Course natural history and prognosis Parkinsons disease 1











- Slides: 34

Course, natural history and prognosis Parkinson’s disease 1

The course of Parkinson’s disease 2

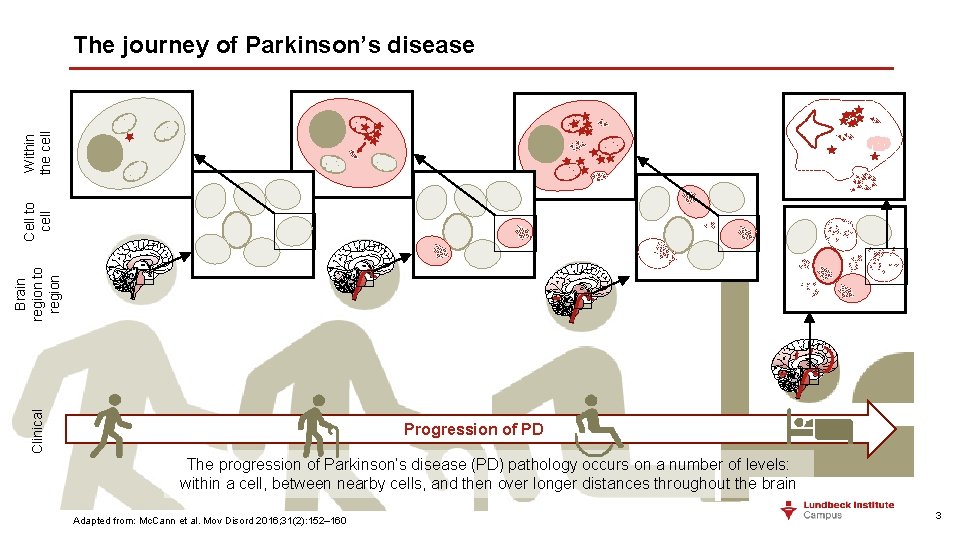
Clinical Brain region to region Cell to cell Within the cell The journey of Parkinson’s disease Progression of PD The progression of Parkinson’s disease (PD) pathology occurs on a number of levels: within a cell, between nearby cells, and then over longer distances throughout the brain Adapted from: Mc. Cann et al. Mov Disord 2016; 31(2): 152– 160 3

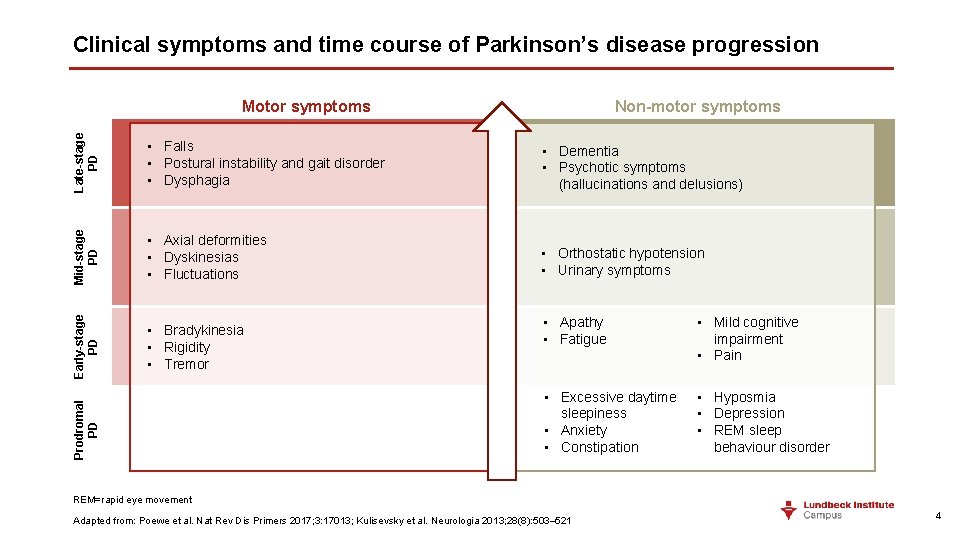
Clinical symptoms and time course of Parkinson’s disease progression Non-motor symptoms Late-stage PD • Dementia • Psychotic symptoms (hallucinations and delusions) Mid-stage PD • Axial deformities • Dyskinesias • Fluctuations • Orthostatic hypotension • Urinary symptoms • Bradykinesia • Rigidity • Tremor Prodromal PD • Falls • Postural instability and gait disorder • Dysphagia Early-stage PD Motor symptoms • Apathy • Fatigue • Mild cognitive impairment • Pain • Excessive daytime sleepiness • Anxiety • Constipation • Hyposmia • Depression • REM sleep behaviour disorder REM=rapid eye movement Adapted from: Poewe et al. Nat Rev Dis Primers 2017; 3: 17013; Kulisevsky et al. Neurologia 2013; 28(8): 503– 521 4

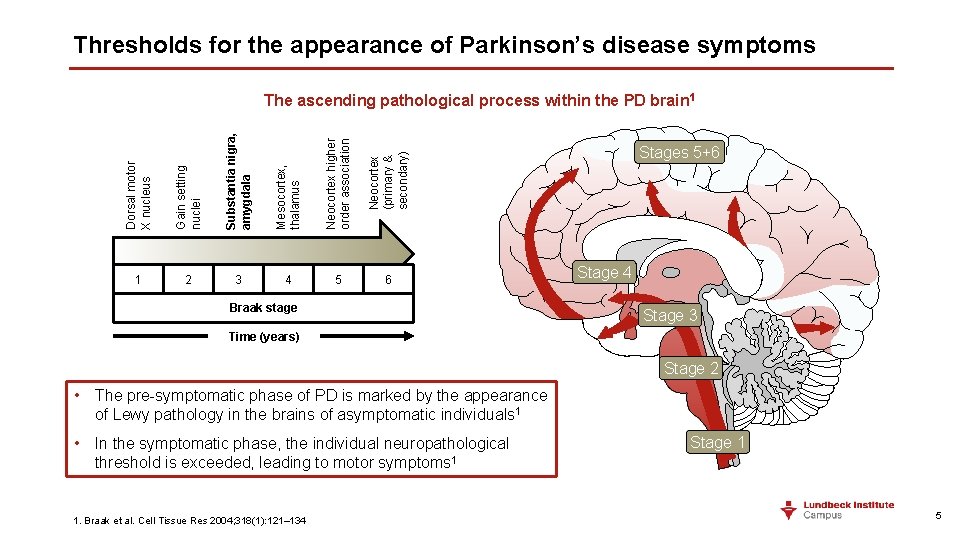
Thresholds for the appearance of Parkinson’s disease symptoms Dorsal motor X nucleus Gain setting nuclei Substantia nigra, amygdala Mesocortex, thalamus Neocortex higher order association Neocortex (primary & secondary) The ascending pathological process within the PD brain 1 1 2 3 4 5 6 Braak stage Stages 5+6 Stage 4 Stage 3 Time (years) Stage 2 • The pre-symptomatic phase of PD is marked by the appearance of Lewy pathology in the brains of asymptomatic individuals 1 • In the symptomatic phase, the individual neuropathological threshold is exceeded, leading to motor symptoms 1 1. Braak et al. Cell Tissue Res 2004; 318(1): 121– 134 Stage 1 5

