Moya on MRI and MRA e Ed E










- Slides: 27

Moya on MRI and MRA e. Ed. E#: e. Ed. E-183 Control #: 2500 Steven Sogge, MD Krish Thamburaj, MD

Financial Disclosures • We do not have any financial disclosures.

Objectives • Review the classification and pathophysiology of Moya • Review the various neuroimaging features of Moya disease on MRI and MRA

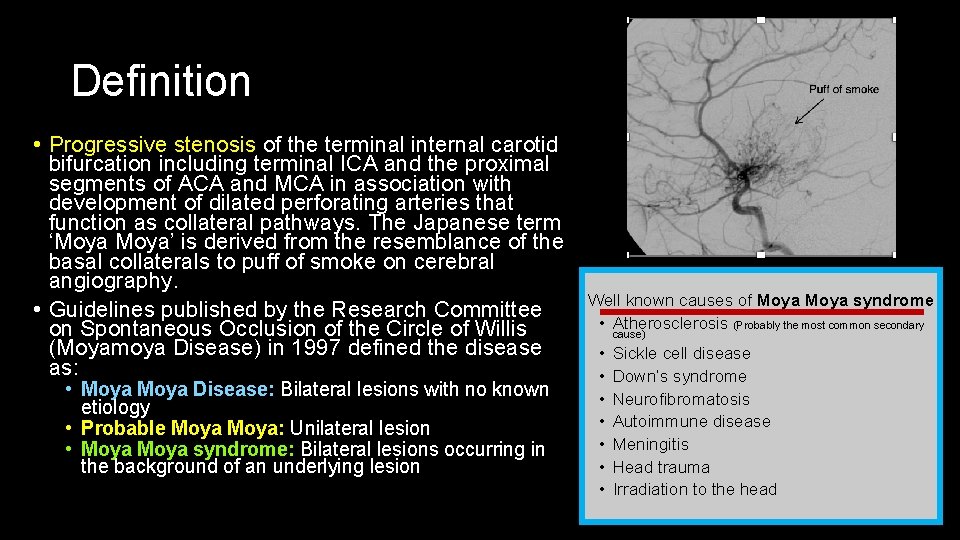
Definition • Progressive stenosis of the terminal internal carotid bifurcation including terminal ICA and the proximal segments of ACA and MCA in association with development of dilated perforating arteries that function as collateral pathways. The Japanese term ‘Moya’ is derived from the resemblance of the basal collaterals to puff of smoke on cerebral angiography. • Guidelines published by the Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) in 1997 defined the disease as: • Moya Disease: Bilateral lesions with no known etiology • Probable Moya: Unilateral lesion • Moya syndrome: Bilateral lesions occurring in the background of an underlying lesion Puff of smoke Well known causes of Moya syndrome • Atherosclerosis (Probably the most common secondary cause) • • Sickle cell disease Down’s syndrome Neurofibromatosis Autoimmune disease Meningitis Head trauma Irradiation to the head

Epidemiology • Observed throughout the world. High incidence in East Asia. In Japan, the annual prevalence and incidence have been estimated at 3· 16 and 0· 35 per 100000, respectively. • Incidence from western parts of USA 0. 086/100, 000. Incidencerate ratios reported in Asian Americans 4. 6, Blacks 2. 2, and Hispanics 0. 5, as compared with Whites. • F: M = 1. 8 • Age: Bimodal distribution, primary peak at age 5 with smaller peak at age 40.

Pathophysiology • Not completely understood • Stenosis starts at distal ICA. May start in proximal MCA or ACA before progressing to terminal ICA. • Pathology of affected vessel demonstrates smooth muscle proliferation and intraluminal thrombi. Fragmented undulated internal elastic lamina and thin media are evident. • Caspase-3 -dependent apoptosis might be associated with these histopathological changes. • Moya vessels also demonstrate similar changes in vessel wall; fragility of vessel wall may (Hematoxylin & eosin) of right terminal ICA shows result in microaneurysm formation. These hyperproliferation (black arrow) of the vessel-wall histopathological changes might be closely components and abundant intraluminal thrombi (blue arrow), leading to narrowing and occlusion of the lumen. associated with the onset of ischemic and Scott RM et al. , NEJM 2009; 360: 1226 hemorrhagic stroke.

Clinical presentation • Trasient ischemic attacks (TIA) and ischemic infarcts typically in younger children. Areas affected tends to be in the territory of the ICA, particularly in the frontal lobe. Repeated insults can lead to impairment of higher order functions later in life. • Hyperventilation will induce ischemic attacks secondary to decreased Pa. CO 2 which leads to arterial vasoconstriction. • Intracranial hemorrhage occurs more commonly in older individuals, those typically occurring around the second peak of incidence. There are two causes of bleed; the first is ruptured of dilated fragile moya vessels or rupture of saccular aneurysms that occur in the setting of the disease. • Headache • Epilepsy • Involuntary movements

Diagnostic criteria and classification • Historically, cerebral angiography is the gold standard. • Cerebral angiography is less than ideal in the primary population affected by Moya due to ionizing radiation exposure. • Per guidelines from the Research Committee on Spontaneous Occlusion of the Circle of Willis (Moya Disease), cerebral angiography is not mandatory if MRI and MRA demonstrate: • Stenosis or occlusion at the end of the ICA or at the proximal part of the ACAs and MCAs on MRA, and an • Abnormal vascular network seen in the basal ganglia with MRA. • The new criteria of the Research Committee for Diagnosis of Moyamoya Disease recommend MRA with a 1· 5 T machine, and MRA scans with 0· 5 T or 1· 0 T machines are not recommended • Stenosis or occlusion of the terminal part of the ICA (the C 1–C 2 portion) and the proximal part of the ACAs and MCAs bilaterally. • Stenosis or occlusion of the proximal part of the posterior cerebral artery also affects about 25% of patients with Moya disease.

