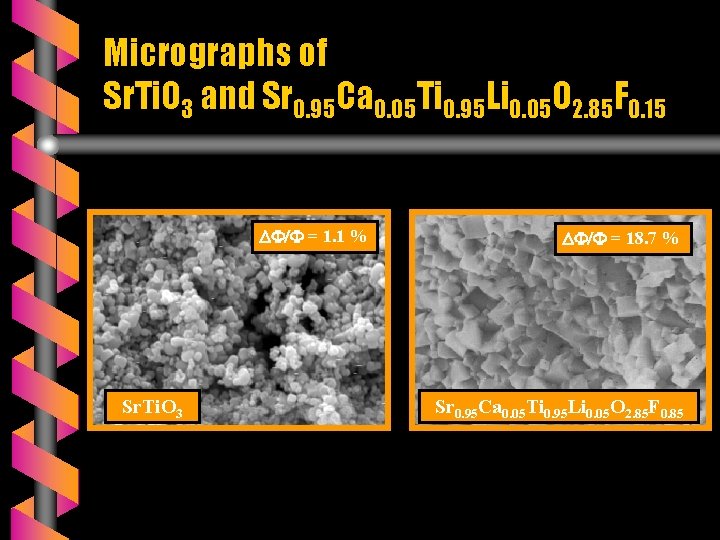
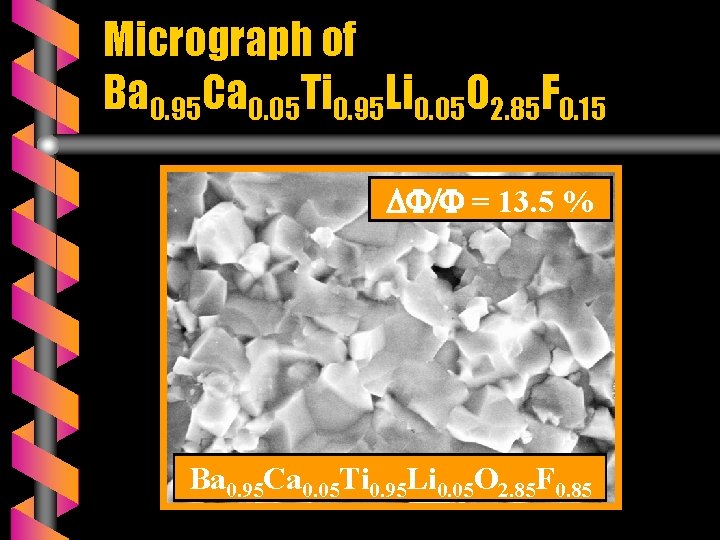
SEM OBSERVATIONS v The micrograhs of A(Ti, Li)(O, F)3 ceramics are monophasic. v ATi. O 3 ceramics are very brittle ( ΔΦ/Φ < 3 % ) and porous whereas the fluoridated ceramics are compact and very hard ( 13 % ΔΦ/Φ 22 % ). v The fluoride mixture Ca. F 2 + Li. F plays a double role: - as substituant and - as sintering agent.

Micrographs of Ca. Ti. O 3 and Ca. Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 = 2. 1 % Ca. Ti. O 3 = 21. 9 % Ca. Ti 0. 95 Li 0. 05 O 2. 85 F 0. 75

Micrographs of Sr. Ti. O 3 and Sr 0. 95 Ca 0. 05 Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 = 1. 1 % Sr. Ti. O 3 = 18. 7 % Sr 0. 95 Ca 0. 05 Ti 0. 95 Li 0. 05 O 2. 85 F 0. 85

Micrograph of Ba 0. 95 Ca 0. 05 Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 = 13. 5 % Ba 0. 95 Ca 0. 05 Ti 0. 95 Li 0. 05 O 2. 85 F 0. 85