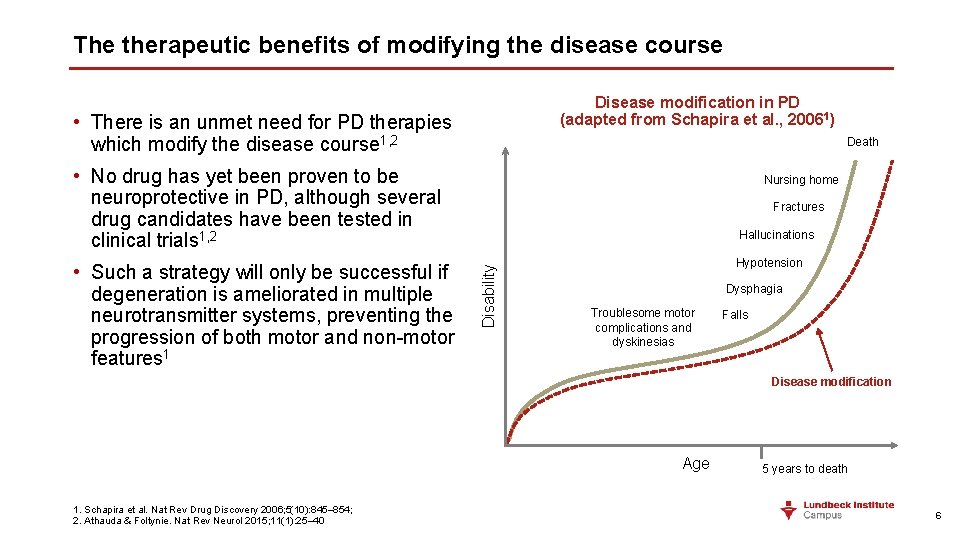
The therapeutic benefits of modifying the disease course Disease modification in PD (adapted from Schapira et al. , 20061) • There is an unmet need for PD therapies which modify the disease course 1, 2 Death • No drug has yet been proven to be neuroprotective in PD, although several drug candidates have been tested in clinical trials 1, 2 Fractures Hallucinations Disability • Such a strategy will only be successful if degeneration is ameliorated in multiple neurotransmitter systems, preventing the progression of both motor and non-motor features 1 Nursing home Hypotension Dysphagia Troublesome motor complications and dyskinesias Falls Disease modification Age 1. Schapira et al. Nat Rev Drug Discovery 2006; 5(10): 845– 854; 2. Athauda & Foltynie. Nat Rev Neurol 2015; 11(1): 25– 40 5 years to death 6

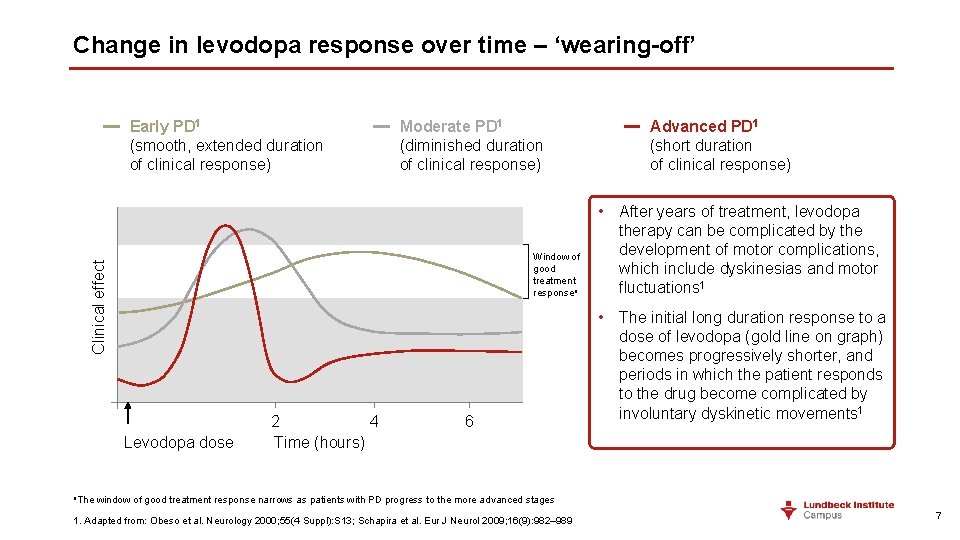
Change in levodopa response over time – ‘wearing-off’ Early PD 1 (smooth, extended duration of clinical response) Moderate PD 1 (diminished duration of clinical response) Clinical effect Window of good treatment responsea Levodopa dose 2 4 Time (hours) 6 Advanced PD 1 (short duration of clinical response) • After years of treatment, levodopa therapy can be complicated by the development of motor complications, which include dyskinesias and motor fluctuations 1 • The initial long duration response to a dose of levodopa (gold line on graph) becomes progressively shorter, and periods in which the patient responds to the drug become complicated by involuntary dyskinetic movements 1 a. The window of good treatment response narrows as patients with PD progress to the more advanced stages 1. Adapted from: Obeso et al. Neurology 2000; 55(4 Suppl): S 13; Schapira et al. Eur J Neurol 2009; 16(9): 982– 989 7

Definitions of terms used in Parkinson’s disease ON/OFF • ON time – period of relatively good motor symptom control, when medication seems to be working well 1 • OFF time – period of relatively poor motor symptom control 1 Wearing off • Generally, the first motor complication is predictable wearing off – recurrence of motor and non-motor symptoms preceding scheduled doses of levodopa 2, 3 • Patients typically report a predictable return of motor or non-motor symptoms occurring before their next scheduled medication dose, these symptoms then improve 15– 45 minutes after the next dose of medication 3 Dyskinesia • Dyskinesia refers to a broad clinical spectrum of different types of involuntary movements ranging from chorea affecting limbs and trunk, slow dystonic movements, fixed dystonic postures, or (more rarely) myoclonus or ballism 4 • Troublesome dyskinesias have been defined as those that are painful, impair balance, or are excessive to the point of causing impairment in coordination or general function 5 1. Kalia & Lang. Lancet 2015; 386: 896– 912; 2. Stacy et al. Mov Disord 2005; 20(6): 726– 733; 3. Stacy & Hauser. J Neural Transm 2007; 114: 211– 217; 4. Hametner et al. J Neurol 2010; 257(Suppl 2): S 268– 275; 5. Parkinson Study Group. Arch Neurol 2005; 62(2): 241– 248 8

Drug-induced dyskinesias in Parkinson’s disease ON-period dyskinesias (‘interdose’)1 • Phasic (choreic) limb movements • Dystonic cranio–cervical movements • More pronounced on side initially affected by PD Biphasic dyskinesias 1 • At onset or wearing-off (or both) of clinical benefits of a dose of levodopa • Mix of phasic and dystonic movements (‘mobile dystonia’) OFF-period dystonia 1 • Most often distal limb (feet) • Painful 1. Hametner et al. J Neurol 2010; 257(Suppl 2): S 268– 275 9

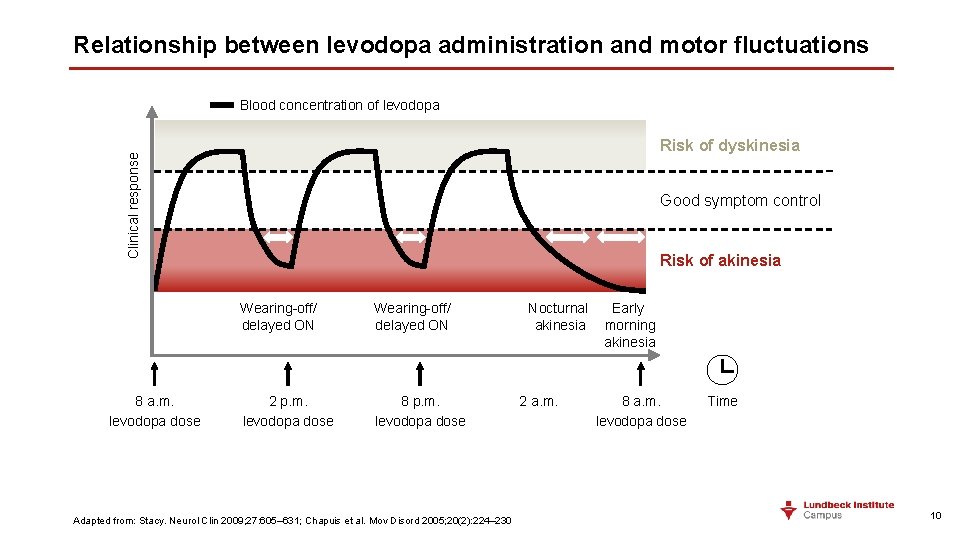
Relationship between levodopa administration and motor fluctuations Blood concentration of levodopa Clinical response Risk of dyskinesia 8 a. m. levodopa dose Good symptom control Risk of akinesia Wearing-off/ delayed ON 2 p. m. levodopa dose 8 p. m. levodopa dose Adapted from: Stacy. Neurol Clin 2009; 27: 605– 631; Chapuis et al. Mov Disord 2005; 20(2): 224– 230 Nocturnal Early akinesia morning akinesia 2 a. m. 8 a. m. levodopa dose Time 10