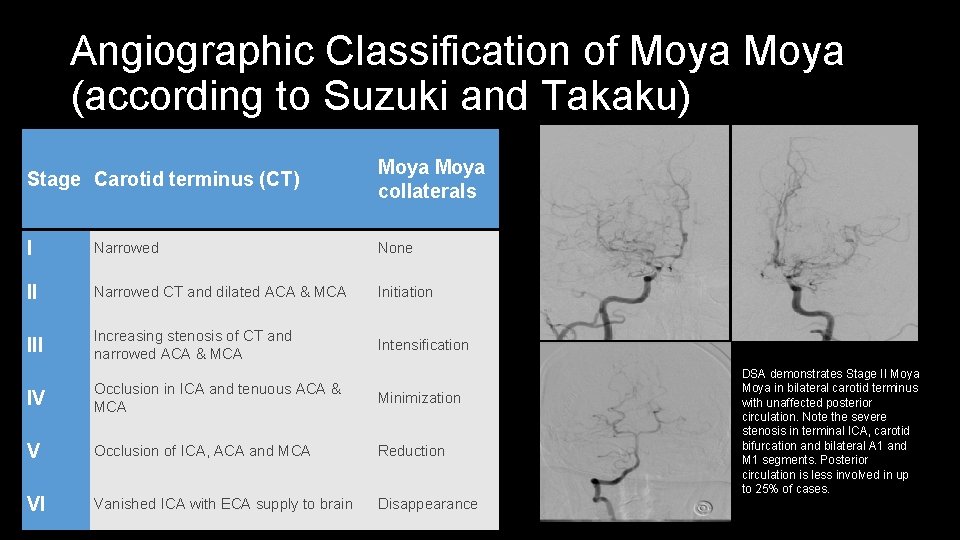
Angiographic Classification of Moya (according to Suzuki and Takaku) Stage Carotid terminus (CT) Moya collaterals I Narrowed None II Narrowed CT and dilated ACA & MCA Initiation III Increasing stenosis of CT and narrowed ACA & MCA Intensification IV Occlusion in ICA and tenuous ACA & MCA Minimization V Occlusion of ICA, ACA and MCA Reduction VI Vanished ICA with ECA supply to brain Disappearance DSA demonstrates Stage II Moya in bilateral carotid terminus with unaffected posterior circulation. Note the severe stenosis in terminal ICA, carotid bifurcation and bilateral A 1 and M 1 segments. Posterior circulation is less involved in up to 25% of cases.

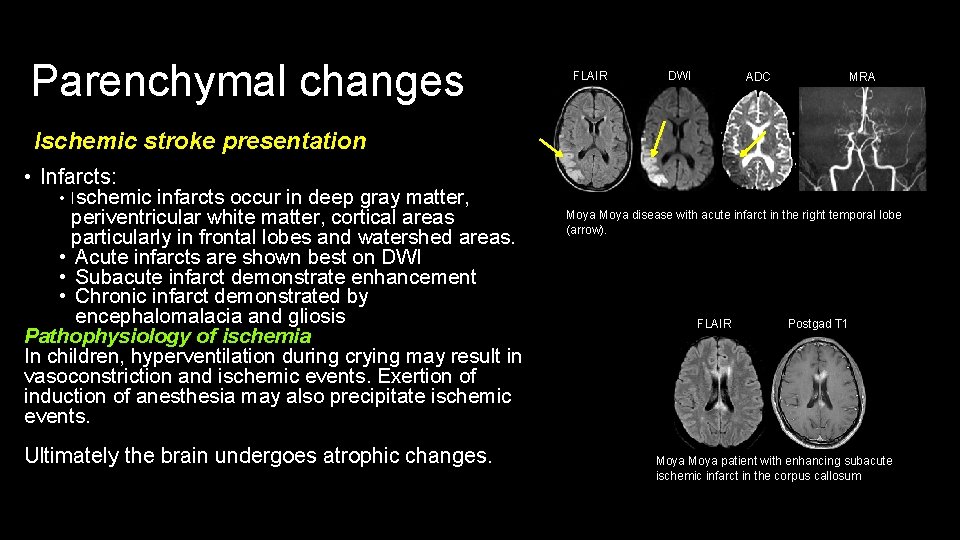
Parenchymal changes FLAIR DWI ADC MRA Ischemic stroke presentation • Infarcts: • Ischemic infarcts occur in deep gray matter, periventricular white matter, cortical areas particularly in frontal lobes and watershed areas. • Acute infarcts are shown best on DWI • Subacute infarct demonstrate enhancement • Chronic infarct demonstrated by encephalomalacia and gliosis Pathophysiology of ischemia In children, hyperventilation during crying may result in vasoconstriction and ischemic events. Exertion of induction of anesthesia may also precipitate ischemic events. Ultimately the brain undergoes atrophic changes. Moya disease with acute infarct in the right temporal lobe (arrow). FLAIR Postgad T 1 Moya patient with enhancing subacute ischemic infarct in the corpus callosum