DIELECTRIC PROPERTIES OF A(Ti, Li)(O, F)3 CERAMICS

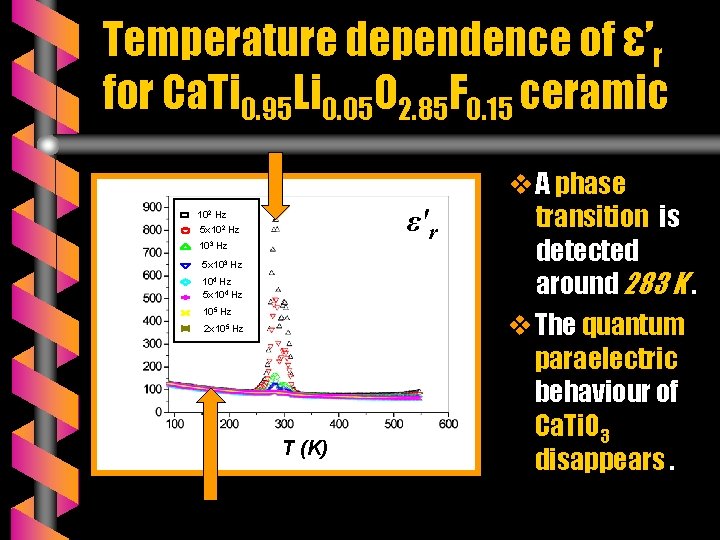
Temperature dependence of ε’r for Ca. Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 ceramic ε'r 102 Hz 5 x 102 Hz 103 Hz 5 x 103 Hz 104 Hz 5 x 104 Hz 105 Hz 2 x 105 Hz T (K) v A phase transition is detected around 283 K. v The quantum paraelectric behaviour of Ca. Ti. O 3 disappears.

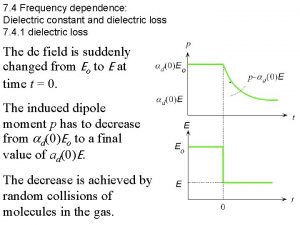
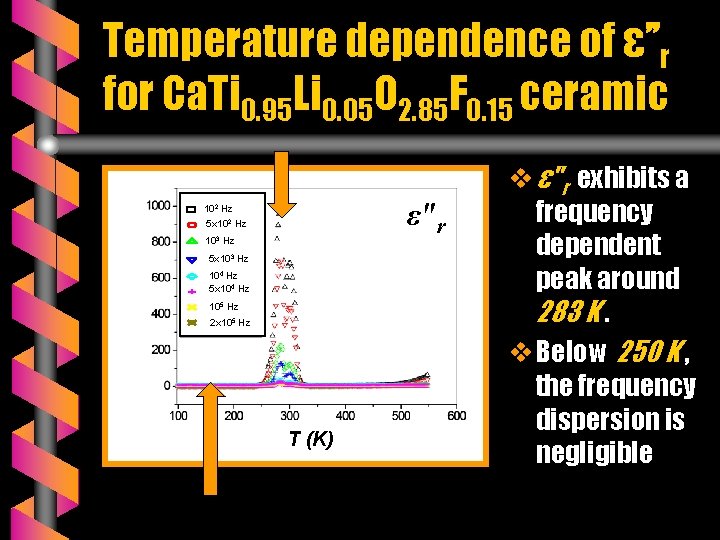
Temperature dependence of ε’’r for Ca. Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 ceramic ε"r 102 Hz 5 x 102 Hz 103 Hz 5 x 103 Hz 104 Hz 5 x 104 Hz 105 Hz 2 x 105 Hz T (K) v ε"r exhibits a frequency dependent peak around 283 K. v Below 250 K , the frequency dispersion is negligible

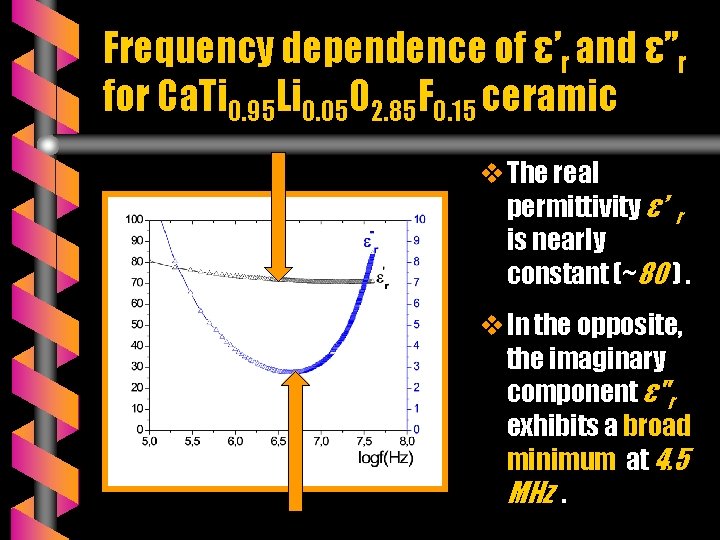
Frequency dependence of ε’r and ε’’r for Ca. Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 ceramic v The real permittivity ε’ r is nearly constant (~80 ). v In the opposite, the imaginary component ε"r exhibits a broad minimum at 4. 5 MHz.