Prodromal Parkinson’s disease 11

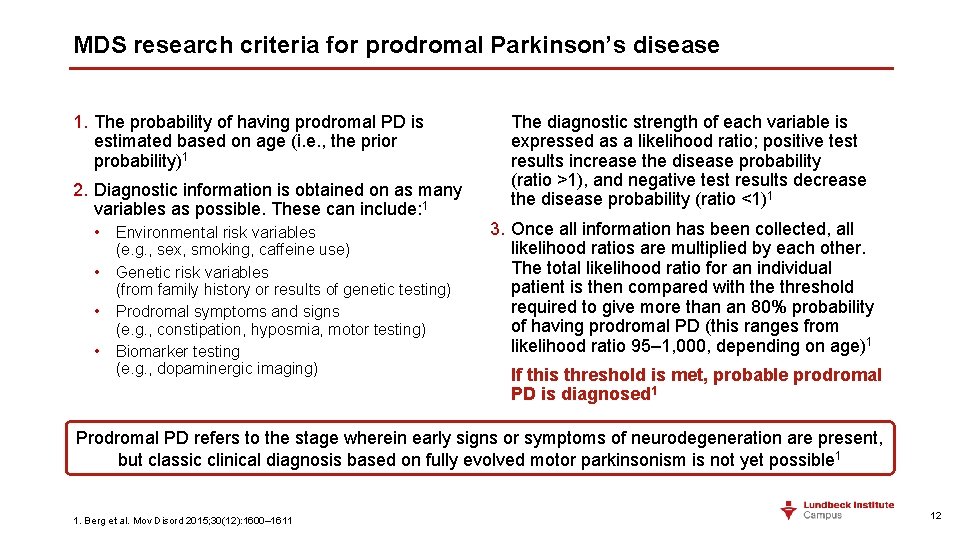
MDS research criteria for prodromal Parkinson’s disease 1. The probability of having prodromal PD is estimated based on age (i. e. , the prior probability)1 2. Diagnostic information is obtained on as many variables as possible. These can include: 1 • Environmental risk variables (e. g. , sex, smoking, caffeine use) • Genetic risk variables (from family history or results of genetic testing) • Prodromal symptoms and signs (e. g. , constipation, hyposmia, motor testing) • Biomarker testing (e. g. , dopaminergic imaging) The diagnostic strength of each variable is expressed as a likelihood ratio; positive test results increase the disease probability (ratio >1), and negative test results decrease the disease probability (ratio <1)1 3. Once all information has been collected, all likelihood ratios are multiplied by each other. The total likelihood ratio for an individual patient is then compared with the threshold required to give more than an 80% probability of having prodromal PD (this ranges from likelihood ratio 95– 1, 000, depending on age)1 If this threshold is met, probable prodromal PD is diagnosed 1 Prodromal PD refers to the stage wherein early signs or symptoms of neurodegeneration are present, but classic clinical diagnosis based on fully evolved motor parkinsonism is not yet possible 1 1. Berg et al. Mov Disord 2015; 30(12): 1600– 1611 12

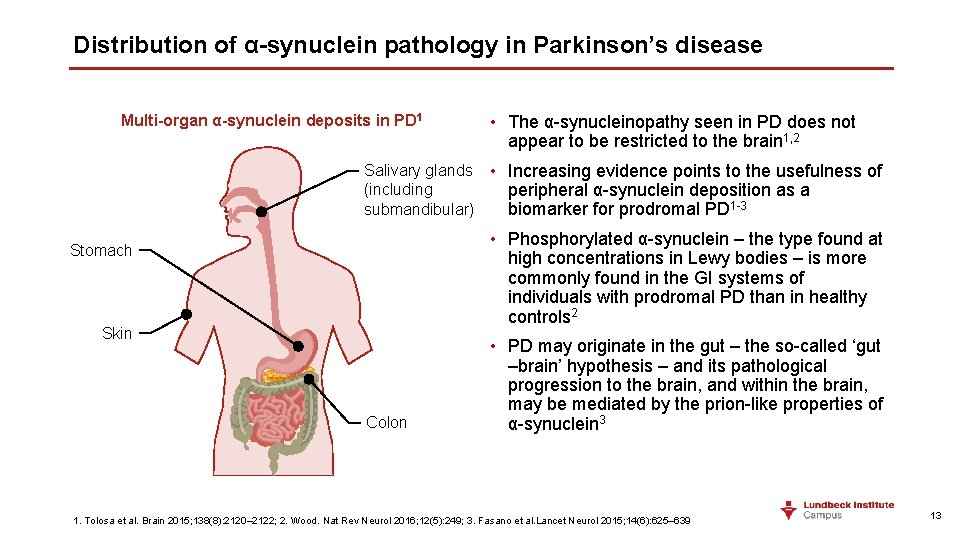
Distribution of α-synuclein pathology in Parkinson’s disease Multi-organ α-synuclein deposits in PD 1 • The α-synucleinopathy seen in PD does not appear to be restricted to the brain 1, 2 Salivary glands • Increasing evidence points to the usefulness of (including peripheral α-synuclein deposition as a submandibular) biomarker for prodromal PD 1 -3 • Phosphorylated α-synuclein – the type found at high concentrations in Lewy bodies – is more commonly found in the GI systems of individuals with prodromal PD than in healthy controls 2 Stomach Skin Colon • PD may originate in the gut – the so-called ‘gut –brain’ hypothesis – and its pathological progression to the brain, and within the brain, may be mediated by the prion-like properties of α-synuclein 3 1. Tolosa et al. Brain 2015; 138(8): 2120– 2122; 2. Wood. Nat Rev Neurol 2016; 12(5): 249; 3. Fasano et al. Lancet Neurol 2015; 14(6): 625– 639 13

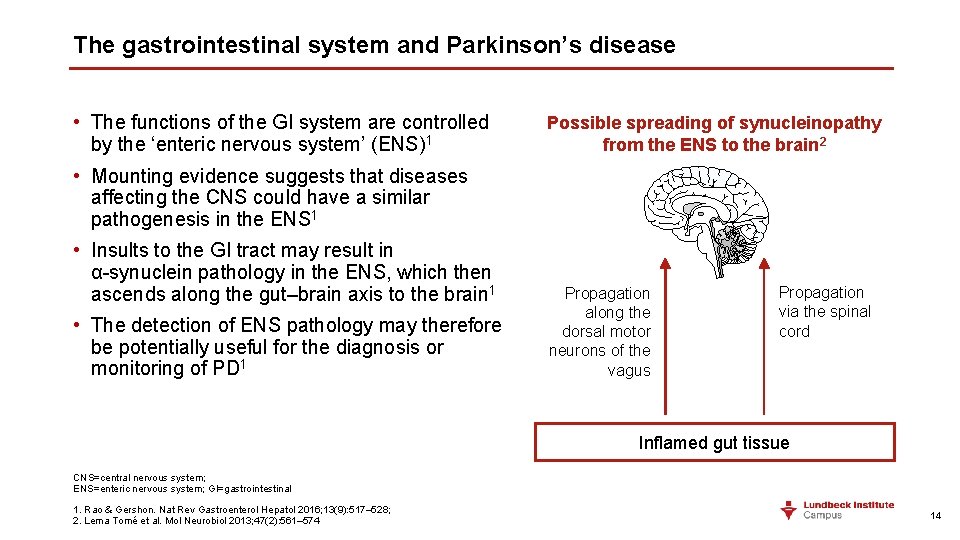
The gastrointestinal system and Parkinson’s disease • The functions of the GI system are controlled by the ‘enteric nervous system’ (ENS)1 Possible spreading of synucleinopathy from the ENS to the brain 2 • Mounting evidence suggests that diseases affecting the CNS could have a similar pathogenesis in the ENS 1 • Insults to the GI tract may result in α-synuclein pathology in the ENS, which then ascends along the gut–brain axis to the brain 1 • The detection of ENS pathology may therefore be potentially useful for the diagnosis or monitoring of PD 1 Propagation along the dorsal motor neurons of the vagus Propagation via the spinal cord Inflamed gut tissue CNS=central nervous system; ENS=enteric nervous system; GI=gastrointestinal 1. Rao & Gershon. Nat Rev Gastroenterol Hepatol 2016; 13(9): 517– 528; 2. Lema Tomé et al. Mol Neurobiol 2013; 47(2): 561– 574 14

