Control of MultiDrug Resistant Microorganisms in Health Care




























- Slides: 54

Control of Multi-Drug Resistant Micro-organisms in Health Care Settings Session 5 a: Antimicrobial resistance surveillance Version: 10 April 2018

Objectives Specific objectives of this session: 1. Identify surveillance systems both local and national in each member state 2. Critically evaluate methods of surveillance for MDRO acquired infections in healthcare settings 3. Critically review systems for local microbiological surveillance and early warning systems 4. Propose strategies for use in clinical areas 2

Outline This session consists of the following elements: 1. Introduction to the antimicrobial resistance surveillance 2. Explanation of the methods of the antimicrobial resistance surveillance 3. Group exercise integrating the antimicrobial resistance surveillance practices and good international examples 3

You can’t manage, what you can’t measure 4

Definition of surveillance Surveillance is the ongoing systematic collection, analysis, and interpretation of health data essential to the planning, implementation and evaluation of public health practice, closely integrated with the timely dissemination of these data to those who need to know. The final link of the surveillance chain is the application of these data to prevention and control. 5

Antimicrobial resistance surveillance

Goals of antimicrobial resistance surveillance • • • To To To monitor trends for local prescribing guidance monitor trends to advise on public health strategies identify new/emerging resistance assess the impact of interventions educate – clinicians, general public, politicians There can be more than one goal embedded within a surveillance programme. 7

Scopes of antimicrobial resistance surveillance • Local - supports local prescribing measures, formulary development, infection control activities • National - provide standardized information to support long term strategic planning, implementation and evaluation of antimicrobial prescribing and infection control activities. • International - limited ability to compare but early alert to emerging trends and potential for spread across borders 8

Define what it is you wish to monitor Examples: • Trends in resistance – decide on organism, specimen type, antimicrobial resistance to be monitored • New mechanisms of resistance • Impact of other activities (e. g. , reducing C. difficile infection through altered prescribing with potential to select different antimicrobial resistance) The main public health concern is the emergence and spread of multi-resistant strains of bacteria. 9

Requirements of antimicrobial resistance surveillance • Must be able to detect significant differences in antimicrobial resistence between locations (e. g. , hospital and community, regions) • Must be able to detect shifts in susceptibility to antimicrobial agents • Must define which combination of organisms and antimicrobials to monitor • Must define population to measure • Must define target audience for reports and publications 10

Methods of antimicrobial resistance surveillance Incidence: number of new cases (resistant organisms) in a defined period Prevalence: number of cases (resistant organisms) at a specific time point Continuous: capture information on an ongoing basis Interrupted: e. g. , repeated point prevalence surveys Laboratory based: less labour intensive Patient based: high data quality, resource-intensive Choice of method depends on aims and available resources. 11

Scopes of antimicrobial resistance surveillance (2) • Active, passive • Department-wide, facility-wide • Selected departments (e. g. , Intensive Care Unit, Haematology unit) • All samples • Blood (and cerebrospinal fluid? ) samples – invasive isolates • Active surveillance (screening) 12

Example of microorganisms included in a surveillance programme using a core data set European Antimicrobial Resistance Surveillance System (EARSS) scheme of blood culture isolates: • • Streptococcus pneumoniae Staphylococcus aureus Enterococcus faecalis Enterococcus faecium Escherichia coli Klebsiella pneumoniae Pseudomonas aeruginosa Acinetobacter baumanii Organisms where antimicrobial resistance is of public health concern 13

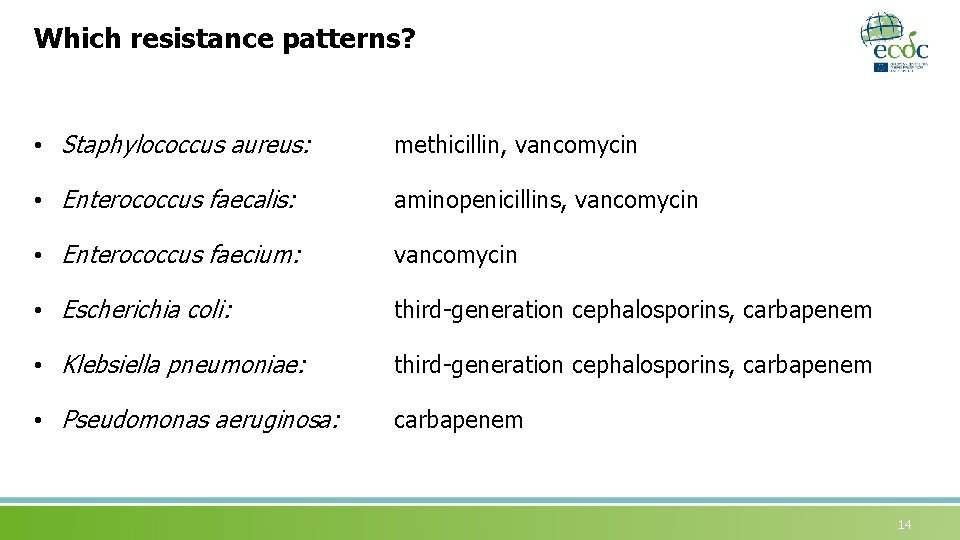
Which resistance patterns? • Staphylococcus aureus: methicillin, vancomycin • Enterococcus faecalis: aminopenicillins, vancomycin • Enterococcus faecium: vancomycin • Escherichia coli: third-generation cephalosporins, carbapenem • Klebsiella pneumoniae: third-generation cephalosporins, carbapenem • Pseudomonas aeruginosa: carbapenem 14