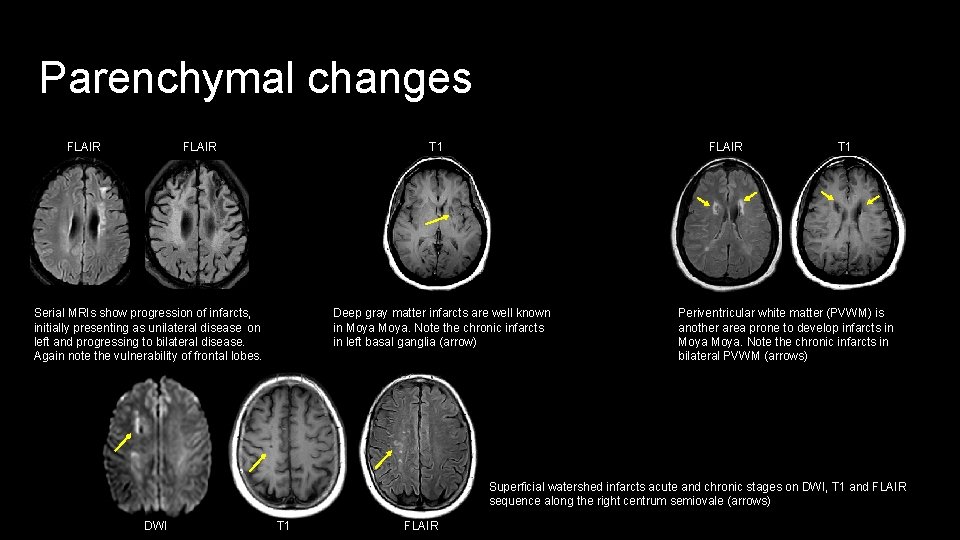
Parenchymal changes FLAIR T 1 Deep gray matter infarcts are well known in Moya. Note the chronic infarcts in left basal ganglia (arrow) Serial MRIs show progression of infarcts, initially presenting as unilateral disease on left and progressing to bilateral disease. Again note the vulnerability of frontal lobes. T 1 Periventricular white matter (PVWM) is another area prone to develop infarcts in Moya. Note the chronic infarcts in bilateral PVWM (arrows) Superficial watershed infarcts acute and chronic stages on DWI, T 1 and FLAIR sequence along the right centrum semiovale (arrows) DWI T 1 FLAIR

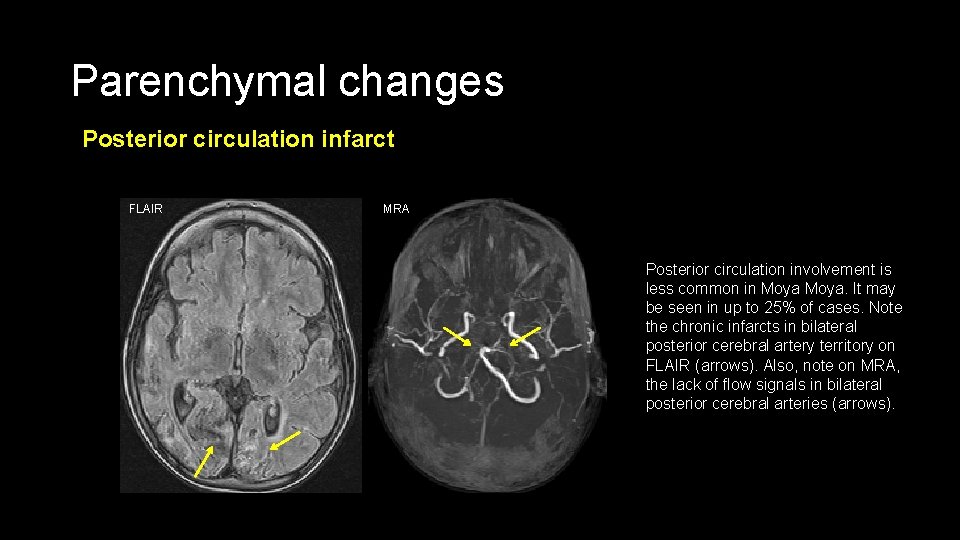
Parenchymal changes Posterior circulation infarct FLAIR MRA Posterior circulation involvement is less common in Moya. It may be seen in up to 25% of cases. Note the chronic infarcts in bilateral posterior cerebral artery territory on FLAIR (arrows). Also, note on MRA, the lack of flow signals in bilateral posterior cerebral arteries (arrows).

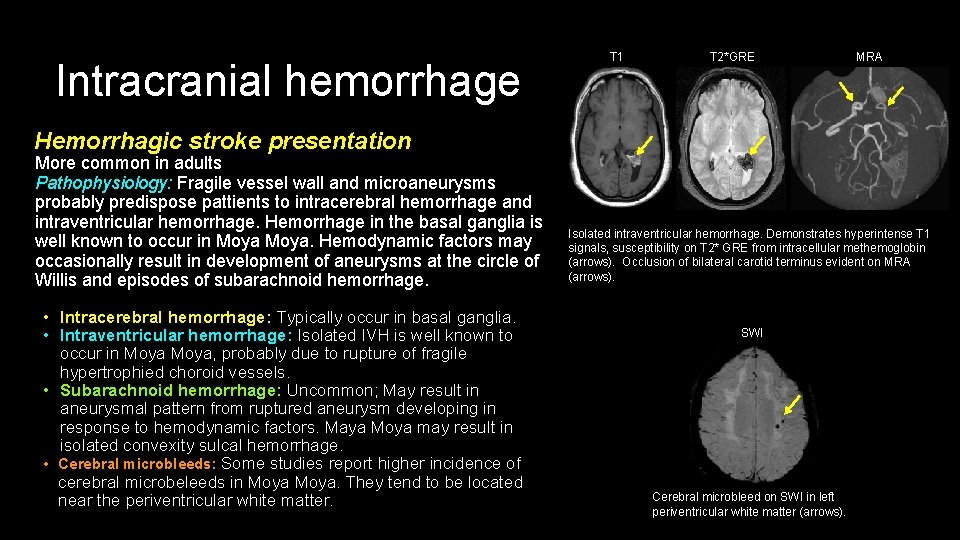
Intracranial hemorrhage T 1 T 2*GRE MRA Hemorrhagic stroke presentation More common in adults Pathophysiology: Fragile vessel wall and microaneurysms probably predispose pattients to intracerebral hemorrhage and intraventricular hemorrhage. Hemorrhage in the basal ganglia is well known to occur in Moya. Hemodynamic factors may occasionally result in development of aneurysms at the circle of Willis and episodes of subarachnoid hemorrhage. • Intracerebral hemorrhage: Typically occur in basal ganglia. • Intraventricular hemorrhage: Isolated IVH is well known to occur in Moya, probably due to rupture of fragile hypertrophied choroid vessels. • Subarachnoid hemorrhage: Uncommon; May result in aneurysmal pattern from ruptured aneurysm developing in response to hemodynamic factors. Maya Moya may result in isolated convexity sulcal hemorrhage. • Cerebral microbleeds: Some studies report higher incidence of cerebral microbeleeds in Moya. They tend to be located near the periventricular white matter. Isolated intraventricular hemorrhage. Demonstrates hyperintense T 1 signals, susceptibility on T 2* GRE from intracellular methemoglobin (arrows). Occlusion of bilateral carotid terminus evident on MRA (arrows). SWI Cerebral microbleed on SWI in left periventricular white matter (arrows).