The stages of Parkinson’s disease 15

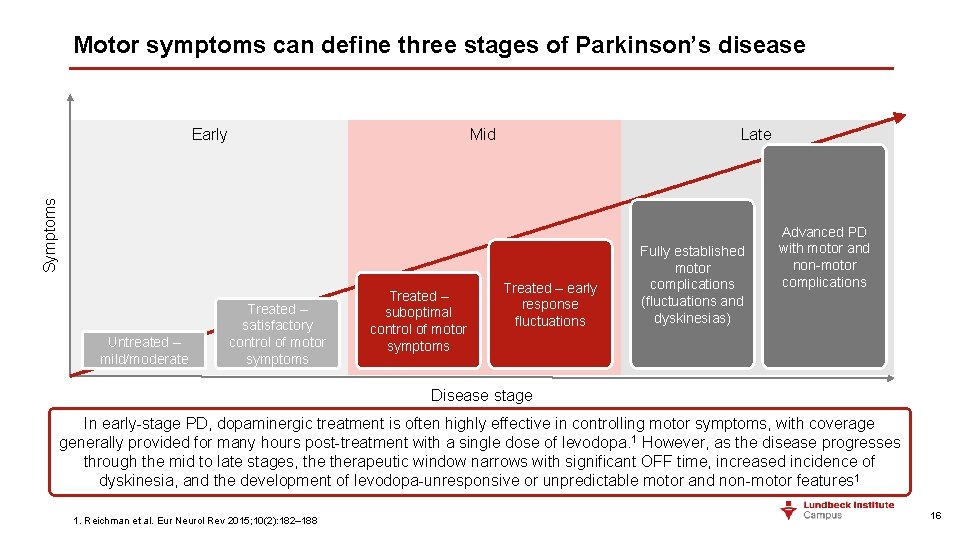
Motor symptoms can define three stages of Parkinson’s disease Mid Late Symptoms Early Untreated – mild/moderate Treated – satisfactory control of motor symptoms Treated – suboptimal control of motor symptoms Treated – early response fluctuations Fully established motor complications (fluctuations and dyskinesias) Advanced PD with motor and non-motor complications Disease stage In early-stage PD, dopaminergic treatment is often highly effective in controlling motor symptoms, with coverage generally provided for many hours post-treatment with a single dose of levodopa. 1 However, as the disease progresses through the mid to late stages, therapeutic window narrows with significant OFF time, increased incidence of dyskinesia, and the development of levodopa-unresponsive or unpredictable motor and non-motor features 1 1. Reichman et al. Eur Neurol Rev 2015; 10(2): 182– 188 16

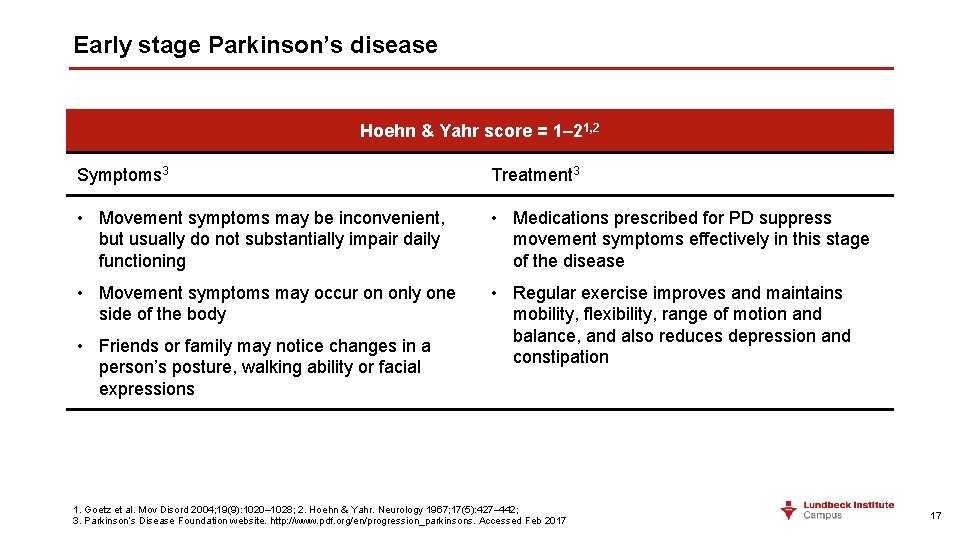
Early stage Parkinson’s disease Hoehn & Yahr score = 1– 21, 2 Symptoms 3 Treatment 3 • Movement symptoms may be inconvenient, but usually do not substantially impair daily functioning • Medications prescribed for PD suppress movement symptoms effectively in this stage of the disease • Movement symptoms may occur on only one side of the body • Regular exercise improves and maintains mobility, flexibility, range of motion and balance, and also reduces depression and constipation • Friends or family may notice changes in a person’s posture, walking ability or facial expressions 1. Goetz et al. Mov Disord 2004; 19(9): 1020– 1028; 2. Hoehn & Yahr. Neurology 1967; 17(5): 427– 442; 3. Parkinson’s Disease Foundation website. http: //www. pdf. org/en/progression_parkinsons. Accessed Feb 2017 17

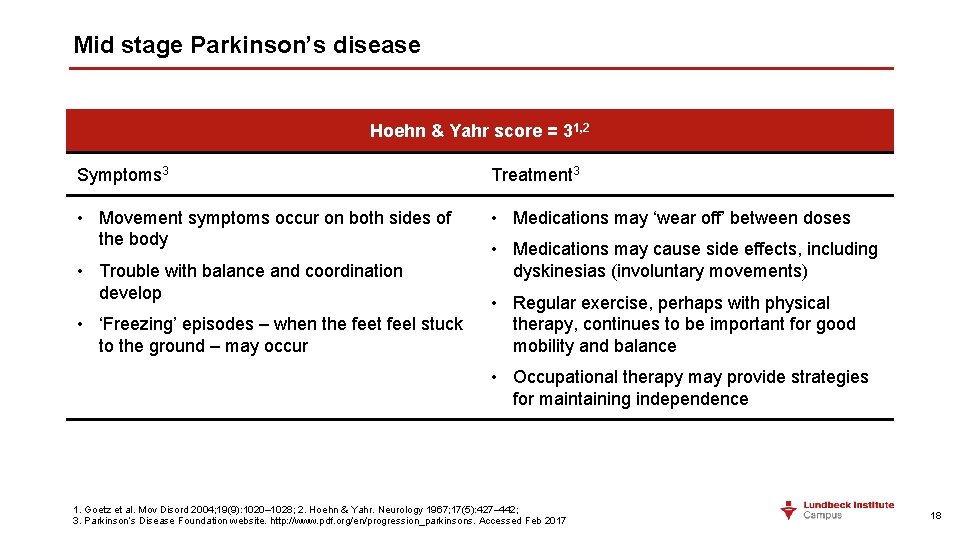
Mid stage Parkinson’s disease Hoehn & Yahr score = 31, 2 Symptoms 3 Treatment 3 • Movement symptoms occur on both sides of the body • Medications may ‘wear off’ between doses • Trouble with balance and coordination develop • ‘Freezing’ episodes – when the feet feel stuck to the ground – may occur • Medications may cause side effects, including dyskinesias (involuntary movements) • Regular exercise, perhaps with physical therapy, continues to be important for good mobility and balance • Occupational therapy may provide strategies for maintaining independence 1. Goetz et al. Mov Disord 2004; 19(9): 1020– 1028; 2. Hoehn & Yahr. Neurology 1967; 17(5): 427– 442; 3. Parkinson’s Disease Foundation website. http: //www. pdf. org/en/progression_parkinsons. Accessed Feb 2017 18