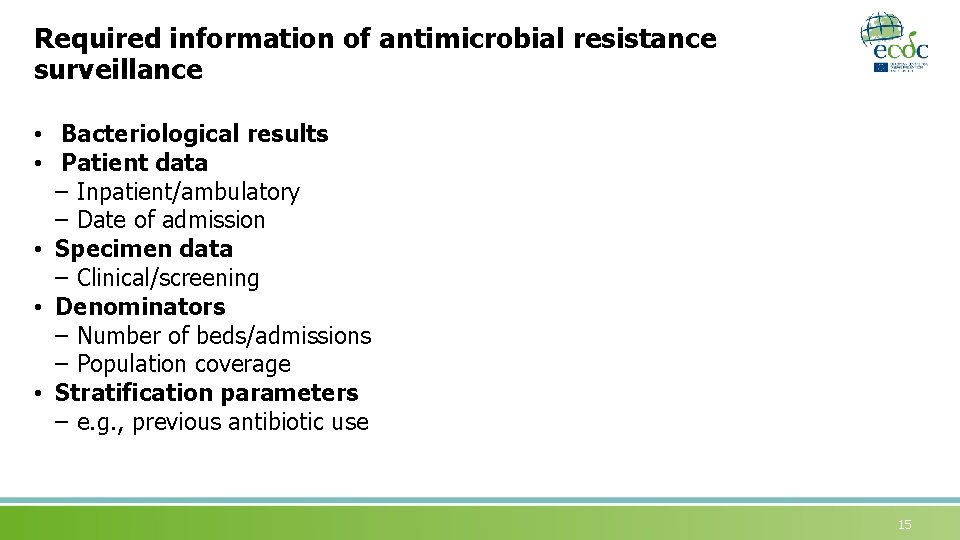
Required information of antimicrobial resistance surveillance • Bacteriological results • Patient data – Inpatient/ambulatory – Date of admission • Specimen data – Clinical/screening • Denominators – Number of beds/admissions – Population coverage • Stratification parameters – e. g. , previous antibiotic use 15

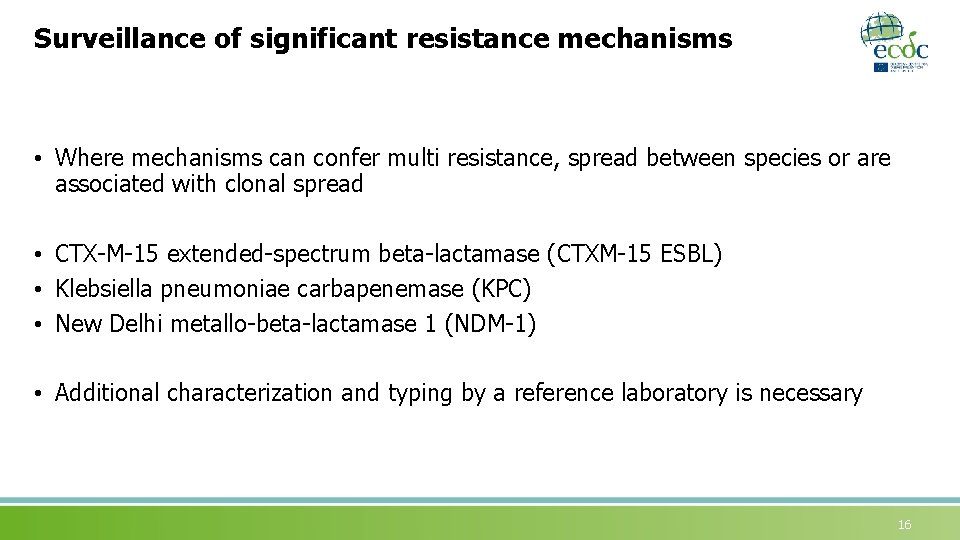
Surveillance of significant resistance mechanisms • Where mechanisms can confer multi resistance, spread between species or are associated with clonal spread • CTX-M-15 extended-spectrum beta-lactamase (CTXM-15 ESBL) • Klebsiella pneumoniae carbapenemase (KPC) • New Delhi metallo-beta-lactamase 1 (NDM-1) • Additional characterization and typing by a reference laboratory is necessary 16

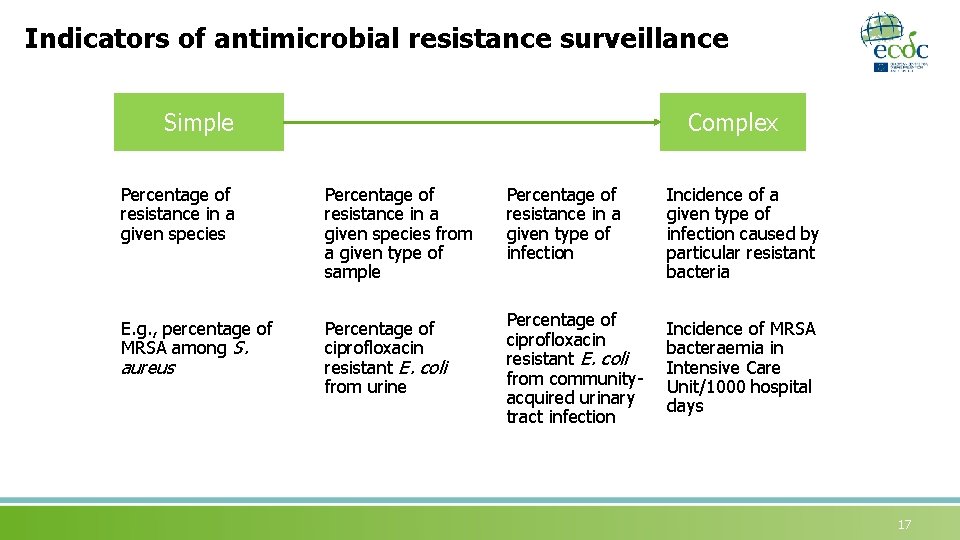
Indicators of antimicrobial resistance surveillance Simple Complex Percentage of resistance in a given species from a given type of sample Percentage of resistance in a given type of infection Incidence of a given type of infection caused by particular resistant bacteria E. g. , percentage of MRSA among S. Percentage of ciprofloxacin resistant E. coli from urine Percentage of ciprofloxacin resistant E. coli from communityacquired urinary tract infection Incidence of MRSA bacteraemia in Intensive Care Unit/1000 hospital days aureus 17