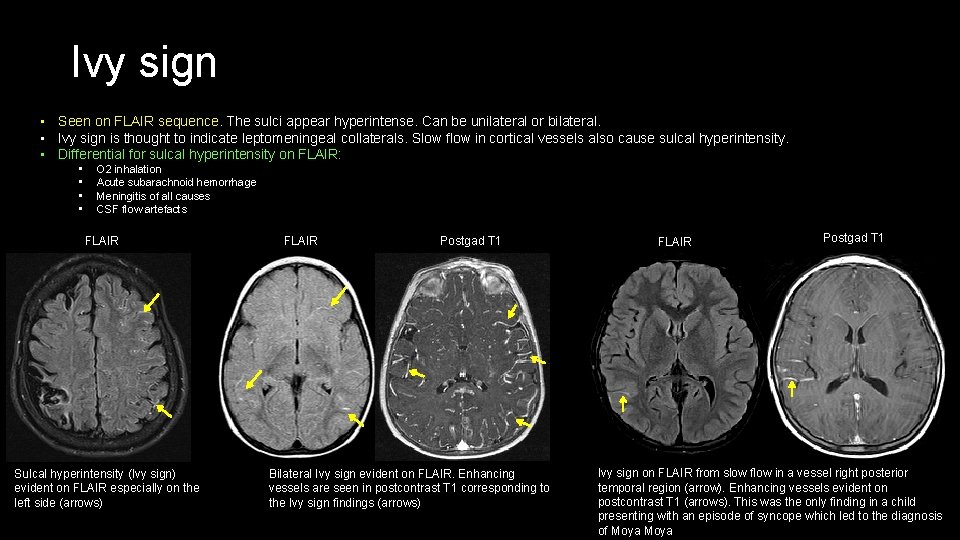
Ivy sign • Seen on FLAIR sequence. The sulci appear hyperintense. Can be unilateral or bilateral. • Ivy sign is thought to indicate leptomeningeal collaterals. Slow flow in cortical vessels also cause sulcal hyperintensity. • Differential for sulcal hyperintensity on FLAIR: • • O 2 inhalation Acute subarachnoid hemorrhage Meningitis of all causes CSF flow artefacts FLAIR Sulcal hyperintensity (Ivy sign) evident on FLAIR especially on the left side (arrows) FLAIR Postgad T 1 Bilateral Ivy sign evident on FLAIR. Enhancing vessels are seen in postcontrast T 1 corresponding to the Ivy sign findings (arrows) FLAIR Postgad T 1 Ivy sign on FLAIR from slow flow in a vessel right posterior temporal region (arrow). Enhancing vessels evident on postcontrast T 1 (arrows). This was the only finding in a child presenting with an episode of syncope which led to the diagnosis of Moya

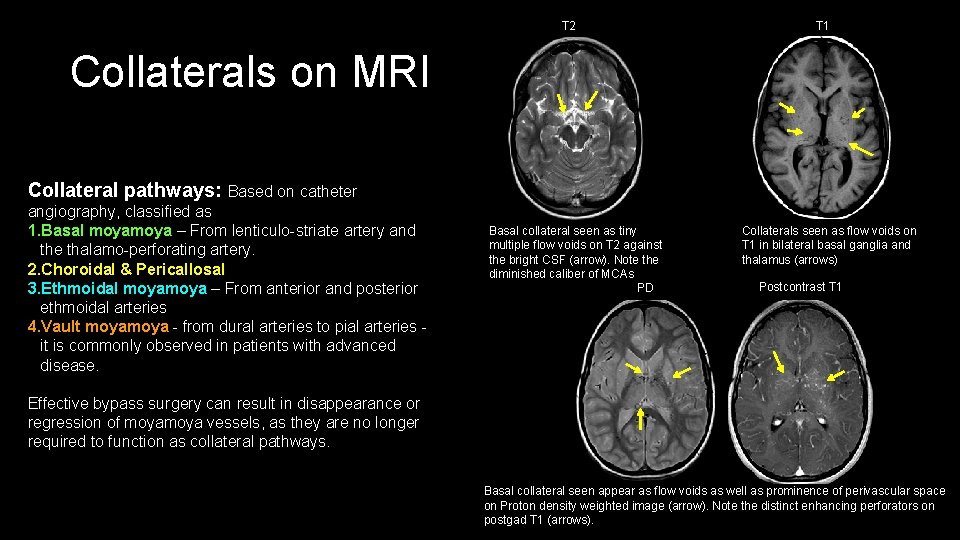
T 2 T 1 Collaterals on MRI Collateral pathways: Based on catheter angiography, classified as 1. Basal moya – From lenticulo-striate artery and the thalamo-perforating artery. 2. Choroidal & Pericallosal 3. Ethmoidal moya – From anterior and posterior ethmoidal arteries 4. Vault moya - from dural arteries to pial arteries - it is commonly observed in patients with advanced disease. Basal collateral seen as tiny multiple flow voids on T 2 against the bright CSF (arrow). Note the diminished caliber of MCAs PD Collaterals seen as flow voids on T 1 in bilateral basal ganglia and thalamus (arrows) Postcontrast T 1 Effective bypass surgery can result in disappearance or regression of moya vessels, as they are no longer required to function as collateral pathways. Basal collateral seen appear as flow voids as well as prominence of perivascular space on Proton density weighted image (arrow). Note the distinct enhancing perforators on postgad T 1 (arrows).