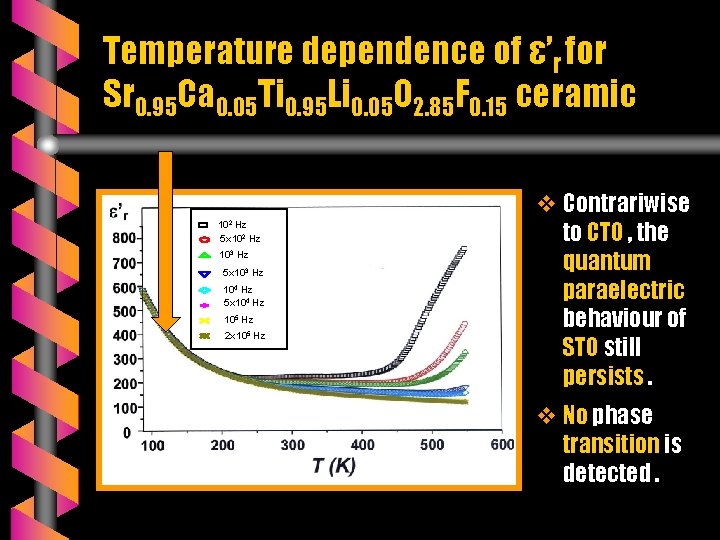
Temperature dependence of ε’r for Sr 0. 95 Ca 0. 05 Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 ceramic 102 Hz 5 x 102 Hz 103 Hz 5 x 103 Hz 104 Hz 5 x 104 Hz 105 Hz 2 x 105 Hz v Contrariwise to CTO , the quantum paraelectric behaviour of STO still persists. v No phase transition is detected.

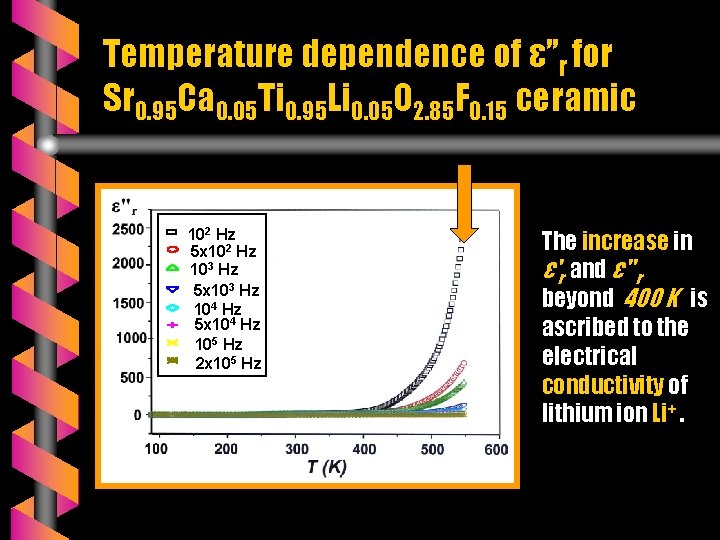
Temperature dependence of ε’’r for Sr 0. 95 Ca 0. 05 Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 ceramic 102 Hz 5 x 102 Hz 103 Hz 5 x 103 Hz 104 Hz 5 x 104 Hz 105 Hz 2 x 105 Hz The increase in ε'r and ε" r beyond 400 K is ascribed to the electrical conductivity of lithium ion Li+.