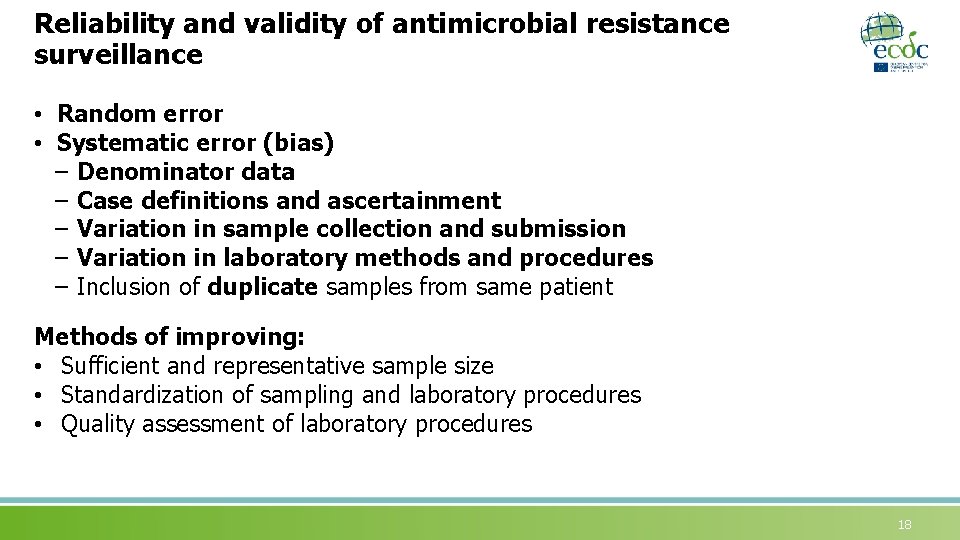
Reliability and validity of antimicrobial resistance surveillance • Random error • Systematic error (bias) – Denominator data – Case definitions and ascertainment – Variation in sample collection and submission – Variation in laboratory methods and procedures – Inclusion of duplicate samples from same patient Methods of improving: • Sufficient and representative sample size • Standardization of sampling and laboratory procedures • Quality assessment of laboratory procedures 18

Timeframe for data collection and analysis • Long time intervals possible for • Cumulative susceptibility report to inform prescription practices locally • Assessment of scale of problem at local, national or international level • Shorter time intervals if alert function • Availability of data and resources influence the reporting period 19

Quality assurance and control • Appropriateness of samples • Accuracy of pathogen identification and susceptibility testing • Completeness of reporting 20

Interpretation of antimicrobial resistance surveillance data

Interpretation of antimicrobial resistance surveillance data Consider factors that could be responsible for emerging and continuing antimicrobial resistance. Risk factors for developing an infection with a resistant pathogen in hospitals can broadly be classified into four categories: • Antimicrobial misuse • Suboptimal infection control • Patient risk factors, including severity of disease and use of medical devices • Imported resistance (e. g. , resistant organisms in primary care, animals, food products, water supply, other region/country/healthcare facility) 22

Communication of antimicrobial resistance surveillance data An essential component of any surveillance system for antimicrobial use and resistance is the way in which information is made available to prescribers, other clinical staff, health care planners and the general public 23

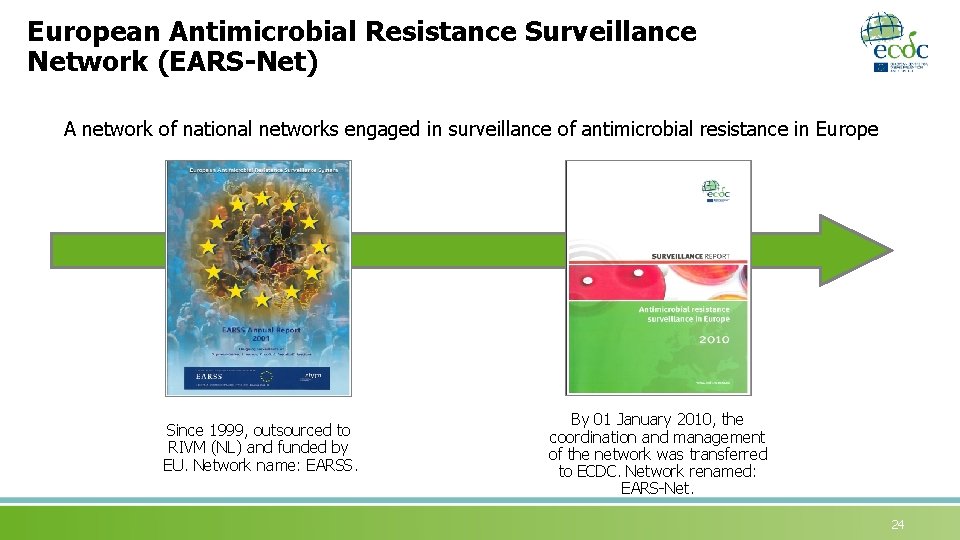
European Antimicrobial Resistance Surveillance Network (EARS-Net) A network of national networks engaged in surveillance of antimicrobial resistance in Europe Since 1999, outsourced to RIVM (NL) and funded by EU. Network name: EARSS. By 01 January 2010, the coordination and management of the network was transferred to ECDC. Network renamed: EARS-Net. 24