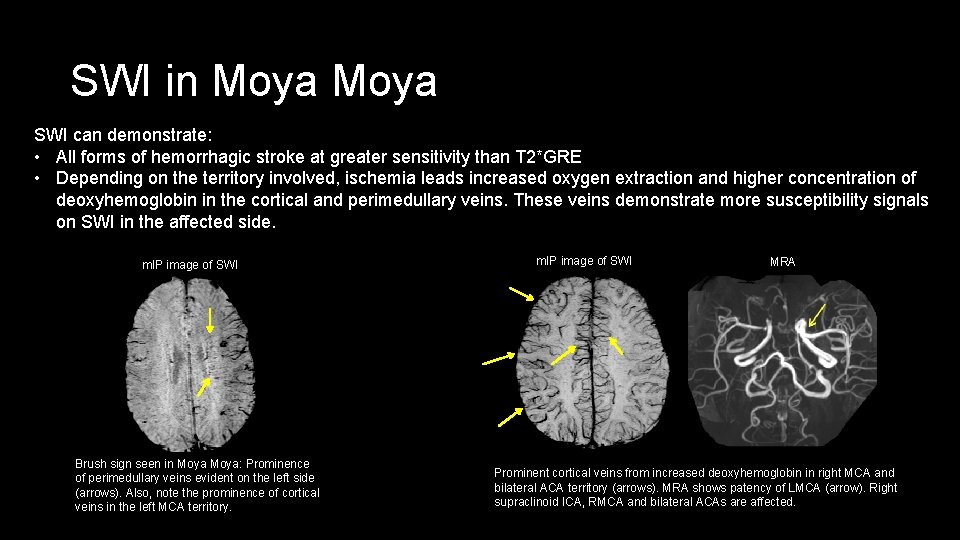
SWI in Moya SWI can demonstrate: • All forms of hemorrhagic stroke at greater sensitivity than T 2*GRE • Depending on the territory involved, ischemia leads increased oxygen extraction and higher concentration of deoxyhemoglobin in the cortical and perimedullary veins. These veins demonstrate more susceptibility signals on SWI in the affected side. m. IP image of SWI Brush sign seen in Moya: Prominence of perimedullary veins evident on the left side (arrows). Also, note the prominence of cortical veins in the left MCA territory. m. IP image of SWI MRA Prominent cortical veins from increased deoxyhemoglobin in right MCA and bilateral ACA territory (arrows). MRA shows patency of LMCA (arrow). Right supraclinoid ICA, RMCA and bilateral ACAs are affected.

MRA in Moya • Technique: Time of flight is the most commonly used technique. Lack of ionizing radiation and lack of IV contrast are major advantages. • TOF MRA may overestimate stenosis as occlusion. • MRA can also can be used to identify Moya collaterals. • MRA scores correlated well with the six-stage classification on cerebral angiography, with a high sensitivity and specificity. • Can be used to monitor the disease progression as well as post surgical changes. • In accordance with the guidelines of the Research Committee on Spontaneous Occlusion of the Circle of Willis (Moya Disease), cerebral angiography is not mandatory if MRI and MRA show all of the following findings: • Stenosis or occlusion at the end of the ICA or at the proximal part of the ACAs and MCAs on MRA, and an • Abnormal vascular network seen in the basal ganglia with MRA. • The new criteria of the Research Committee for Diagnosis of Moya Disease recommend MRA with a 1. 5 T machine, and MRA scans with 0. 5 T or 1. 0 T machines are not recommended.