Late stage Parkinson’s disease Hoehn & Yahr score = 4– 51, 2 Symptoms 3 Treatment • Great difficulty walking; in wheelchair or bed most of the day • Balancing the benefits of medications with their side effects (e. g. , motor complications, orthostatic hypotension, psychosis) at this stage of the disease becomes more challenging 3, 4 • Not able to live alone • Assistance needed with many or all daily activities • Cognitive–behavioural problems may be prominent, including hallucinations and delusions • Physiotherapy may still be beneficial at this stage, with the aim of improving functional independence, including mobility, activities of daily living, and maintaining flexibility 5, 6 • Unpredictable treatment response 6 1. Goetz et al. Mov Disord 2004; 19(9): 1020– 1028; 2. Hoehn & Yahr. Neurology 1967; 17(5): 427– 442; 3. Parkinson’s Disease Foundation website. http: //www. pdf. org/en/progression_parkinsons. Accessed Feb 2017; 4. Sinemet. Summary of Product Characteristics. 2017; 5. Kulisevsky et al. Neurologia 2013; 28(9): 558– 583; 6. Kulisevsky et al. Neurologia 2013; 28(8): 503– 521 19

Identifying biomarkers of Parkinson’s disease progression using observational cohorts 20

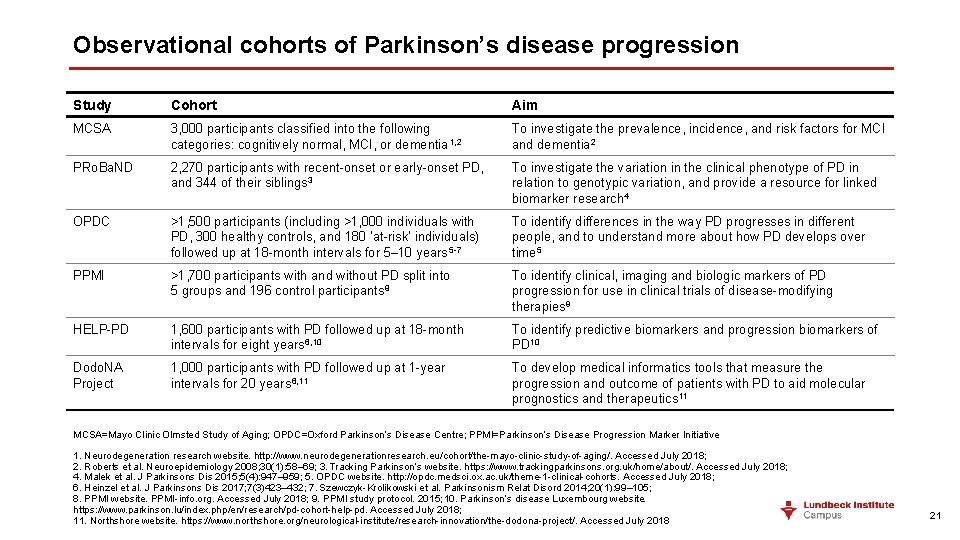
Observational cohorts of Parkinson’s disease progression Study Cohort Aim MCSA 3, 000 participants classified into the following categories: cognitively normal, MCI, or dementia 1, 2 To investigate the prevalence, incidence, and risk factors for MCI and dementia 2 PRo. Ba. ND 2, 270 participants with recent-onset or early-onset PD, and 344 of their siblings 3 To investigate the variation in the clinical phenotype of PD in relation to genotypic variation, and provide a resource for linked biomarker research 4 OPDC >1, 500 participants (including >1, 000 individuals with PD, 300 healthy controls, and 180 ‘at-risk’ individuals) followed up at 18 -month intervals for 5– 10 years 5 -7 To identify differences in the way PD progresses in different people, and to understand more about how PD develops over time 5 PPMI >1, 700 participants with and without PD split into 5 groups and 196 control participants 8 To identify clinical, imaging and biologic markers of PD progression for use in clinical trials of disease-modifying therapies 9 HELP-PD 1, 600 participants with PD followed up at 18 -month intervals for eight years 6, 10 To identify predictive biomarkers and progression biomarkers of PD 10 Dodo. NA Project 1, 000 participants with PD followed up at 1 -year intervals for 20 years 6, 11 To develop medical informatics tools that measure the progression and outcome of patients with PD to aid molecular prognostics and therapeutics 11 MCSA=Mayo Clinic Olmsted Study of Aging; OPDC=Oxford Parkinson’s Disease Centre; PPMI=Parkinson’s Disease Progression Marker Initiative 1. Neurodegeneration research website. http: //www. neurodegenerationresearch. eu/cohort/the-mayo-clinic-study-of-aging/. Accessed July 2018; 2. Roberts et al. Neuroepidemiology 2008; 30(1): 58– 69; 3. Tracking Parkinson’s website. https: //www. trackingparkinsons. org. uk/home/about/. Accessed July 2018; 4. Malek et al. J Parkinsons Dis 2015; 5(4): 947– 959; 5. OPDC website. http: //opdc. medsci. ox. ac. uk/theme-1 -clinical-cohorts. Accessed July 2018; 6. Heinzel et al. J Parkinsons Dis 2017; 7(3)423– 432; 7. Szewczyk-Krolikowski et al. Parkinsonism Relat Disord 2014; 20(1): 99– 105; 8. PPMI website. PPMI-info. org. Accessed July 2018; 9. PPMI study protocol. 2015; 10. Parkinson’s disease Luxembourg website. https: //www. parkinson. lu/index. php/en/research/pd-cohort-help-pd. Accessed July 2018; 11. Northshore website. https: //www. northshore. org/neurological-institute/research-innovation/the-dodona-project/. Accessed July 2018 21

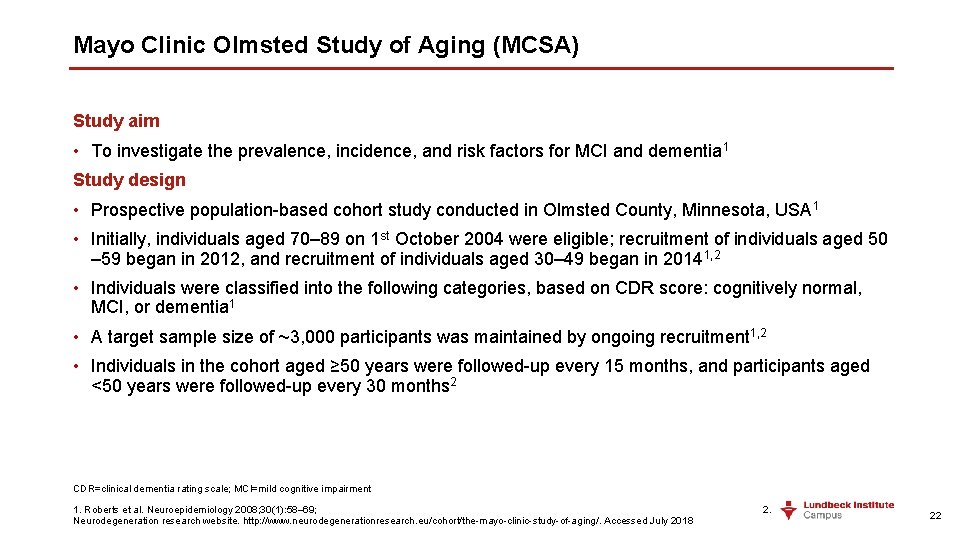
Mayo Clinic Olmsted Study of Aging (MCSA) Study aim • To investigate the prevalence, incidence, and risk factors for MCI and dementia 1 Study design • Prospective population-based cohort study conducted in Olmsted County, Minnesota, USA 1 • Initially, individuals aged 70– 89 on 1 st October 2004 were eligible; recruitment of individuals aged 50 – 59 began in 2012, and recruitment of individuals aged 30– 49 began in 20141, 2 • Individuals were classified into the following categories, based on CDR score: cognitively normal, MCI, or dementia 1 • A target sample size of ~3, 000 participants was maintained by ongoing recruitment 1, 2 • Individuals in the cohort aged ≥ 50 years were followed-up every 15 months, and participants aged <50 years were followed-up every 30 months 2 CDR=clinical dementia rating scale; MCI=mild cognitive impairment 1. Roberts et al. Neuroepidemiology 2008; 30(1): 58– 69; 2. Neurodegeneration research website. http: //www. neurodegenerationresearch. eu/cohort/the-mayo-clinic-study-of-aging/. Accessed July 2018 22