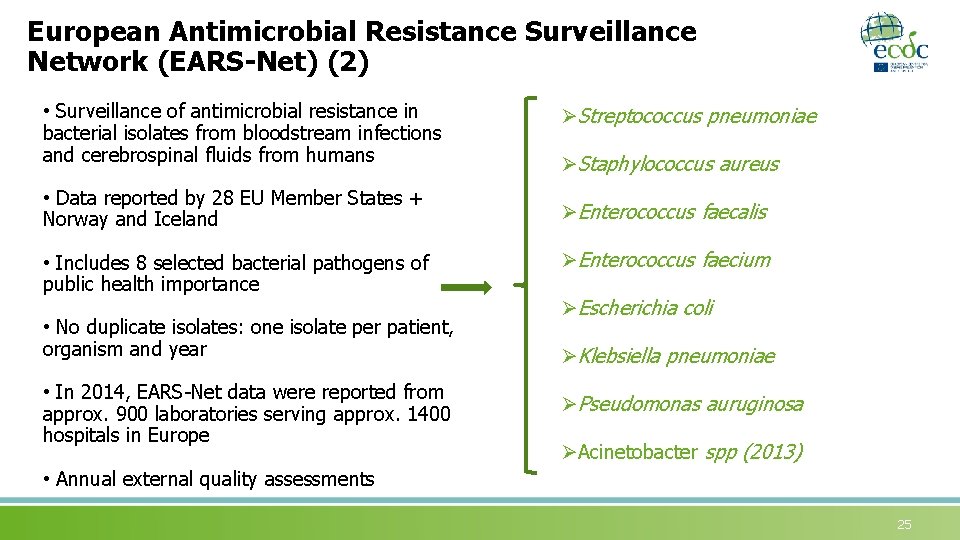
European Antimicrobial Resistance Surveillance Network (EARS-Net) (2) • Surveillance of antimicrobial resistance in bacterial isolates from bloodstream infections and cerebrospinal fluids from humans • Data reported by 28 EU Member States + Norway and Iceland • Includes 8 selected bacterial pathogens of public health importance • No duplicate isolates: one isolate per patient, organism and year • In 2014, EARS-Net data were reported from approx. 900 laboratories serving approx. 1400 hospitals in Europe ØStreptococcus pneumoniae ØStaphylococcus aureus ØEnterococcus faecalis ØEnterococcus faecium ØEscherichia coli ØKlebsiella pneumoniae ØPseudomonas auruginosa ØAcinetobacter spp (2013) • Annual external quality assessments 25

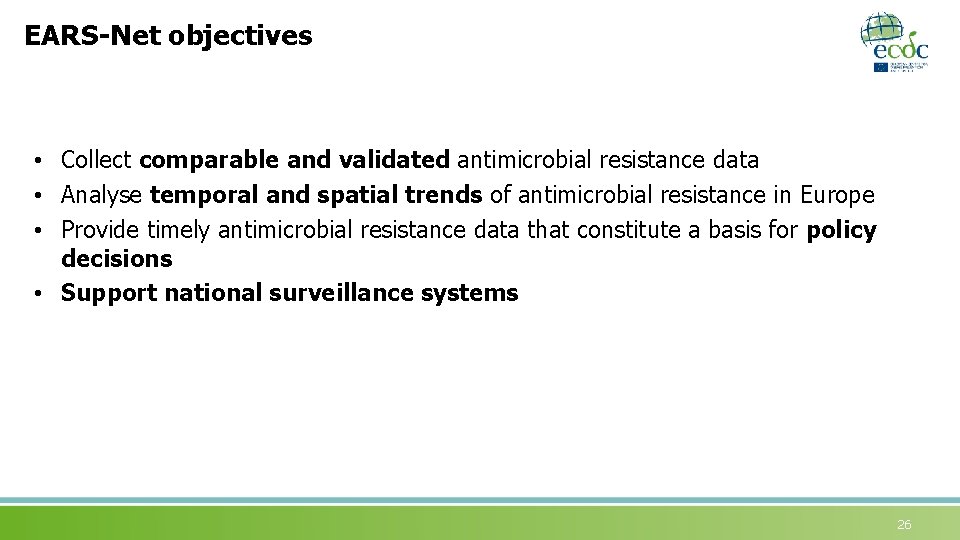
EARS-Net objectives • Collect comparable and validated antimicrobial resistance data • Analyse temporal and spatial trends of antimicrobial resistance in Europe • Provide timely antimicrobial resistance data that constitute a basis for policy decisions • Support national surveillance systems 26

Success factors Easy data access and data for action • Timely feedback and fast analysis after data is approved by the member states • Easy accessible outputs Interactive public database Annual reports Special analyses

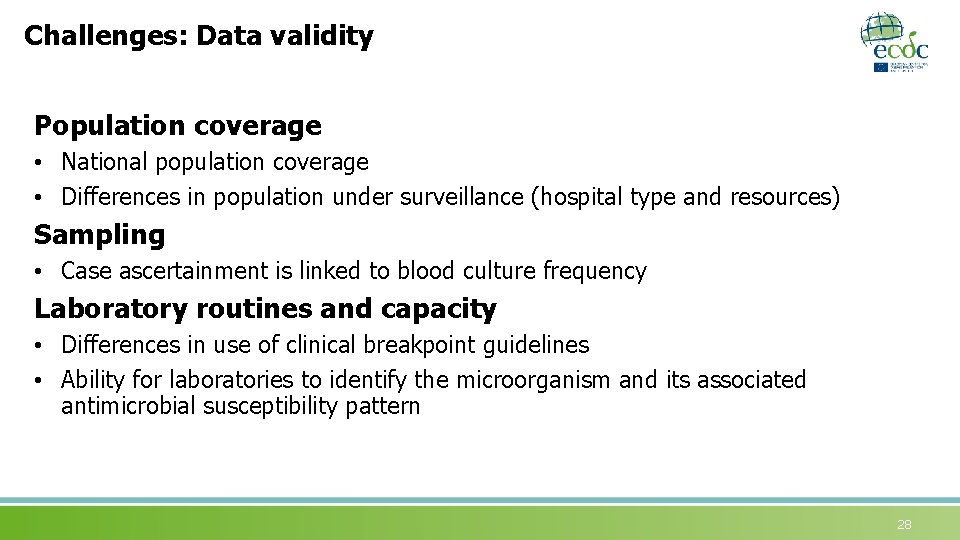
Challenges: Data validity Population coverage • National population coverage • Differences in population under surveillance (hospital type and resources) Sampling • Case ascertainment is linked to blood culture frequency Laboratory routines and capacity • Differences in use of clinical breakpoint guidelines • Ability for laboratories to identify the microorganism and its associated antimicrobial susceptibility pattern 28