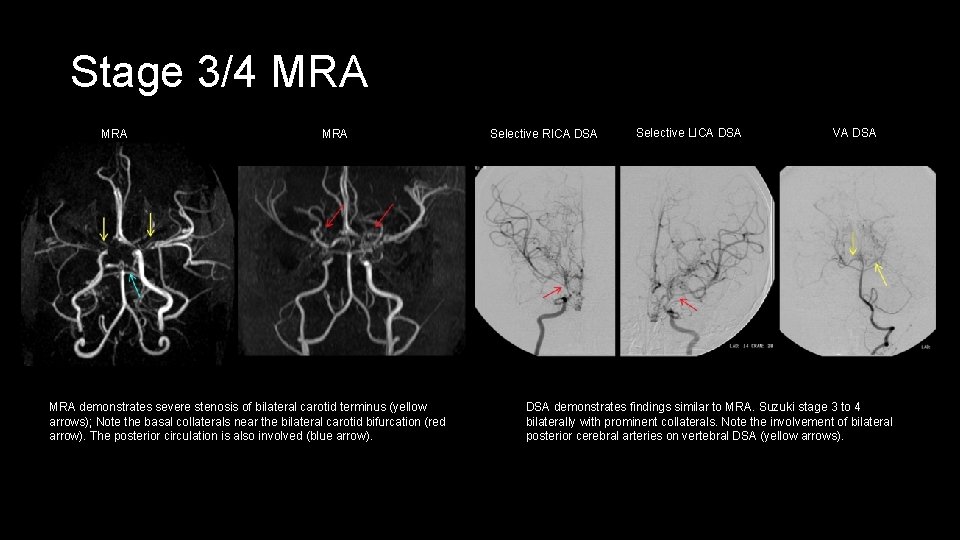
Stage 3/4 MRA MRA demonstrates severe stenosis of bilateral carotid terminus (yellow arrows); Note the basal collaterals near the bilateral carotid bifurcation (red arrow). The posterior circulation is also involved (blue arrow). Selective RICA DSA Selective LICA DSA VA DSA demonstrates findings similar to MRA. Suzuki stage 3 to 4 bilaterally with prominent collaterals. Note the involvement of bilateral posterior cerebral arteries on vertebral DSA (yellow arrows).

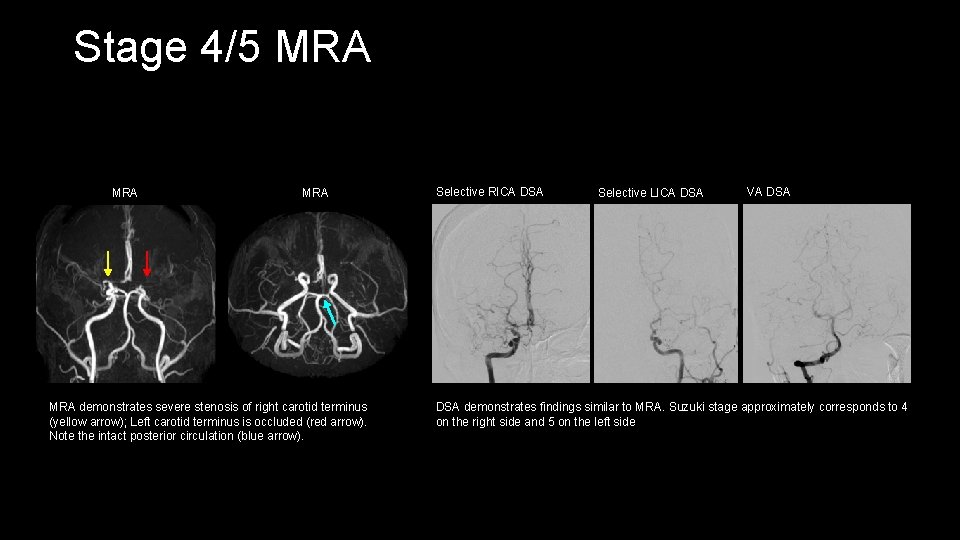
Stage 4/5 MRA MRA demonstrates severe stenosis of right carotid terminus (yellow arrow); Left carotid terminus is occluded (red arrow). Note the intact posterior circulation (blue arrow). Selective RICA DSA Selective LICA DSA VA DSA demonstrates findings similar to MRA. Suzuki stage approximately corresponds to 4 on the right side and 5 on the left side

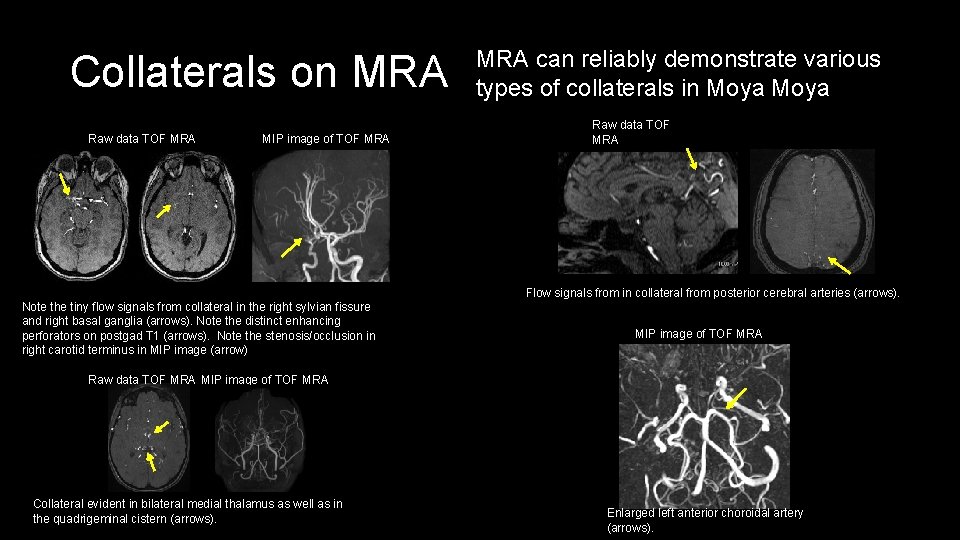
Collaterals on MRA Raw data TOF MRA MIP image of TOF MRA can reliably demonstrate various types of collaterals in Moya Raw data TOF MRA Flow signals from in collateral from posterior cerebral arteries (arrows). Note the tiny flow signals from collateral in the right sylvian fissure and right basal ganglia (arrows). Note the distinct enhancing perforators on postgad T 1 (arrows). Note the stenosis/occlusion in right carotid terminus in MIP image (arrow) MIP image of TOF MRA Raw data TOF MRA MIP image of TOF MRA Collateral evident in bilateral medial thalamus as well as in the quadrigeminal cistern (arrows). Enlarged left anterior choroidal artery (arrows).