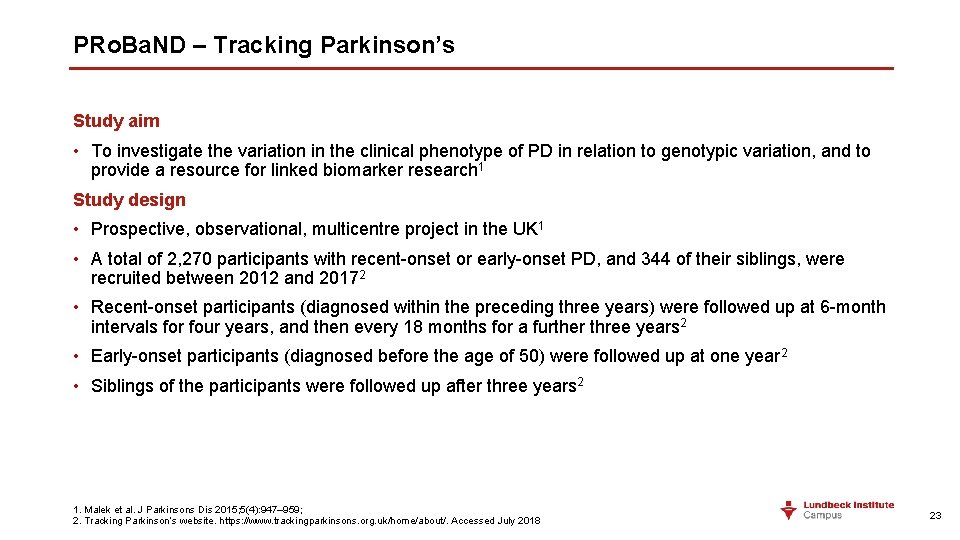
PRo. Ba. ND – Tracking Parkinson’s Study aim • To investigate the variation in the clinical phenotype of PD in relation to genotypic variation, and to provide a resource for linked biomarker research 1 Study design • Prospective, observational, multicentre project in the UK 1 • A total of 2, 270 participants with recent-onset or early-onset PD, and 344 of their siblings, were recruited between 2012 and 20172 • Recent-onset participants (diagnosed within the preceding three years) were followed up at 6 -month intervals for four years, and then every 18 months for a further three years 2 • Early-onset participants (diagnosed before the age of 50) were followed up at one year 2 • Siblings of the participants were followed up after three years 2 1. Malek et al. J Parkinsons Dis 2015; 5(4): 947– 959; 2. Tracking Parkinson’s website. https: //www. trackingparkinsons. org. uk/home/about/. Accessed July 2018 23

Oxford Parkinson’s Disease Centre (OPDC) Study aim • To identify differences in the way PD progresses in different people, and to understand more about how PD develops over time 1 Study design • Prospective, longitudinal study conducted in the UK 2 • Over 1, 500 participants, including >1, 000 individuals with early idiopathic PD, 300 healthy controls and 180 ‘at-risk’ individuals have been recruited 1 • Participants were to be followed up initially at 18 -month intervals for 5– 10 years 2, 3 • Analysis of initial data from this cohort has allowed identification of some important differences in the way PD progresses in different people 1 1. OPDC website. http: //opdc. medsci. ox. ac. uk/theme-1 -clinical-cohorts. Accessed July 2018; 2. Szewczyk-Krolikowski et al. Parkinsonism Relat Disord 2014; 20(1): 99– 105; 3. Heinzel et al. J Parkinsons Dis 2017; 7(3): 423– 432 24

What is the Parkinson’s disease progression marker initiative (PPMI)? • The PPMI is a landmark international trial, sponsored by the Michael J. Fox foundation, that is following thousands of individuals over many years 1, 2 Study aim • To identify clinical, imaging and biologic markers of PD progression for use in clinical trials of disease-modifying therapies 1 • Once these biomarkers are defined, they can be used in therapeutic studies, which is the ultimate goal 2 Study design • Longitudinal, multi-centre study to assess progression of clinical features, imaging and biologic markers in patients with PD compared with healthy controls 1 1. PPMI study protocol. 2015; 2. PPMI website. PPMI-info. org. Accessed July 2018 25

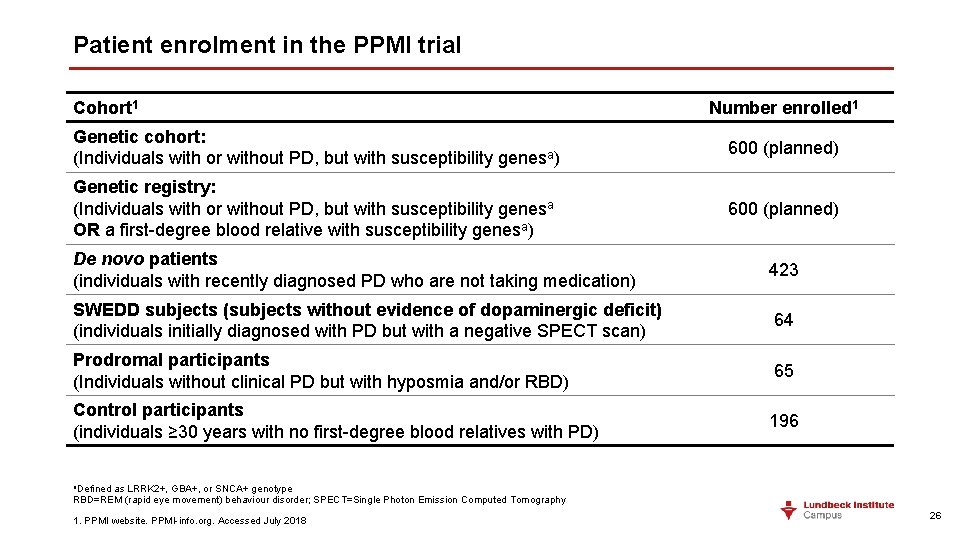
Patient enrolment in the PPMI trial Cohort 1 Number enrolled 1 Genetic cohort: (Individuals with or without PD, but with susceptibility genesa) 600 (planned) Genetic registry: (Individuals with or without PD, but with susceptibility genesa OR a first-degree blood relative with susceptibility genesa) 600 (planned) De novo patients (individuals with recently diagnosed PD who are not taking medication) 423 SWEDD subjects (subjects without evidence of dopaminergic deficit) (individuals initially diagnosed with PD but with a negative SPECT scan) 64 Prodromal participants (Individuals without clinical PD but with hyposmia and/or RBD) 65 Control participants (individuals ≥ 30 years with no first-degree blood relatives with PD) 196 a. Defined as LRRK 2+, GBA+, or SNCA+ genotype RBD=REM (rapid eye movement) behaviour disorder; SPECT=Single Photon Emission Computed Tomography 1. PPMI website. PPMI-info. org. Accessed July 2018 26