Example of international comparisons

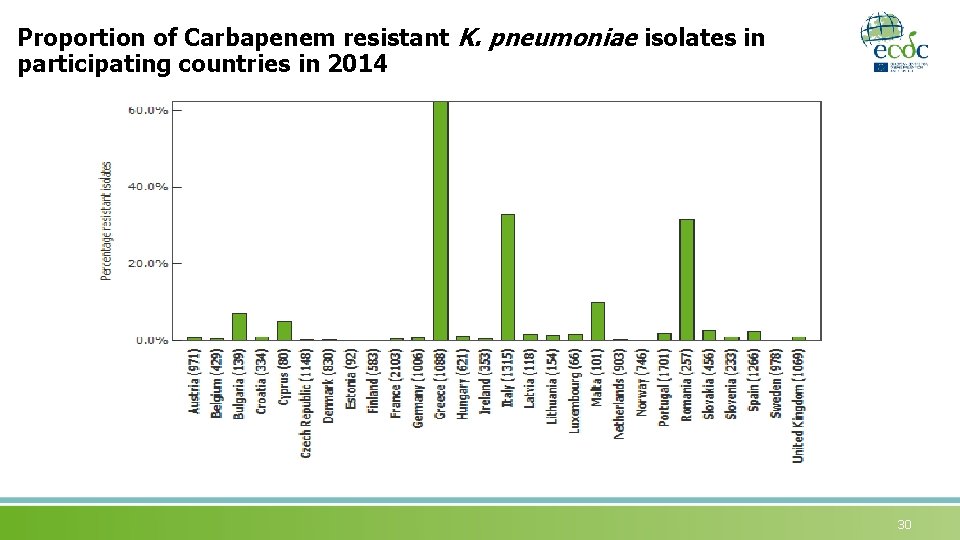
Proportion of Carbapenem resistant K. pneumoniae isolates in participating countries in 2014 30

Trends in K. pneumoniae resistant to third-generation cephalosporins 31

Examples of national aantimicrobial resistance surveillance DANMAP Danish National surveillance system monitors antimicrobial consumption and resistance trends in humans and food animals http: //www. danmap. org STRAMA Swedish strategic programme against antibiotics resistance monitors trends in human antimicrobial consumption and resistance http: //www. strama. se/dyn//, 226, 18, 77. html Scot. MARAP-2/SAPG Scottish monitoring of antimicrobial resistance and antimicrobial prescribing overseen by Scottish Antimicrobial Prescribing Group http: //www. scottishmedicines. org. uk/SAPG/Scottish_Antimicrobial_Prescribing_Group_SAPG 32

33

Monitoring Multi-drug resistance at a national level Scot. MARAP-2: Non-susceptible K. pneumoniae bacteraemias 34

Automated electronic antimicrobial resistance surveillance • Facilitates the timely detection of unusual phenotypes or emerging clusters • Automates exchange of information • Allows rapid communication and alerting NB: validity and interpretation of data 35

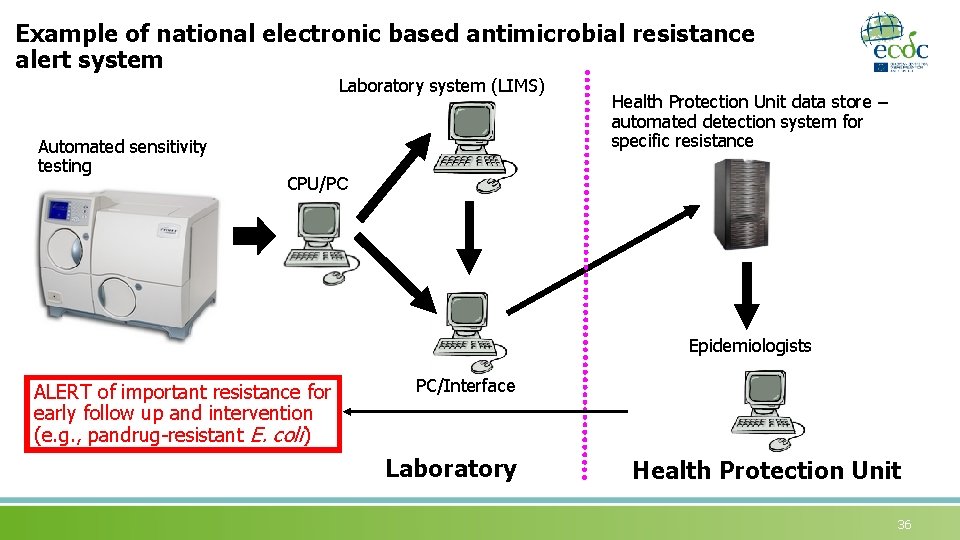
Example of national electronic based antimicrobial resistance alert system Laboratory system (LIMS) Automated sensitivity testing Health Protection Unit data store – automated detection system for specific resistance CPU/PC Epidemiologists ALERT of important resistance for early follow up and intervention (e. g. , pandrug-resistant E. coli) PC/Interface Laboratory Health Protection Unit 36