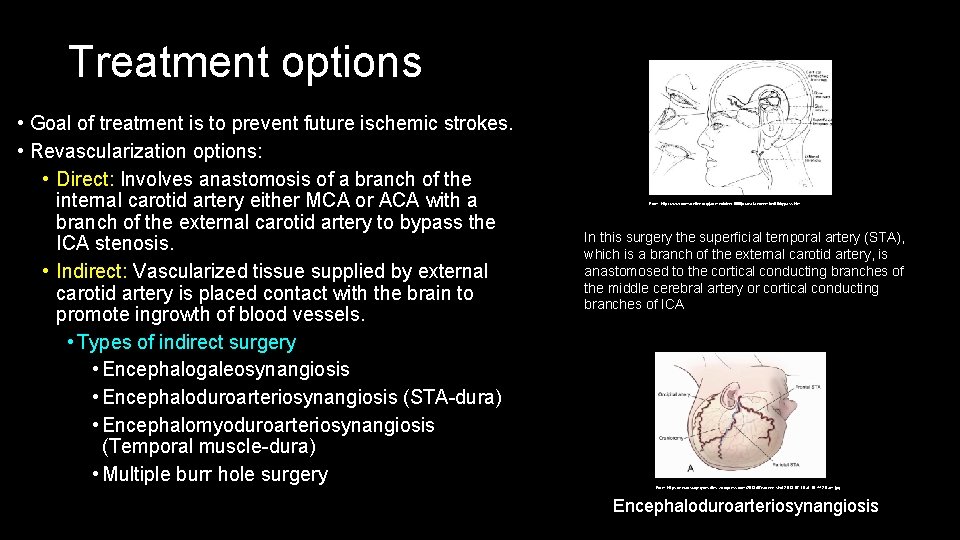
Treatment options • Goal of treatment is to prevent future ischemic strokes. • Revascularization options: • Direct: Involves anastomosis of a branch of the internal carotid artery either MCA or ACA with a branch of the external carotid artery to bypass the ICA stenosis. • Indirect: Vascularized tissue supplied by external carotid artery is placed contact with the brain to promote ingrowth of blood vessels. • Types of indirect surgery • Encephalogaleosynangiosis • Encephaloduroarteriosynangiosis (STA-dura) • Encephalomyoduroarteriosynangiosis (Temporal muscle-dura) • Multiple burr hole surgery From: http: //www. dcmsonline. org/jax-medicine/1998 journals/november 98/bypass. htm In this surgery the superficial temporal artery (STA), which is a branch of the external carotid artery, is anastomosed to the cortical conducting branches of the middle cerebral artery or cortical conducting branches of ICA From: https: //neurosurgerycns. files. wordpress. com/2013/07/screen-shot-2013 -07 -16 -at-10 -44 -29 -am. jpg Encephaloduroarteriosynangiosis

MRA and post surgical changes • Encephaloduroarteriosynangiosis (EDAS): - Enlargement of the superficial temporal artery (STA) and middle meningeal artery (MMA) can be observed; Approximately 3 months later, well developed collaterals can be seen on MRA. • EDAS has a high success rate in pediatric cases. In adults, 40 -50% of cases may not develop adequate collaterals. • STA-MCA bypass: Patency can be assessed on MRA. Aneurysm may develop rarely at the site of anastomosis. MRA can help identify the complications. • Post treatment changes: • Moya vessels start regressing 1 month after combined bypass surgery. STA and MMA increase their caliber in 3 months after surgery. Stenotic change in the carotid terminations quickly progresses after surgery. There is a reciprocal relation between neovascularization and the regression of moya vessels.

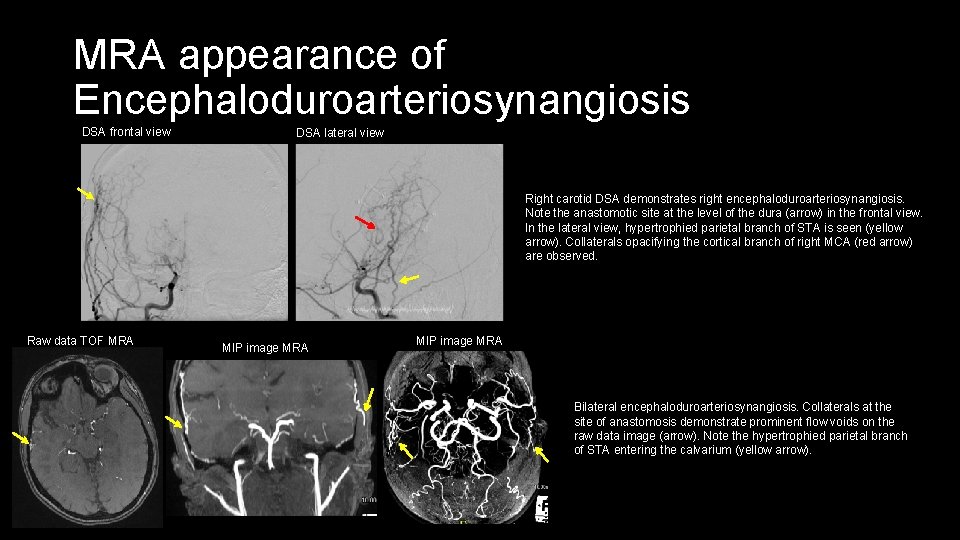
MRA appearance of Encephaloduroarteriosynangiosis DSA frontal view DSA lateral view Right carotid DSA demonstrates right encephaloduroarteriosynangiosis. Note the anastomotic site at the level of the dura (arrow) in the frontal view. In the lateral view, hypertrophied parietal branch of STA is seen (yellow arrow). Collaterals opacifying the cortical branch of right MCA (red arrow) are observed. Raw data TOF MRA MIP image MRA Bilateral encephaloduroarteriosynangiosis. Collaterals at the site of anastomosis demonstrate prominent flow voids on the raw data image (arrow). Note the hypertrophied parietal branch of STA entering the calvarium (yellow arrow).

