Dengue Chikungunya Zika Knowledge gaps Research priorities and


























- Slides: 45

Dengue, Chikungunya & Zika: Knowledge gaps, Research priorities and Way forward Prepared by Mrs Rani Chandran


Chikungunya Fever The disease mostly occurs in Africa, Asia and the Indian subcontinent. However a major outbreak in 2015 affected several countries of the Region of the Americas. Chikungunya is a viral disease transmitted to humans by infected Aedes mosquitoes. It causes fever and severe joint pain. Other symptoms include muscle pain, headache, nausea, fatigue and rash. First outbreak was reported in southern Tanzania in 1952. The name “chikungunya” derives from a word in the Kimakonde language, meaning “to become contorted”, and describes the stooped appearance of sufferers with joint pain (arthralgia). Chikungunya is rarely fatal. Symptoms are generally self-limiting and last for 2– 3 days. The virus remains in the human system for 5 -7 days.

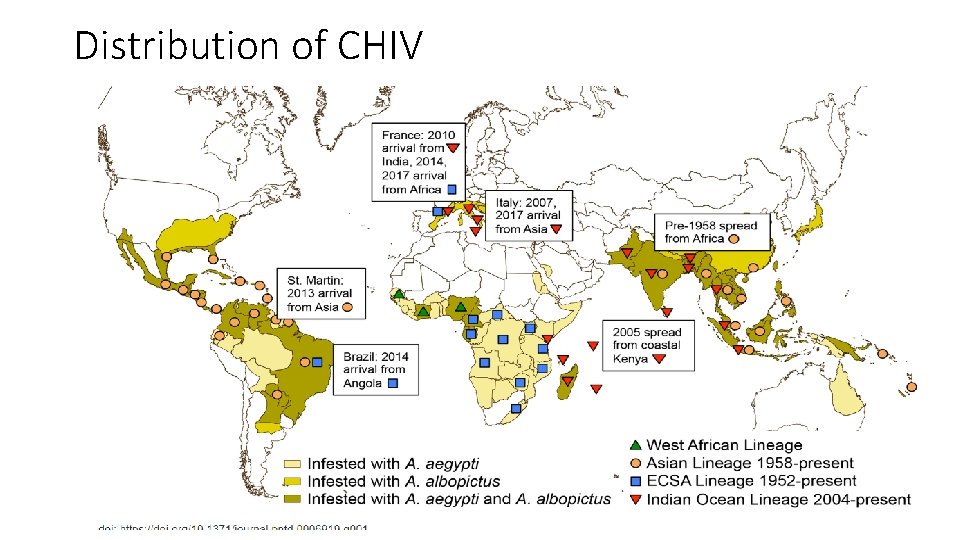
Recent outbreaks of CHIKV • 2005 -2006: More than 272 000 people were infected during an outbreak of Chikungunya in the Indian Ocean islands of Réunion and Mauritius where Ae. albopictus was the presumed vector. • 2006: Outbreak in India, more than 1 500 000 cases of chikungunya were reported with Ae. aegypti and Ae. albopictus implicated as the vector. • 2007: Migration of infected people introduced the infection in a coastal village in Italy. This outbreak (197 cases) confirmed that mosquito-borne outbreaks by Ae. albopictus are plausible in Europe.

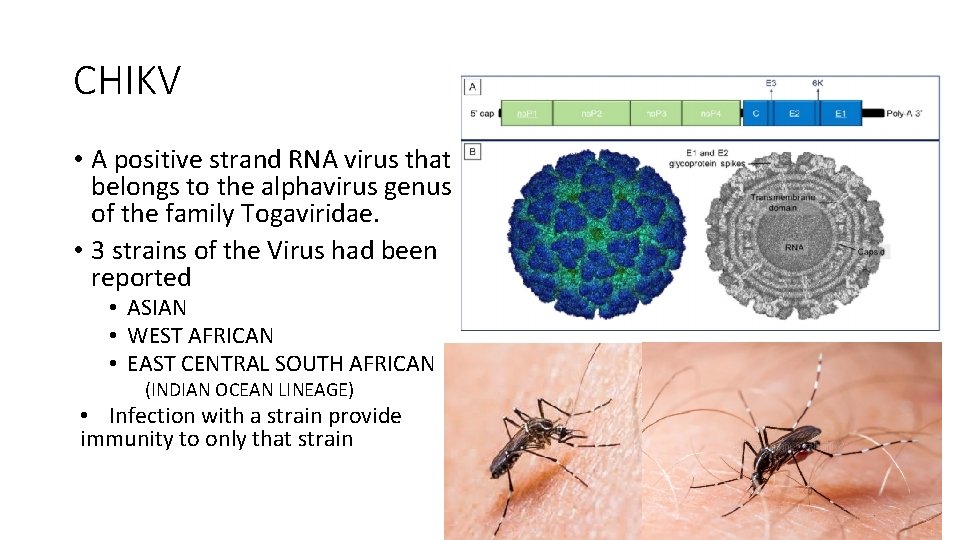
CHIKV • A positive strand RNA virus that belongs to the alphavirus genus of the family Togaviridae. • 3 strains of the Virus had been reported • ASIAN • WEST AFRICAN • EAST CENTRAL SOUTH AFRICAN (INDIAN OCEAN LINEAGE) • Infection with a strain provide immunity to only that strain