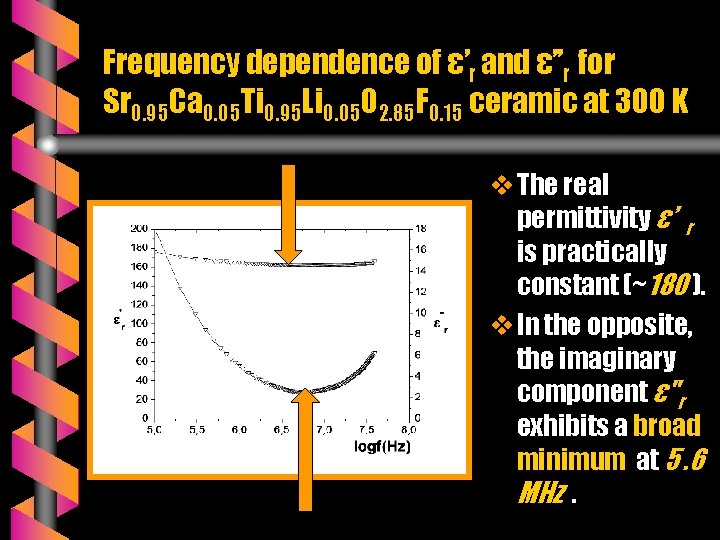
Frequency dependence of ε’r and ε’’r for Sr 0. 95 Ca 0. 05 Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 ceramic at 300 K v The real permittivity ε’ r is practically constant (~180 ). v In the opposite, the imaginary component ε"r exhibits a broad minimum at 5. 6 MHz.

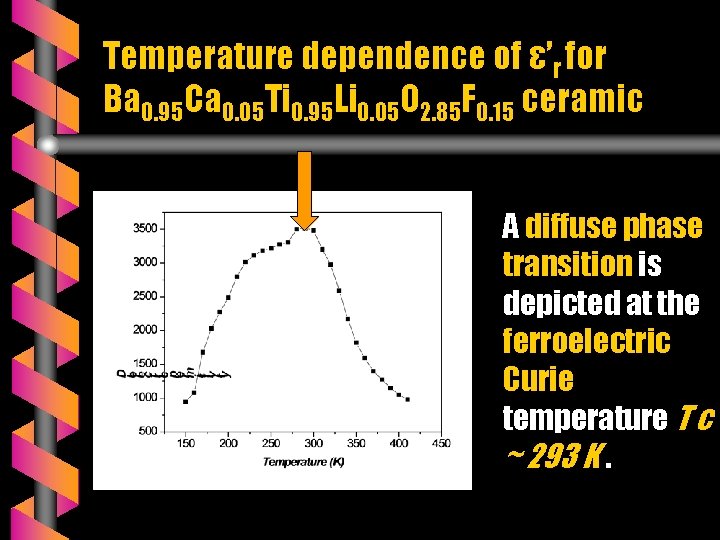
Temperature dependence of ε’r for Ba 0. 95 Ca 0. 05 Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 ceramic A diffuse phase transition is depicted at the ferroelectric Curie temperature T c ~ 293 K.

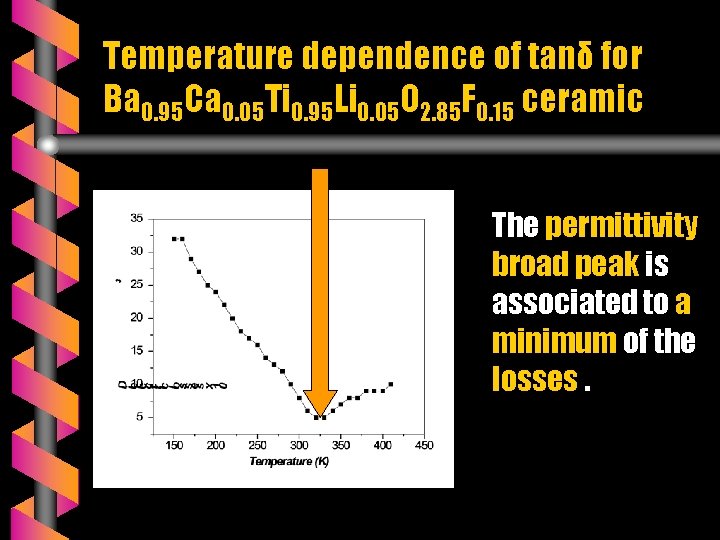
Temperature dependence of tanδ for Ba 0. 95 Ca 0. 05 Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 ceramic The permittivity broad peak is associated to a minimum of the losses.