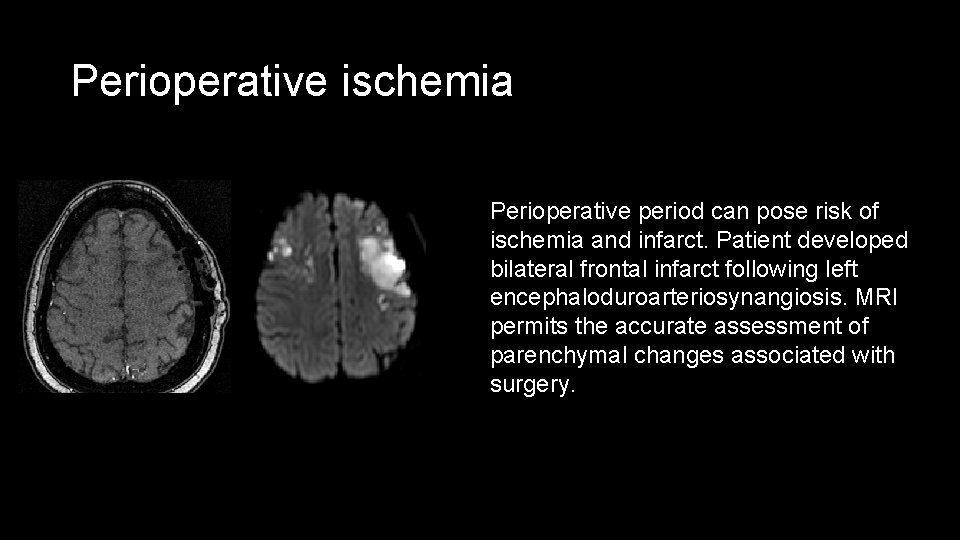
Perioperative ischemia Perioperative period can pose risk of ischemia and infarct. Patient developed bilateral frontal infarct following left encephaloduroarteriosynangiosis. MRI permits the accurate assessment of parenchymal changes associated with surgery.

MR perfusion in Moya Several techniques permit assessment of cerebral perfusion including MRI, CT, SPECT and PET. MR perfusion can be assessed with postcontrast dynamic perfusion and arterial spin labelling technique. Diamox challenge can help identify tissue at risk and guide treatment decisions. MR perfusion permits assessment of cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT) and time to peak (TTP) maps. DSA MRA Note the excellent correlation between DSA and MRA. Severe stenosis is evident in the right carotid terminus with nonvisualization of RA 1 from occlusion. Left is also occluded with failure to visualize the ACA branches on bilateral carotid injections. Note the fetal origin of left PCA. The posterior circulation is intact with left P 1 hypoplasia from developmental variation. Dynamic contrast enhanced MR perfusion. CBF demonstrates decrease in BF RMCA territory particularly in the frontal lobe (arrow). CBV shows increased BV in right MCA and bilateral ACA, particularly on the right side. MTT shows delayed transit RMCA and bilateral ACA. (arrows) 1523366

Conclusions • Moya disease is an uncommon condition. • The disease can present with ischemic or hemorrhagic stroke, with ischemic strokes more common in the pediatric population. • It is characterized by development of progressive stenosis carotid terminus with development of collaterals. • DSA is considered the gold standard to diagnose. MRI is the noninvasive imaging technique of choice. MRI and MRA can reliably demonstrate several neuroimaging features, satisfying the diagnostic criteria for Moya disease. • MRI and MRA can be used to monitor the progress of the disease and guide treatment. MR perfusion may help identify cerebral parenchyma at risk. • Post surgical changes and associated improvement and complications can be recognized with MRI and MRA.

References • Scott RM, and Smith ER. Moyamoya Disease and Moyamoya Syndrome. N Engl J Med 2009; 360: 1226 -37. • Kuroda S(1), Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008 Nov; 7(11): 1056 -66. doi: 10. 1016/S 1474 -4422(08)70240 -0. • Suzuki J, Takaku A. Cerebrovascular "moya" disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969; 20 (3): 288 -99. • Mugikura S, Takahashi S, Higano S et-al. The relationship between cerebral infarction and angiographic characteristics in childhood moya disease. AJNR Am J Neuroradiol. 1999; 20 (2): 336 -43. • Yoon HK, Shin HJ, Chang YW. "Ivy sign" in childhood moya disease: depiction on FLAIR and contrastenhanced T 1 -weighted MR images. Radiology. 2002; 223 (2): 384 -9. • Bruno A, Adams HP, Biller J et-al. Cerebral infarction due to moya disease in young adults. Stroke. 1988; 19 (7): 826 -33. • Horie N, Morikawa M, Nozaki A et-al. "Brush Sign" on susceptibility-weighted MR imaging indicates the severity of moya disease. AJNR Am J Neuroradiol. 2011; 32 (9): 1697 -702. • Ryoo S, Cha J, Kim SJ, et al. High-Resolution Magnetic Resonance Wall Imaging Findings of Moyamya disease. Stroke 2014; 45: 2457 -2460. • Lin R, Xie Z, Zhang J, Xu H, Su H, Tan X, et al. Clinical and immunopathological features of moya disease. Plo. S one. 2012; 7: e 36386