Distribution of CHIV

CHIKV India • Phylogenetic analysis based on partial sequences of NS 4 and E 1 genes showed that all earlier isolates (1963– 1973) were Asian genotype, whereas the 2006 and 2000 isolates were African genotype. The progenitor of the 2005– 2007 viruses was found to have existed around 9 years ago and may have originated from Uganda • The Indian 2006 isolates were closer to the Reunion islands isolates (99. 9% identity) than to the 2000 isolate, confirming involvement of the Reunion virus in the outbreak. •

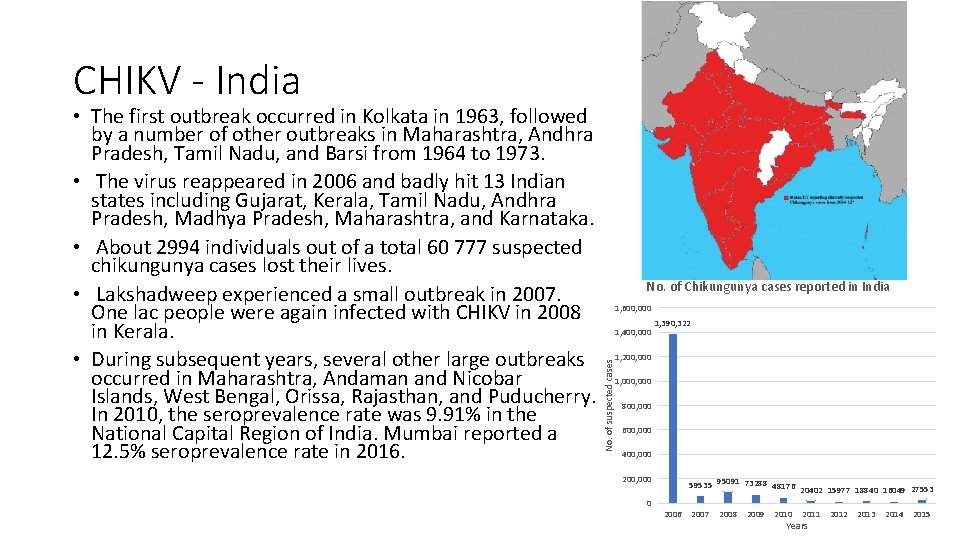
CHIKV - India No. of Chikungunya cases reported in India 1, 600, 000 1, 400, 000 1, 390, 322 1, 200, 000 No. of suspected cases • The first outbreak occurred in Kolkata in 1963, followed by a number of other outbreaks in Maharashtra, Andhra Pradesh, Tamil Nadu, and Barsi from 1964 to 1973. • The virus reappeared in 2006 and badly hit 13 Indian states including Gujarat, Kerala, Tamil Nadu, Andhra Pradesh, Madhya Pradesh, Maharashtra, and Karnataka. • About 2994 individuals out of a total 60 777 suspected chikungunya cases lost their lives. • Lakshadweep experienced a small outbreak in 2007. One lac people were again infected with CHIKV in 2008 in Kerala. • During subsequent years, several other large outbreaks occurred in Maharashtra, Andaman and Nicobar Islands, West Bengal, Orissa, Rajasthan, and Puducherry. In 2010, the seroprevalence rate was 9. 91% in the National Capital Region of India. Mumbai reported a 12. 5% seroprevalence rate in 2016. 1, 000 800, 000 600, 000 400, 000 200, 000 0 59535 95091 73288 48176 20402 15977 18840 16049 27553 2006 2007 2008 2009 2010 2011 Years 2012 2013 2014 2015

CHIK Fever symptoms • People of all ages can become infected with CHIKV and suffer a debilitating illness, but only 20% of the people that are infected will suffer any illness symptoms. • Infants, senior citizens, and people already suffering from other underlying illnesses are at greater risk of a severe illness. • Most people infected with CHIKV have a fever that may be accompanied by joint pain or swelling in multiple joints, body aches, headache, nausea, back pain, and rash. • most commonly occurs in joints of the hands, feet, wrists, and ankles, or in more proximal joints like the elbows and knees. Joint pain may be severe. • The initial illness may last for up to 10 days, but joint pains often last longer. Some patients may recover, then experience a relapse of joint pain two to three months after the initial illness. The joint pain may become chronic and last for several years after the acute illness. • Fatalities are uncommon, but are more likely to occur in elderly patients or people with underlying health conditions. • There is no specific treatment available for CHIK. Healthcare providers primarily provide supportive care and work to relieve the symptoms of the illness.

A 226 V mutation in the Envelope gene of CHIKV The A 226 V shift observed with the progression of the epidemic in Reunion Island was absent in the Indian isolates (Kumar et al. , 2007, Arankalle et al. , 2008) but was present in isolates obtained from 2007 onwards in different parts of India initially in Kerala (Kumar et al. , 2008) and later in other states as Orissa, Tamil Nadu etc. Reported initially from Kerala during 2006 This enabled the CHIKV to multiply and disseminate in Ae. albopictus, the predominant species in Kerala Later it spread to different regions of te Country wherever Ae. albopictus was prevalent.