Active surveillance of antimicrobial resistance (screening for MDROs) • Greater proportion of patients colonised with MDROs than have clinical infection • Screening for carriage associated with other control measures may reduce risk of transmission • Must be clear what screening for and measures to be taken when carriage confirmed (e. g. , eradication of MRSA, transmission based precautions of Klebsiella pneumoniae carbapenemase-producing Klebsiella) 37

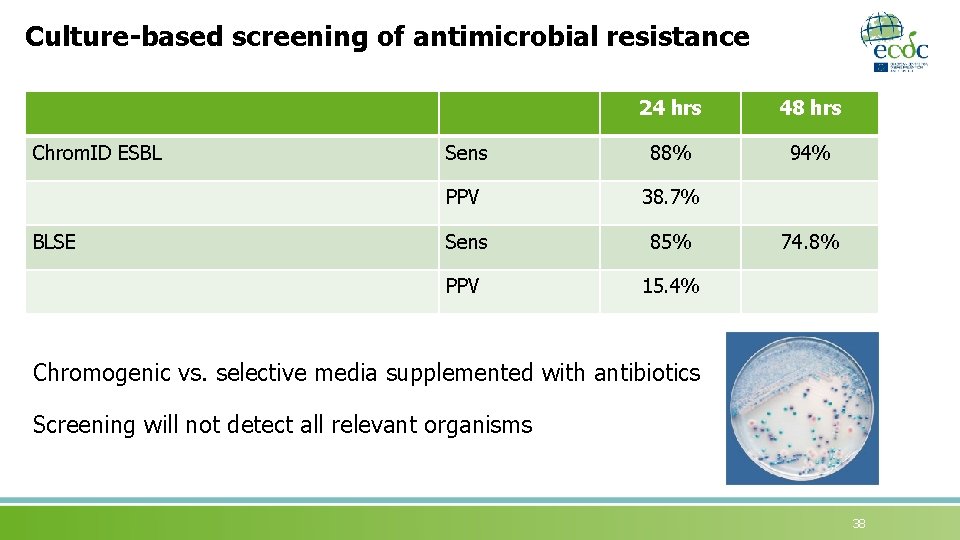
Culture-based screening of antimicrobial resistance Chrom. ID ESBL BLSE 24 hrs 48 hrs Sens 88% 94% PPV 38. 7% Sens 85% PPV 15. 4% 74. 8% Chromogenic vs. selective media supplemented with antibiotics Screening will not detect all relevant organisms 38

Rapid detection tests for active screening of Gramnegative MDROs • Multiple targets • Unclear clinical significance (e. g. , chromosomal Amp. C gene) • Possible developments in microarrays and real-time polymerase chain reaction 39

Active screening for Methicillin-resistant Staphylococcus aureus (MRSA) Major pitfalls: quasi-experimental design and not accounting for secular trends or concomitant measures Glick SB. Am J Infect Contr 2014; 42: 148 -55 40

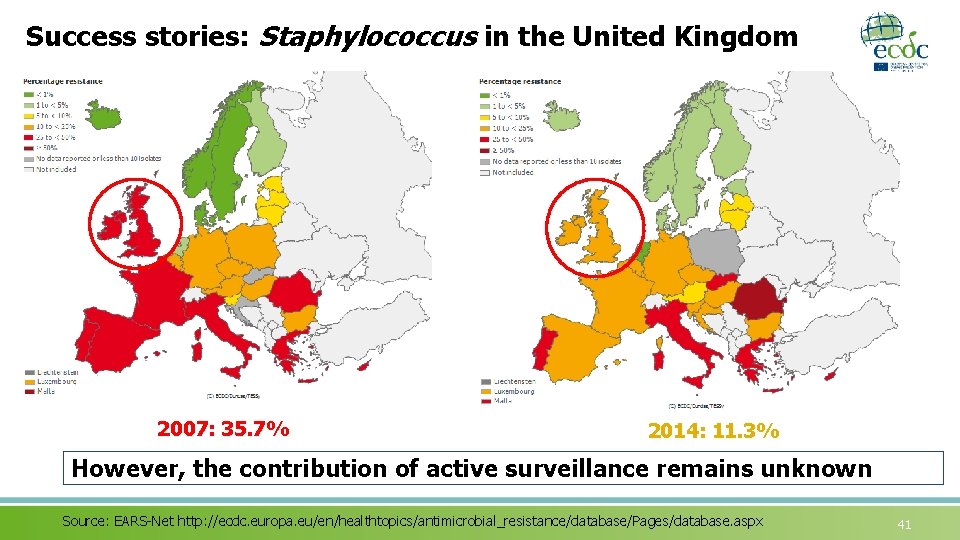
Success stories: Staphylococcus in the United Kingdom 2007: 35. 7% 2014: 11. 3% However, the contribution of active surveillance remains unknown Source: EARS-Net http: //ecdc. europa. eu/en/healthtopics/antimicrobial_resistance/database/Pages/database. aspx 41