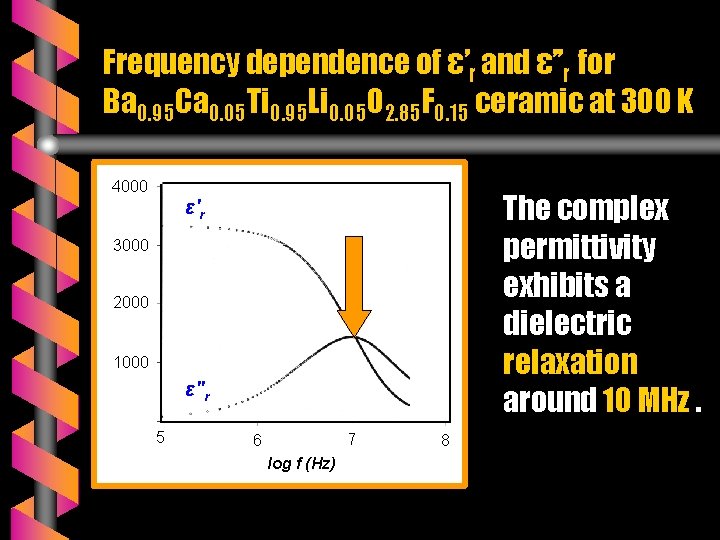
Frequency dependence of ε’r and ε’’r for Ba 0. 95 Ca 0. 05 Ti 0. 95 Li 0. 05 O 2. 85 F 0. 15 ceramic at 300 K 4000 The complex permittivity exhibits a dielectric relaxation around 10 MHz. ε'r 3000 2 1000 ε"r 5 7 6 log f (Hz) 8

CONCLUSION

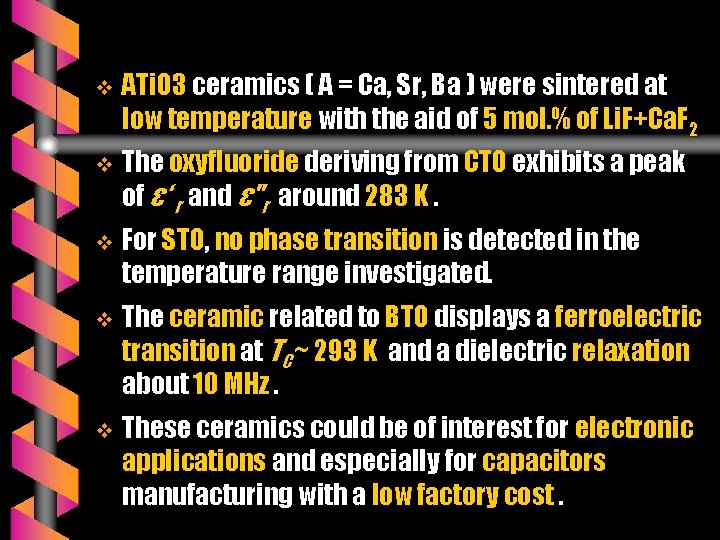
v ATi. O 3 ceramics ( A = Ca, Sr, Ba ) were sintered at low temperature with the aid of 5 mol. % of Li. F+Ca. F 2 v The oxyfluoride deriving from CTO exhibits a peak of ε‘ r and ε"r around 283 K. v For STO, no phase transition is detected in the temperature range investigated. v The ceramic related to BTO displays a ferroelectric transition at TC ~ 293 K and a dielectric relaxation about 10 MHz. v These ceramics could be of interest for electronic applications and especially for capacitors manufacturing with a low factory cost.

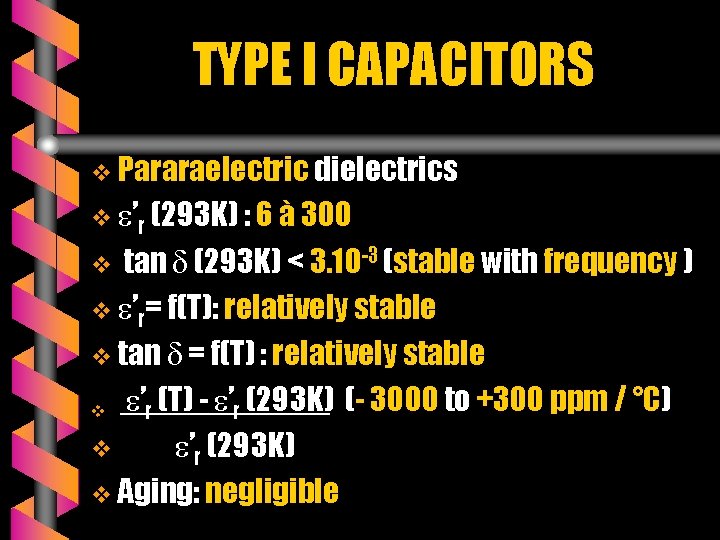
TYPE I CAPACITORS v Pararaelectric dielectrics v ’r (293 K) : 6 à 300 tan (293 K) < 3. 10 -3 (stable with frequency ) v ’r= f(T): relatively stable v tan = f(T) : relatively stable v ’r (T) - ’r (293 K) (- 3000 to +300 ppm / °C) v ’r (293 K) v Aging: negligible v