Fitness for infection of Ae. albopictus increased 40 fold due to A 226 V mutation While there was a slight decrease in the fitness of Ae. aegypti (Weaver et al. , 2010)

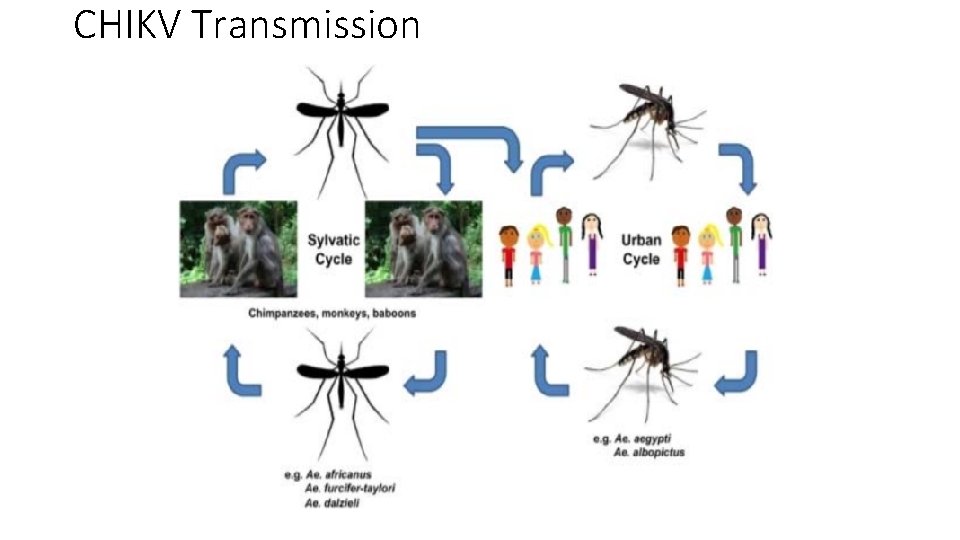
CHIKV Transmission

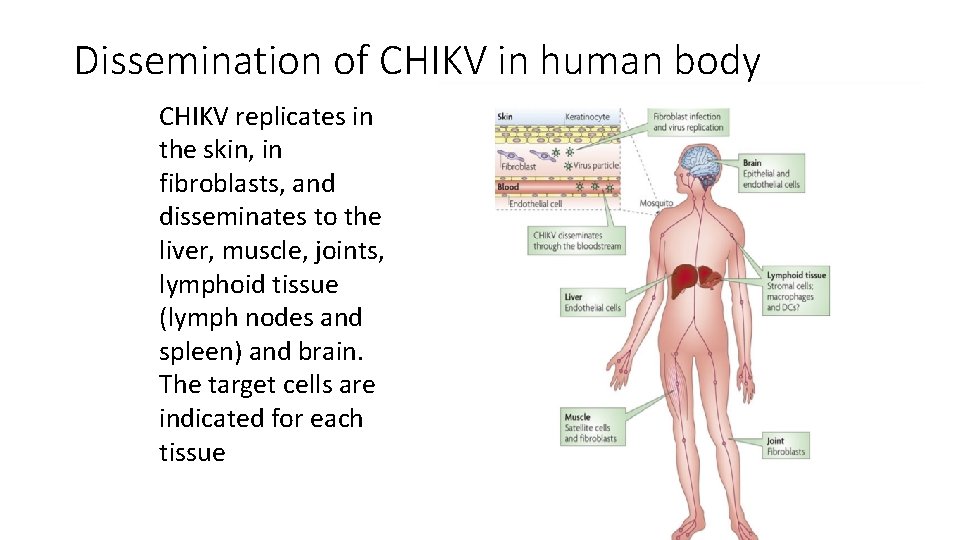
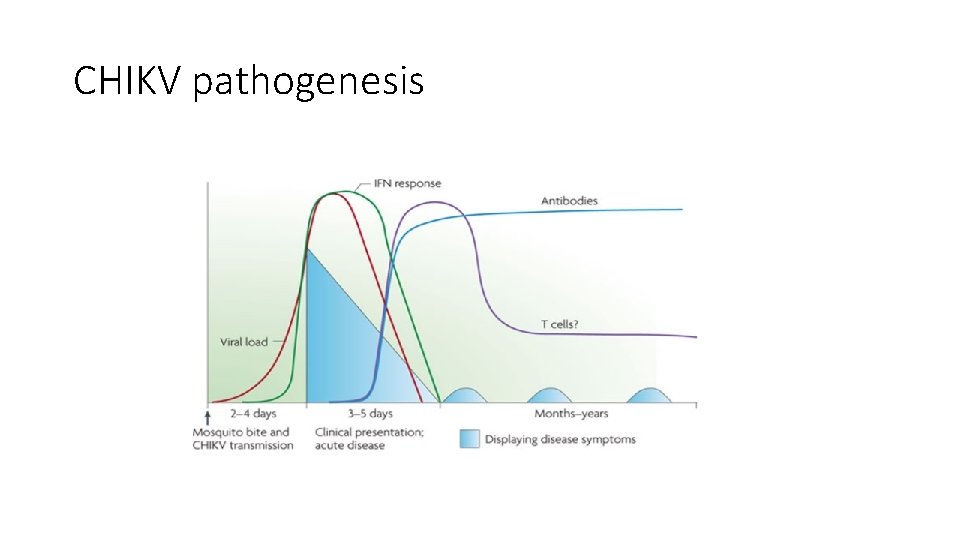
Dissemination of CHIKV in human body CHIKV replicates in the skin, in fibroblasts, and disseminates to the liver, muscle, joints, lymphoid tissue (lymph nodes and spleen) and brain. The target cells are indicated for each tissue