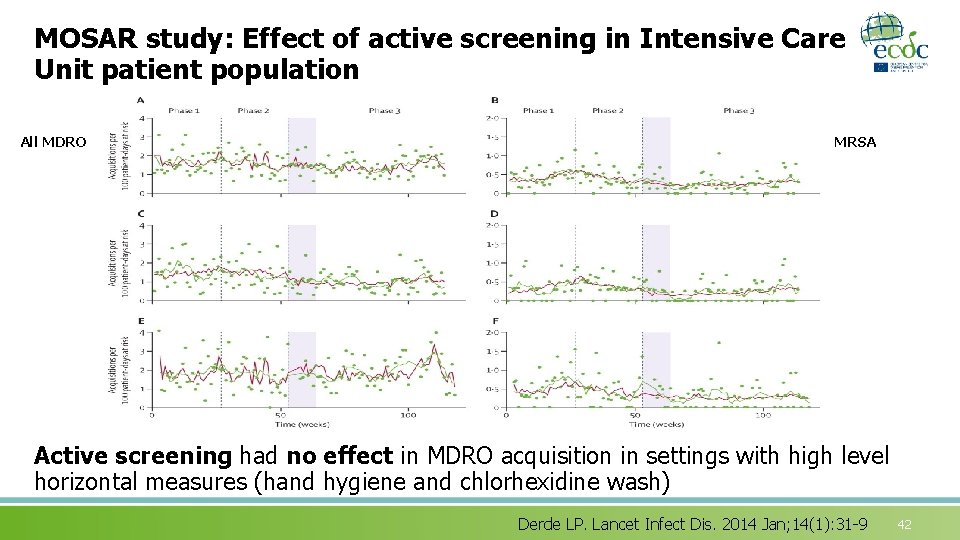
MOSAR study: Effect of active screening in Intensive Care Unit patient population All MDRO MRSA Active screening had no effect in MDRO acquisition in settings with high level horizontal measures (hand hygiene and chlorhexidine wash) Derde LP. Lancet Infect Dis. 2014 Jan; 14(1): 31 -9 42

Active screening of Gram-negative MDROs recommendations of European Society of Clinical Microbiology and Infectious Diseases (ESCMID) • Recommended for epidemic settings • Moderate level of evidence for K. pneumoniae and A. baumannii • Unclear for endemic settings • Depending on organism • But contact precautions for colonized patients recommended • Active surveillance on admission and weekly in the Intensive Care Unit was part of the strategy used in Israel Tacconelli et al. ESCMID Guidelines to reduce the hospital spread of MDR-GNB. Clin Microbiol Infect 2014; 20 (Suppl. 1): 1– 55 Cohen et al. Infect Control Hosp Epidemiol 2011; 32(7): 673 -8 43

Active surveillance for Gram-negative MDROs There is evidence from studies that report multi-faceted infection control bundles for the effectiveness of early implementation of: a) active surveillance … on admission to specific wards/units b) pre-emptive isolation of high-risk patients upon admission and c) active surveillance during outbreaks (evidence level ++). 44

Situations where active surveillance is recommended or can be considered • Admission after overseas travel to or hospitalization in areas with endemic MDRO • Admission from Nursing homes • High risk patients (e. g. , Intensive Care Units, Haematology units) • Where newly identified organism for the institution (e. g. , Vancomycin-resistant Enterococcus with potential to prevent further spread) 45

Factors to consider for planning active surveillance (screening for colonisation) • Define the facility and regional epidemiology (e. g. , endemic) • Who? • All admissions • Admissions in certain departments (e. g. , Intensive Care Units) • Admissions from certain facilities (e. g. , Nursing homes) • Targeted screening of admissions with risk factors • When? • On admission • On regular intervals during hospitalization • How? • Sample (e. g. , rectal, perianal, stool) • Detection method (culture in antibiotic supplemented medium, polymerase chain reaction) 46

In summary List of learning points in this session: • Surveillance of antimicrobial resistance - essential component of infection control, antimicrobial prescribing and public health • Objectives must be clearly defined • Bias and error need to be carefully addressed • Results must be communicated in a timely fashion • Screening for carriage may be of value in defined situation and where there is an intervention 47

Group exercise Key areas: • Current MDRO surveillance practices in member states, identifying common practice • A standardised approach towards MDRO surveillance in European member states • Different procedures of reporting to public health and alert systems • Approaches to active surveillance (screening) 48

References • Diekema DJ, Pfaller MA. Rapid detection of antibiotic-resistant organism carriage for infection prevention. Clin Infect Dis. 2013 June; 56(11): 1614 -20. • Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988 Jun; 16(3): 128 -40. • Drees M, Gerber J, Morgan D, Lee G. Research Methods in Healthcare Epidemiology and Antimicrobial Stewardship: Use of Administrative and Surveillance Databases. Infect Control Hosp Epidemiol. 2016 Nov; 37(11): 1278 -87. • European Centre for Disease Prevention and Control (ECDC). Data from the ECDC Surveillance Atlas - Antimicrobial resistance. Available at: http: //ecdc. europa. eu/en/healthtopics/antimicrobial_resistance/database/Pages/graph_reports. as px • Cohen et al. Infect Control Hosp Epidemiol 2011; 32(7): 673 -8 49

References (2) • Health Protection Scotland (HPS). Report on Antimicrobial Use and Resistance in Humans in 2013. Health Protection Scotland Information Services Division 2015. Available at: http: //www. isdscotland. org/Health-Topics/Prescribing-and-Medicines/Publications/2015 -0127/2015 -01 -27 -SAPG-2013 -Report. pdf • Glick SB, Samson DJ, Huang ES, Vats V, Aronson N, Weber SG. Screening for methicillin-resistant Staphylococcus aureus: a comparative effectiveness review. Am J Infect Control. 2014 Feb; 42(2): 148 -55. • Duerden B, Fry C, Johnson AP, Wilcox MH. The Control of Methicillin-Resistant Staphylococcus aureus Blood Stream Infections in England. Open Forum Infect Dis. 2015 Mar 12; 2(2): ofv 035. • Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014 Jan; 14(1): 31 -9. • EARS-Net http: //ecdc. europa. eu/en/healthtopics/antimicrobial_resistance/database/Pages/database. aspx 50