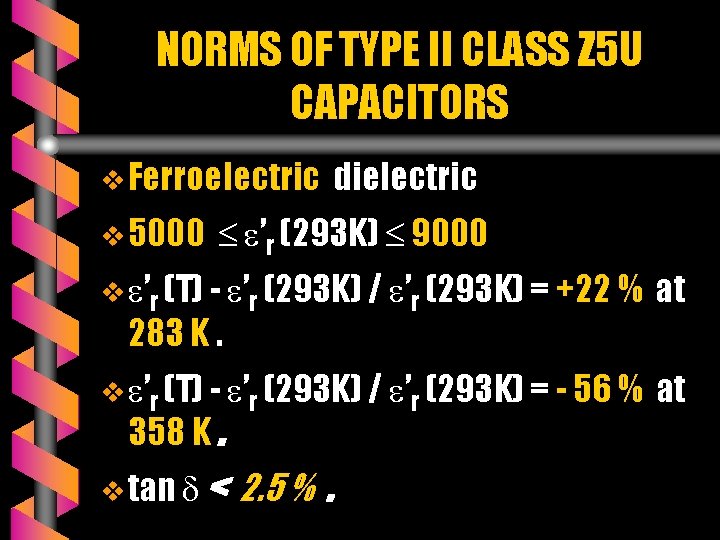
NORMS OF TYPE II CLASS Z 5 U CAPACITORS v Ferroelectric v 5000 dielectric ’r (293 K) 9000 v ’r (T) - ’r (293 K) / ’r (293 K) = +22 % at v ’r (T) - ’r (293 K) / ’r (293 K) = - 56 % at 283 K. 358 K. v tan < 2. 5 %.

THANK YOU FOR YOUR ATTENTION
 Disupear
Disupear Structure of ceramics
Structure of ceramics Structures and properties of ceramics
Structures and properties of ceramics Pcc academic advisor
Pcc academic advisor Aca international certification
Aca international certification Aca student liability insurance
Aca student liability insurance Photoshop study guide
Photoshop study guide Airman comprehensive assessment
Airman comprehensive assessment Aca.cl
Aca.cl Acá
Acá Aca 100
Aca 100 Aca code of ethics 2014
Aca code of ethics 2014 Imieslowy
Imieslowy California aca reporting requirements
California aca reporting requirements Ppms certification
Ppms certification Aca management information
Aca management information Integrity data aca
Integrity data aca Adaptive conjoint analysis aca
Adaptive conjoint analysis aca Infark
Infark Aca code of ethics 2019
Aca code of ethics 2019 Aca territory stroke
Aca territory stroke Bracketing therapy
Bracketing therapy Blood supply to the brain
Blood supply to the brain Imiesłów przymiotnikowy
Imiesłów przymiotnikowy Aca 221
Aca 221 Aca student liability insurance
Aca student liability insurance Effect of dielectric on capacitance
Effect of dielectric on capacitance Derive gauss law in dielectric medium
Derive gauss law in dielectric medium Normaldand
Normaldand What is dielectric materials
What is dielectric materials Dielectric heating
Dielectric heating Solid dielectric switchgear
Solid dielectric switchgear Boundary conditions for perfect dielectric materials
Boundary conditions for perfect dielectric materials Dielectric constant definition in physics
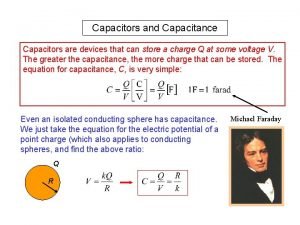
Dielectric constant definition in physics Potential energy of capacitor
Potential energy of capacitor Interlevel dielectric
Interlevel dielectric Conduction and breakdown in commercial liquids
Conduction and breakdown in commercial liquids Langevin debye equation
Langevin debye equation Dielectric heating formula
Dielectric heating formula Dielectric laser accelerator
Dielectric laser accelerator 370hr dielectric constant
370hr dielectric constant