CHIKV pathogenesis

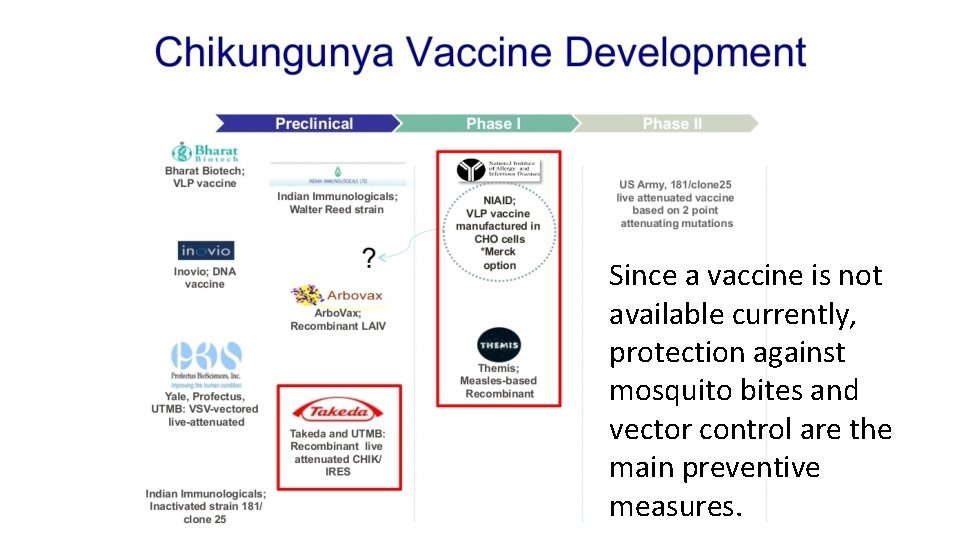
Since a vaccine is not available currently, protection against mosquito bites and vector control are the main preventive measures.

ZIKA FEVER

ZIKA • Zika virus is a mosquito-borne flavivirus that was first identified in Uganda in 1947 in monkeys. It was later identified in humans in 1952 in Uganda and the United Republic of Tanzania. • Outbreaks of Zika virus disease have been recorded in Africa, the Americas, Asia and the Pacific. From the 1960 s to 1980 s, rare sporadic cases of human infections were found across Africa and Asia, typically accompanied by mild illness. • The first recorded outbreak of Zika virus disease was from the Island of Yap (Federated States of Micronesia) in 2007. • This was followed by a large outbreak of Zika virus infection in French Polynesia in 2013 and other countries and territories in the Pacific.

Recent outbreak of ZIKA • In March 2015, Brazil reported a large outbreak of rash illness, soon identified as Zika virus infection. • In July 2015, found to be associated with Guillain-Barré syndrome. • In October 2015, Brazil reported an association between Zika virus infection and microcephaly. Outbreaks and evidence of transmission soon appeared throughout the Americas, Africa, and other regions of the world. • WHO declared a Global Public Health Emergency during Feb. 2016 • To date, a total of 86 countries and territories have reported evidence of mosquito-transmitted Zika infection.

ZIKA Distribution 2004 -2018

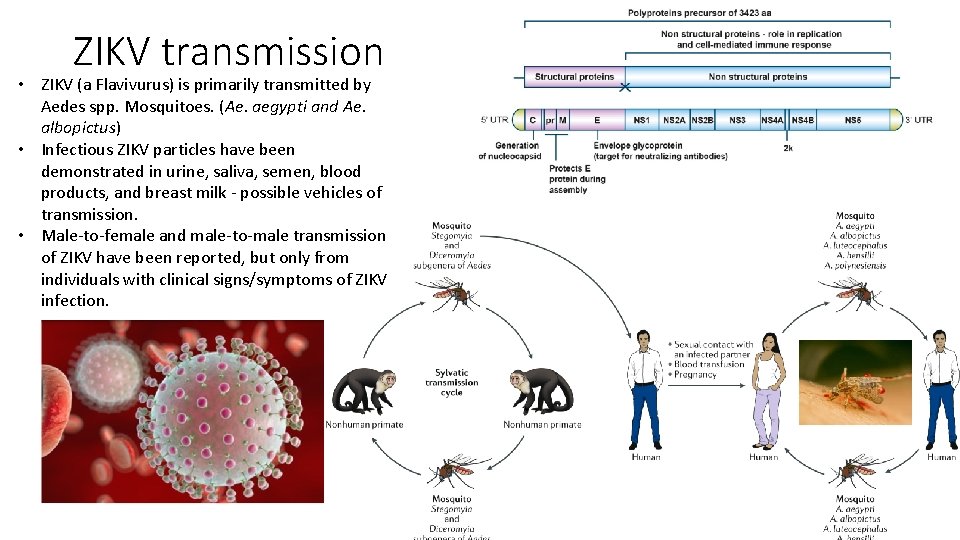
ZIKV transmission • ZIKV (a Flavivurus) is primarily transmitted by Aedes spp. Mosquitoes. (Ae. aegypti and Ae. albopictus) • Infectious ZIKV particles have been demonstrated in urine, saliva, semen, blood products, and breast milk - possible vehicles of transmission. • Male-to-female and male-to-male transmission of ZIKV have been reported, but only from individuals with clinical signs/symptoms of ZIKV infection.