Preliminary results of the PPMI study • Although the project is still in progress, there have already been promising publications using data from the PPMI study, including: • The identification of cerebrospinal fluid biomarkers for early cognitive decline in patients with PD 1, 2 • The suggestions that cerebrospinal fluid levels of Aβ 1 -42, T-tau, P-tau 181, and α-synuclein have prognostic and diagnostic potential in early-stage PD 1, 2 When the PPMI study is completed, a comprehensive database of biological progression data will be developed, comparing those who did and those who did not develop PD – this constitutes an invaluable dataset for exploration of the biomarkers of early PD and disease progression 3 1. Skogseth et al. J Parkinsons Dis 2015; 5(4): 783– 792; 2. Kang et al. JAMA Neurol 2013; 70(10): 1277– 1287; 3. PPMI website. PPMI-info. org. Accessed July 2018 27

Parkinson’s disease prognosis 28

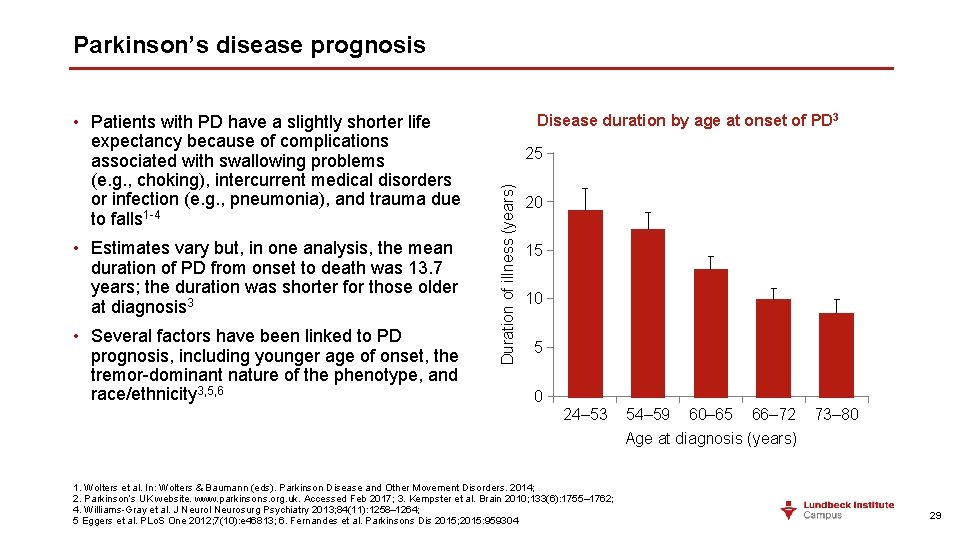
Parkinson’s disease prognosis • Estimates vary but, in one analysis, the mean duration of PD from onset to death was 13. 7 years; the duration was shorter for those older at diagnosis 3 • Several factors have been linked to PD prognosis, including younger age of onset, the tremor-dominant nature of the phenotype, and race/ethnicity 3, 5, 6 Disease duration by age at onset of PD 3 25 Duration of illness (years) • Patients with PD have a slightly shorter life expectancy because of complications associated with swallowing problems (e. g. , choking), intercurrent medical disorders or infection (e. g. , pneumonia), and trauma due to falls 1 -4 20 15 10 5 0 24– 53 1. Wolters et al. In: Wolters & Baumann (eds). Parkinson Disease and Other Movement Disorders. 2014; 2. Parkinson’s UK website. www. parkinsons. org. uk. Accessed Feb 2017; 3. Kempster et al. Brain 2010; 133(6): 1755– 1762; 4. Williams-Gray et al. J Neurol Neurosurg Psychiatry 2013; 84(11): 1258– 1264; 5 Eggers et al. PLo. S One 2012; 7(10): e 46813; 6. Fernandes et al. Parkinsons Dis 2015; 2015: 959304 54– 59 60– 65 66– 72 Age at diagnosis (years) 73– 80 29

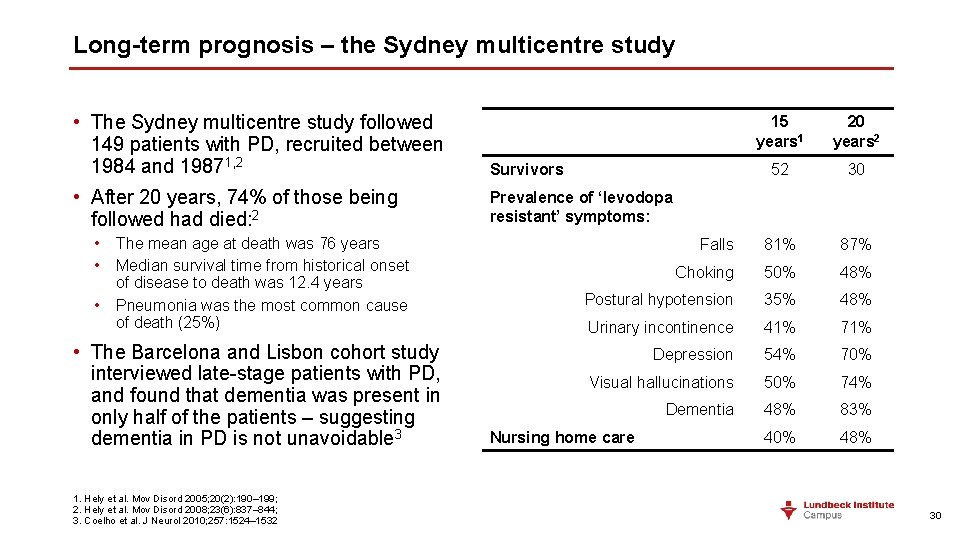
Long-term prognosis – the Sydney multicentre study • The Sydney multicentre study followed 149 patients with PD, recruited between 1984 and 19871, 2 Survivors • After 20 years, 74% of those being followed had died: 2 Prevalence of ‘levodopa resistant’ symptoms: • The mean age at death was 76 years • Median survival time from historical onset of disease to death was 12. 4 years • Pneumonia was the most common cause of death (25%) • The Barcelona and Lisbon cohort study interviewed late-stage patients with PD, and found that dementia was present in only half of the patients – suggesting dementia in PD is not unavoidable 3 1. Hely et al. Mov Disord 2005; 20(2): 190– 199; 2. Hely et al. Mov Disord 2008; 23(6): 837– 844; 3. Coelho et al. J Neurol 2010; 257: 1524– 1532 15 years 1 20 years 2 52 30 Falls 81% 87% Choking 50% 48% Postural hypotension 35% 48% Urinary incontinence 41% 71% Depression 54% 70% Visual hallucinations 50% 74% Dementia 48% 83% 40% 48% Nursing home care 30