References (3) • Tacconelli E, Cataldo MA, Dancer SJ, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014 Jan; 20 Suppl 1: 1 -55. • Mutters NT, Günther F, Frank U, Mischnik A. Costs and possible benefits of a two-tier infection control management strategy consisting of active screening for multidrug-resistant organisms and tailored control measures. J Hosp Infect. 2016 Jun; 93(2): 191 -6. • European Centre for Disease Prevention and Control. Systematic review of the effectiveness of infection control measures to prevent the transmission of extended-spectrum beta-lactamaseproducing Enterobacteriaceae through cross-border transfer of patients. Stockholm: ECDC; 2014. Available at: https: //ecdc. europa. eu/sites/portal/files/media/en/publications/Publications/ESBLsystematic-review-effectiveness-infection-control-measures. pdf 51

References (4) • Faron ML, Ledeboer NA, Buchan BW. Resistance Mechanisms, Epidemiology, and Approaches to Screening for Vancomycin-Resistant Enterococcus in the Health Care Setting. J Clin Microbiol. 2016 Oct; 54(10): 2436– 47. • Perry JD. A Decade of Development of Chromogenic Culture Media for Clinical Microbiology in an Era of Molecular Diagnostics. Clin Microbiol Rev. 2017 Apr; 30(2): 449– 79. 52

Acknowledgements The creation of this training material was commissioned in 2011 by ECDC to Health Protection of Scotland, National Services Scotland, University of Chester and University of Dundee with the direct involvement of Eastaway A, C Wiuff, A Seaton, J Reilly, M Rivett. This lecture was adapted in 2014 by Diamantis Plachouras (ECDC). The revision and update of this training material was commissioned in 2017 by ECDC to Transmissible (Netherlands) with the direct involvement of Rita Szabo, Remco Schrijver and Arnold Bosman 53

This work is licensed under the Creative Commons (http: //creativecommons. org) Attribution 2. 0 Generic license (http: //creativecommons. org/licenses/by/2. 0/deed. en). ---- This last slide or page must always be included with this work ---Authors: A. Eastaway ; M. Rivett ATTENTION AUTHORS & ADAPTERS: All external sources should be mentioned on individual pages, diagrams, tables, photos within the work. Adapted / Modified by: Diamantis Plachouras, ECDC, 2014 Rita Szabo, Remco Schrijver in collaboration with Transmissible (Netherlands), 2018 If you make any modifications to this work, enter your name, organization, date, and event here. ATTENTION READER: The original author may not have endorsed modifications. You are free: to share – to copy, distribute and transmit the work to remix – to adapt the work Under the following conditions: attribution – You must attribute the work in the manner specified by the author or licensor but not : • in any way that suggests that they endorse you or your use of the work. • In any way that suggests you are author of the work The authors of this slide/page encourage educators, trainers, and professionals to include this slide within their documents and presentations for rightful attribution of their works and thus also allow it to be easily shared. (ECV 1 -29/9/11) 54
 Tertiary level of care
Tertiary level of care Care value base health and social care
Care value base health and social care Spill resistant vacuum breaker
Spill resistant vacuum breaker Cyanide-resistant respiration slideshare
Cyanide-resistant respiration slideshare Resistant materials tools
Resistant materials tools Collision resistant hash function
Collision resistant hash function Collision resistant hash function
Collision resistant hash function Rodent resistant fiber optic cable
Rodent resistant fiber optic cable Kyloc usmc
Kyloc usmc Scbf design example
Scbf design example Design of seismic-resistant steel building structures
Design of seismic-resistant steel building structures Does metronidazole contain penicillin
Does metronidazole contain penicillin Define temper evident packaging
Define temper evident packaging Insect-resistant packaging solutions
Insect-resistant packaging solutions Fire resistant cable thai yazaki
Fire resistant cable thai yazaki Flame resistant lab coat amazon
Flame resistant lab coat amazon Savage syndrome
Savage syndrome Tuned mass damper
Tuned mass damper Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Arc duct switchgear
Arc duct switchgear Health and social care component 3
Health and social care component 3 Learning objectives of microorganisms
Learning objectives of microorganisms Microbiology founder
Microbiology founder Are microorganisms helpful or harmful
Are microorganisms helpful or harmful Harmful microorganisms
Harmful microorganisms The five i's of studying microorganisms
The five i's of studying microorganisms Flora ir fauna
Flora ir fauna Factors affecting food spoilage
Factors affecting food spoilage Limitations of light microscope
Limitations of light microscope Microorganisms meaning
Microorganisms meaning Microorganisms 5th grade
Microorganisms 5th grade Where does fermentation occur
Where does fermentation occur Fermentation in microorganisms
Fermentation in microorganisms Microorganisms wanted poster
Microorganisms wanted poster What is microbiology
What is microbiology Classification of microorganisms
Classification of microorganisms Microorganisms
Microorganisms What is the microorganisms
What is the microorganisms Physiology of microorganisms
Physiology of microorganisms Classification of microorganisms
Classification of microorganisms Flora
Flora Wanted poster microorganism
Wanted poster microorganism Morphology of algae
Morphology of algae Major groups of microorganisms
Major groups of microorganisms Oil palm
Oil palm Bacterial growth curve
Bacterial growth curve Microorganisms
Microorganisms Microorganisms species
Microorganisms species Observing microorganisms through a microscope
Observing microorganisms through a microscope Care certificate duty of care
Care certificate duty of care Acul magnetic al unei busole se orienteaza
Acul magnetic al unei busole se orienteaza Palliative care versus hospice care
Palliative care versus hospice care Animale care nasc pui vii
Animale care nasc pui vii Care sunt simturile prin care sunt evocate
Care sunt simturile prin care sunt evocate Care certificate standard 1
Care certificate standard 1