Complications of ZIKA during the current Outbreak CONGENITAL ZIKA SYNDROME – (placental insufficiency, fetal growth restriction, microcephaly, oligohydramnios, ocular disorders, auditory impairments, CNS injury and fetal death. (around 2650 cases reported) Autoimmune neurologic disorders such as Acute Disseminated Encephalomyelitis (ADEM) and Guillain-Barre syndrome (GBS). (20 fold increase during outbreak)

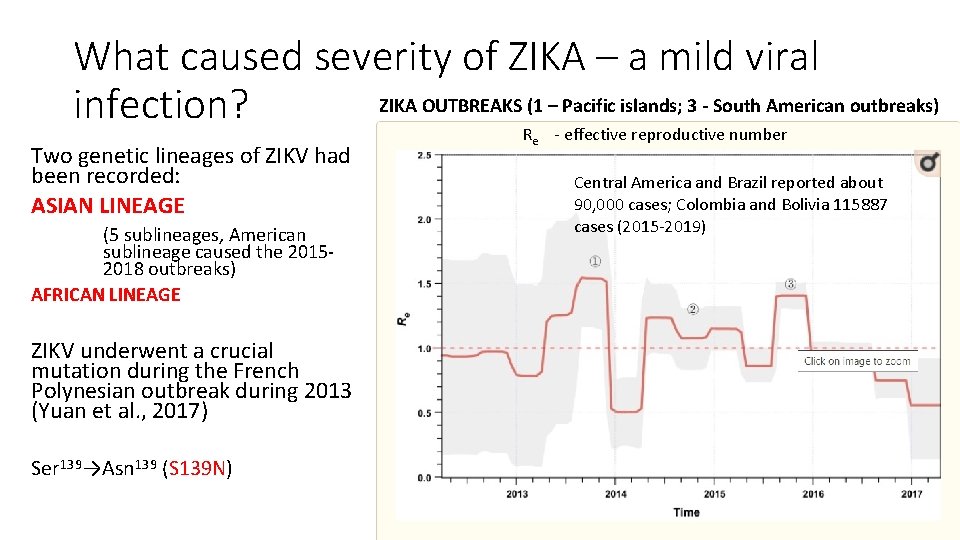
What caused severity of ZIKA – a mild viral ZIKA OUTBREAKS (1 – Pacific islands; 3 - South American outbreaks) infection? Two genetic lineages of ZIKV had been recorded: ASIAN LINEAGE (5 sublineages, American sublineage caused the 20152018 outbreaks) AFRICAN LINEAGE ZIKV underwent a crucial mutation during the French Polynesian outbreak during 2013 (Yuan et al. , 2017) Ser 139→Asn 139 (S 139 N) Re - effective reproductive number Central America and Brazil reported about 90, 000 cases; Colombia and Bolivia 115887 cases (2015 -2019)

ZIKA - VACCINES • Live attenuated (4): Recombinant Chimeri. Vax™ – Sanofi Pasteur; Recombinant dengue-2 – Instituto Butantan/US NIH; Recombinant chimeric 17 DD – Bio-Manguinhos/Fiocruz; Recombinant ZIKV infectious clone – UTMB/Evandro Chagas Institute/Brazil Ministry of Health. • Formalin inactivated purified whole virus (5): Bharat Biotech; Chimeri. Vax – Sanofi Pasteur; Bio-Manguinhos/Fiocruz; New. Link Genetics; Instituto Butantan/ US NIH • Live vectored (4): Measles virus vector - Themis Bioscience/Institut Pasteur; Lentivirus-vector – Institut Pasteur; MVA-VLP – Geo. Vax; Simian Adenovirus – Jenner Institute