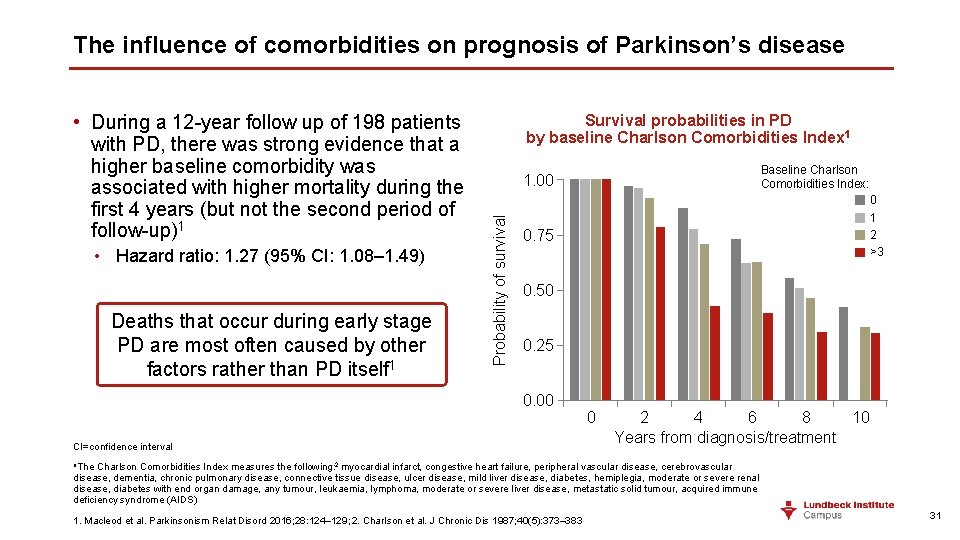
The influence of comorbidities on prognosis of Parkinson’s disease • Hazard ratio: 1. 27 (95% CI: 1. 08– 1. 49) Deaths that occur during early stage PD are most often caused by other factors rather than PD itself 1 Survival probabilities in PD by baseline Charlson Comorbidities Index 1 Baseline Charlson Comorbidities Index: 1. 00 Probability of survival • During a 12 -year follow up of 198 patients with PD, there was strong evidence that a higher baseline comorbidity was associated with higher mortality during the first 4 years (but not the second period of follow-up)1 0 1 2 >3 0. 75 0. 50 0. 25 0. 00 CI=confidence interval 0 2 4 6 8 10 Years from diagnosis/treatment a. The Charlson Comorbidities Index measures the following: 2 myocardial infarct, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, mild liver disease, diabetes, hemiplegia, moderate or severe renal disease, diabetes with end organ damage, any tumour, leukaemia, lymphoma, moderate or severe liver disease, metastatic solid tumour, acquired immune deficiency syndrome (AIDS) 1. Macleod et al. Parkinsonism Relat Disord 2016; 28: 124– 129; 2. Charlson et al. J Chronic Dis 1987; 40(5): 373– 383 31

Cognitive impairment and dementia in Parkinson’s disease • In a study of progression to dementia, patients with PD were compared to control individuals 1 • Another study determined clinical and neuropsychological variables that predicted cognitive decline in PD 2 • After 4 years, 62% of those with PD and mild cognitive impairment (MCI) at baseline developed dementia, compared to 20% of those without MCI (p=0. 001)1 • Of the 126 non-demented patients with PD who were assessed between 3 and 5 years after diagnosis, 10% subsequently developed dementia 2 • Predictive factors for dementia included: 2 The presence of MCI in patients with PD predicts the subsequent development of dementia 1 • • Older age Non-tremor dominant motor phenotype Higher UPDRS-Motor score Below average performance on various scales and measures of verbal fluency and memory UPDRS=Unified Parkinson’s Disease Rating Scale 1. Janvin et al. Mov Dis 2006; 21(9): 1343– 1349; 2. Williams-Gray et al. Brain 2007; 130(Pt 7): 1787– 1798 32

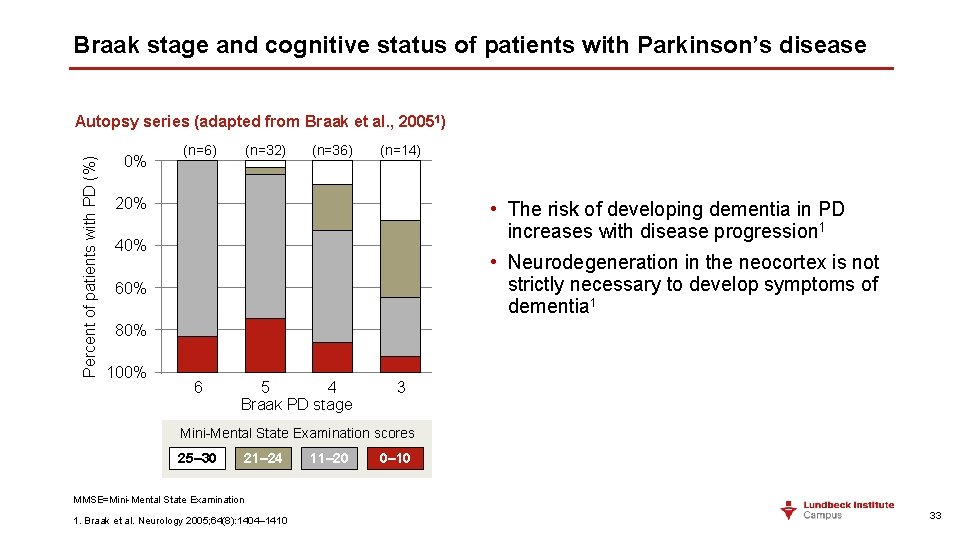
Braak stage and cognitive status of patients with Parkinson’s disease Percent of patients with PD (%) Autopsy series (adapted from Braak et al. , 20051) 0% (n=6) (n=32) (n=36) (n=14) 20% • The risk of developing dementia in PD increases with disease progression 1 40% • Neurodegeneration in the neocortex is not strictly necessary to develop symptoms of dementia 1 60% 80% 100% 6 5 4 Braak PD stage 3 Mini-Mental State Examination scores 25– 30 21– 24 11– 20 0– 10 MMSE=Mini-Mental State Examination 1. Braak et al. Neurology 2005; 64(8): 1404– 1410 33

Clinical approach during late stage Parkinson’s disease • A patient may require palliative care if they meet the following criteria: 1 -3 • • • Lack of response to medication, with marked/rapid decline in physical functioning Decreased independence and needing help for activities of daily living Lack of predictability for ‘OFF’ periods Presence of dysphagia, recurrent pneumonia, stability problems, freezing of gait, and falls Severe dementia Complex psychosocial needs • In the final stages of the disease, specific objectives for caring for patients with PD include the following: 1 • Relieving the patient’s symptoms and the patient’s and carer’s distress • Preserving the patient’s remaining dignity and functions despite the advanced stage of disease • Preventing treatment-related complications 1. Kulisevsky et al. Neurologia 2013; 28(9): 558– 583; 2. Hussain et al. Int J Palliat Nurs 2013; 19(4): 162– 169; 3. Boersma et al. Neurology 2014; 83(6): 561– 567 34
 Parkinsons disease hereditory
Parkinsons disease hereditory Tasha big feet
Tasha big feet Natural history and spectrum of disease
Natural history and spectrum of disease Shy drager syndrome
Shy drager syndrome Rectosigmoid junction
Rectosigmoid junction Katharine hepburn parkinsons
Katharine hepburn parkinsons Behcets disease wiki
Behcets disease wiki Natural history of disease adalah
Natural history of disease adalah Natural history of disease
Natural history of disease Natural history of diseases
Natural history of diseases Natural history of disease is best studied by
Natural history of disease is best studied by Natural history of disease
Natural history of disease Spectrum of disease
Spectrum of disease Bharathi viswanathan
Bharathi viswanathan Orleans hanna algebra prognosis test
Orleans hanna algebra prognosis test Ejemplos de prognosis en trabajo social
Ejemplos de prognosis en trabajo social Ich score prognosis
Ich score prognosis Hellp syndrome
Hellp syndrome Autism prognosis
Autism prognosis Splinter skills
Splinter skills Autism prognosis
Autism prognosis Autism prognosis
Autism prognosis Autism prognosis
Autism prognosis Static-99
Static-99 What is prognosis
What is prognosis Prognosis
Prognosis Child-pugh score prognosis
Child-pugh score prognosis Cholangitis prognosis
Cholangitis prognosis Good prognosis
Good prognosis Prognosis of cystic fibrosis
Prognosis of cystic fibrosis Tubular spine surgery in mumbai
Tubular spine surgery in mumbai Good prognosis
Good prognosis Course number and title
Course number and title T junction english bond
T junction english bond Course interne moyenne externe
Course interne moyenne externe