DENGUE FEVER

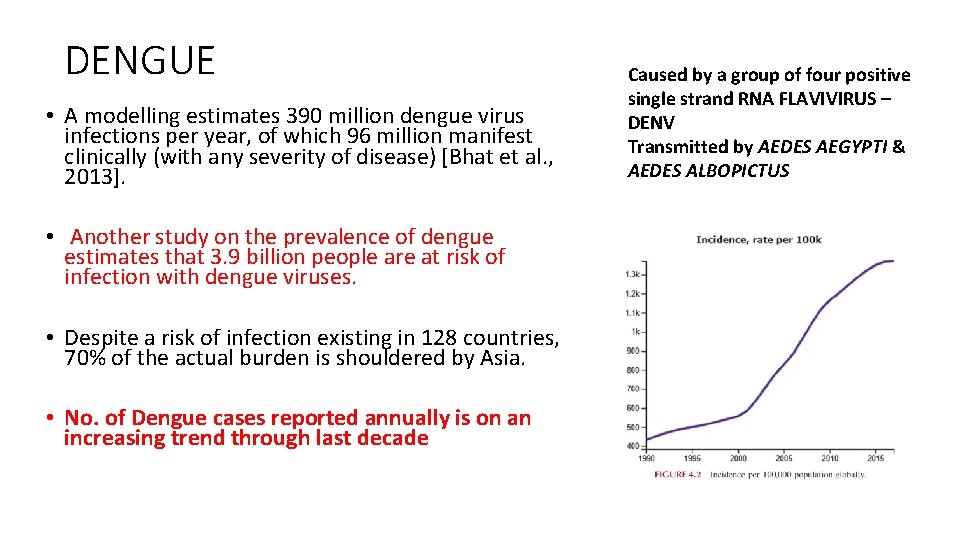
DENGUE • A modelling estimates 390 million dengue virus infections per year, of which 96 million manifest clinically (with any severity of disease) [Bhat et al. , 2013]. • Another study on the prevalence of dengue estimates that 3. 9 billion people are at risk of infection with dengue viruses. • Despite a risk of infection existing in 128 countries, 70% of the actual burden is shouldered by Asia. • No. of Dengue cases reported annually is on an increasing trend through last decade Caused by a group of four positive single strand RNA FLAVIVIRUS – DENV Transmitted by AEDES AEGYPTI & AEDES ALBOPICTUS

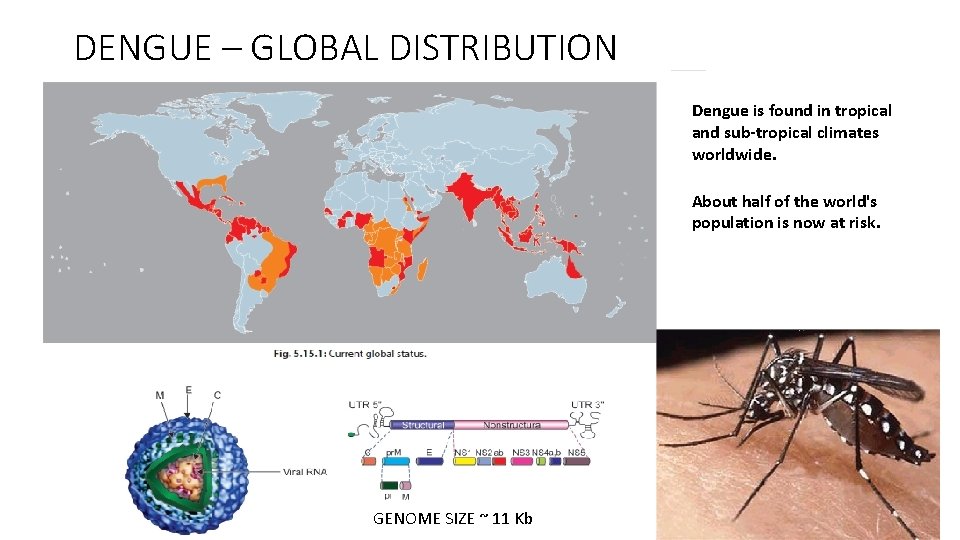
DENGUE – GLOBAL DISTRIBUTION Dengue is found in tropical and sub-tropical climates worldwide. About half of the world's population is now at risk. GENOME SIZE ~ 11 Kb

Indian estimates of DENGUE Quantifying the true burden globally remains elusive because of notoriously poor surveillance systems on capturing all symptomatic dengue infections, resulting in gross under-reporting. This discrepancy is particularly high for India contributed 34 million apparent global dengue infections, a number which stands in blatant contrast to the 12 484 reported cases from India (Bhatt et al. , 2013) Such a mismatch was also reported for India in another study, (Shepard et al. , 2014) in which the actual number of dengue cases were 282 times the number reported by the national vector-borne disease control program Another model (FOI) estimated 8· 8– 12· 9 million cases of Dengue occurred in India during 2017 with 2· 2– 3· 2 million clinical cases (Mukter et al. , 2017). National Vector Borne Disease Control Programme reported 0. 188 million clinical cases This huge underreporting in Dengue surveillance system in India remains the major draw back in management of Dengue control in INDIA

SEROTYPES/GENOTYPES OF DENGUE VIRUS The differential amino acid sequence of the E proteins separates DENV into serotypes • • • Dengue viruses-1: three GENOTYPES Dengue viruses-2: two (one nonhuman primate) GENOTYPES Dengue viruses-3: four GENOTYPES Dengue viruses-4: four (one nonhuman primate) GENOTYPES Recently, a fifth variant of dengue virus (DENV-5) has been isolated in October 2013. This new serotype follows the sylvatic cycle unlike the other four serotypes which follow the human cycle. The likely cause of emergence of the new serotype could be genetic recombination, natural selection, and genetic bottlenecks. There is no indication of the presence of DENV-5 in India till date.

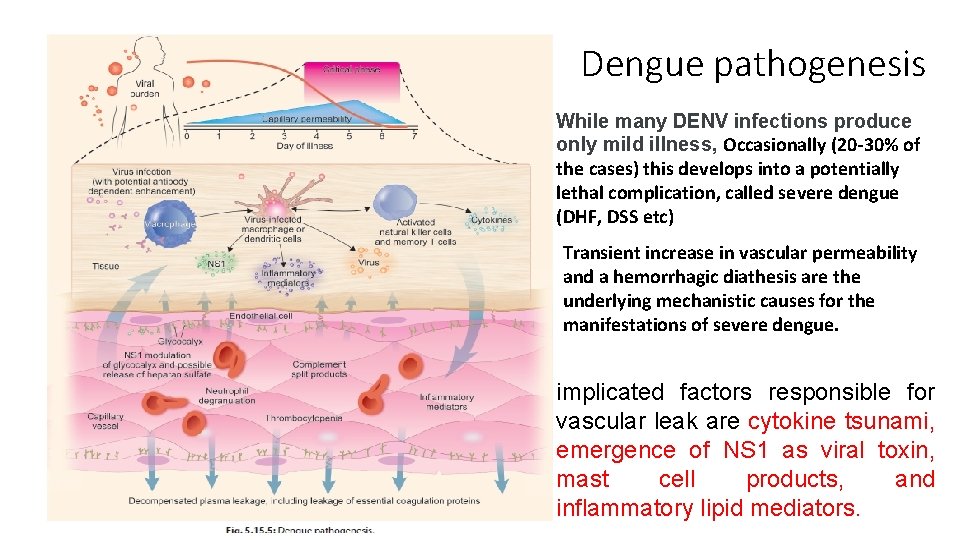
Dengue pathogenesis While many DENV infections produce only mild illness, Occasionally (20 -30% of the cases) this develops into a potentially lethal complication, called severe dengue (DHF, DSS etc) Transient increase in vascular permeability and a hemorrhagic diathesis are the underlying mechanistic causes for the manifestations of severe dengue. implicated factors responsible for vascular leak are cytokine tsunami, emergence of NS 1 as viral toxin, mast cell products, and inflammatory lipid mediators.

WHY AND HOW DENGUE TURNS SEVERE • viral virulence (serotypes/genotypes), virus burden, host immune response, and genetic predisposition, have been implicated as risk factors for DHF • An elevated free s. NS 1 level (600 ng/m. L) within 72 h of illness onset identified patients at risk for developing DHF. Quantitative estimation of NS 1 may prove to be useful prognostic tool for dengue illnesses. • Mast cells (MCs), which line blood vessels and regulate vascular function, are able to detect DENV in vivo and promote vascular leakage. MC-derived protease, tryptase, is consequential for promoting vascular permeability during DENV infection through inducing breakdown of endothelial cell tight junctions. • MC proteases tryptase and chymase promote vascular leakage and induce hypovolemic shock in vivo

CLINICAL MANAGEMENT AND DRUG DEVELOPMENT Access to proper symptomatic medical care lowers fatality rates of severe dengue to below 1% A potent tryptase inhibitor, nafamostat mesylate, blocked DENVinduced vascular leakage in vivo. (MC proteases tryptase and chymase promote vascular leakage and induce hypovolemic shock in vivo (Rathore et al. , 2019))

DENGUE VACCINE Efforts to develop a vaccine against dengue have been ongoing for decades. The biggest challenge has been to find out a tetravalent vaccine which could simultaneously induce long lasting protective immunity against all four viruses. The first vaccine recommended for use is chimeric yellow fever virus. DENV–tetravalent dengue vaccine (marketed as Dengvaxia) a live, attenuated tetravalent product developed by Sanofi Pasteur. Dengvaxia was licensed in December 2015. Expert opinion and a position paper on Dengvaxia were issued by WHO in 2016.

DENGVAXIA • A supplemental statement from WHO on December 22, 2017, verifies that overall Dengvaxia reduces the risk of confirmed severe dengue and hospital admissions; • However, among seronegative recipients regardless of age at vaccination—there was a higher risk of severe dengue disease and hospital admission compared with unvaccinated controls. • Thus, in seronegative individuals, the vaccine seems to enhance the severity of subsequent dengue infection. • Dengvaxia is not yet licensed for use in India.

CONTROL OF AEDES BORNE DISEASES

Control/Management of Aedes-borne Diseases • “Half the world’s population is now at risk of dengue, ” said Dr Soumya Swaminathan, WHO Chief Scientist. “And despite our best efforts, current efforts to control it are falling short. We desperately need new approaches and this initiative is both promising and exciting. ” • Presently, there are no commercially available antivirals or dengue/ Chikungunya/ vaccines, though progress on their development is encouraging. • Hence prevention of these Diseases, is currently limited to Vector control.

Prospects towards control of Aedes borne Diseases • Development of a systematic disease/vector surveillance program • Reduction of man-vector contact • Reduction of human amplification of Flaviviruses/Alphaviruses by using antivirals • Control of transmission by reducing Aedes vector population • Prevention of infection by development of effective vaccines

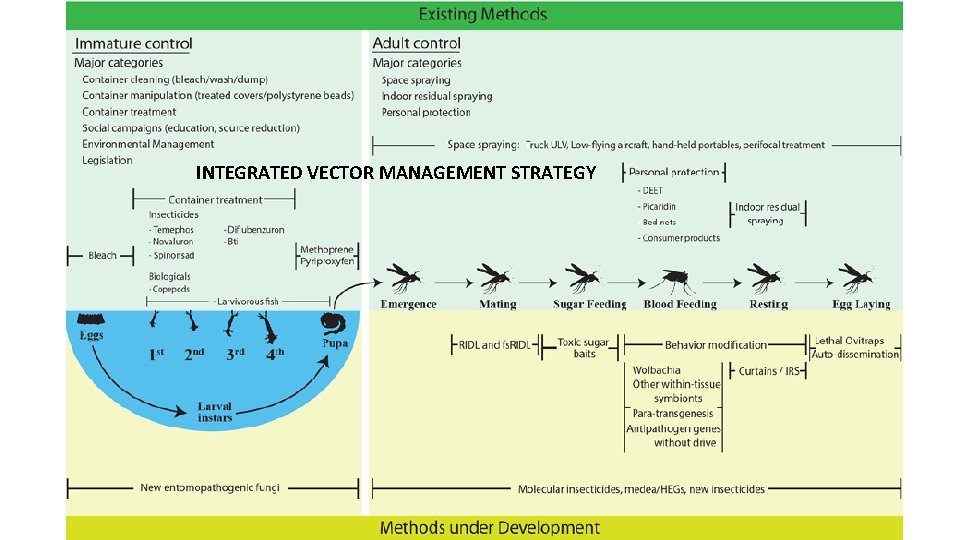
INTEGRATED VECTOR MANAGEMENT STRATEGY

THE CHALLENGE: Controlling mosquito populations is currently the only measure used to reduce risk and incidence of certain vector -borne diseases such as dengue, chikungunya and Zika. So far, the use of insecticides has been the primary vector control method, but due to an increasing trend in insecticide resistance in mosquito vectors, as well as the residual effects of insecticides on the environment, there is an urgent need for alternative methods.





TESTING THE STERILE INSECT TECHNIQUE AS A VECTOR CONTROL TOOL AGAINST AEDESBORNE DISEASES (WHO-TDR) - NOVEMBER 2019


PROMISING FUTURE STRATEGIES • STUDIES ON MICROBIOME • RNAi transmission-blocking strains • micro. RNAs (mi. RNAs) as small non-coding RNAs in regulation of gene expression • A new tool for insect transgenesis and genome editing based on CRISPR/Cas 9 DNA double-strand breaks and repair, has the potential to substantially propel the field of genetic pest management forward • Attracting and then killing female mosquitoes as they lay eggs, via lethal ovitraps or sticky gravid traps • Insect specific Flaviviruses which interact with other pathogenic Flaviviruses
 Chikungunya mauritius
Chikungunya mauritius Is the zika virus lytic or lysogenic
Is the zika virus lytic or lysogenic Zika
Zika Surah al an'am ayat 16 and 17 for dengue
Surah al an'am ayat 16 and 17 for dengue Surveilans terpadu adalah
Surveilans terpadu adalah Malaria and dengue
Malaria and dengue Cadena epidemiologica de la fiebre amarilla
Cadena epidemiologica de la fiebre amarilla Criterios diagnosticos dengue
Criterios diagnosticos dengue Dengue duo
Dengue duo Pathogenesis dengue fever
Pathogenesis dengue fever Etapas del dengue comezón
Etapas del dengue comezón Dengue monitoring chart
Dengue monitoring chart Dengue shock syndrome
Dengue shock syndrome Taksonomi virus dengue
Taksonomi virus dengue Haemaccel in dengue
Haemaccel in dengue Symptoms dengue
Symptoms dengue Abcd dengue
Abcd dengue Definición de capilar
Definición de capilar Dengue
Dengue Sintimas de la gonorrea
Sintimas de la gonorrea Dengue no brasil
Dengue no brasil Dengue
Dengue Measles
Measles Microcefalia
Microcefalia O que é dengue
O que é dengue Ibuprofen
Ibuprofen Todo pernilongo listrado é da dengue
Todo pernilongo listrado é da dengue Knowledge creation and knowledge architecture
Knowledge creation and knowledge architecture Defense priorities and allocations system
Defense priorities and allocations system Goals and priorities
Goals and priorities Goals and priorities
Goals and priorities Goals and priorities
Goals and priorities Goals and priorities
Goals and priorities Personal vs shared knowledge
Personal vs shared knowledge Knowledge shared is knowledge squared
Knowledge shared is knowledge squared Knowledge shared is knowledge multiplied interpretation
Knowledge shared is knowledge multiplied interpretation Contoh shallow knowledge dan deep knowledge
Contoh shallow knowledge dan deep knowledge A priori ne demek
A priori ne demek Book smart vs street smart
Book smart vs street smart Shared knowledge vs personal knowledge
Shared knowledge vs personal knowledge Gertler econ
Gertler econ Activity 5 i fill you
Activity 5 i fill you Read and fill in the gaps with the verbs given in the box
Read and fill in the gaps with the verbs given in the box Fills in gaps in data and fit data into curves
Fills in gaps in data and fit data into curves Giving directions dialogue
Giving directions dialogue Gaps and islands
Gaps and islands