Control of Multidrug Resistant MicroOrganisms in Health Care













- Slides: 28

Control of Multidrug Resistant Micro-Organisms in Health Care Settings Session 2: Laboratory Investigations Diagnostics typing and susceptibility testing Version: 2011

Origins of Antimicrobial Resistance • Bacteria evolved in hostile environments • They developed mechanisms to protect them from harmful toxins • Antibiotics are a naturally occurring group of agents produced by eg Aspergillus species to provide a survival advantage • Other micro-organisms evolved protective mechanisms eg enzymes to protect themselves particularly soil bacteria • These resistance mechanisms have been acquired by human pathogens and then spread. 2

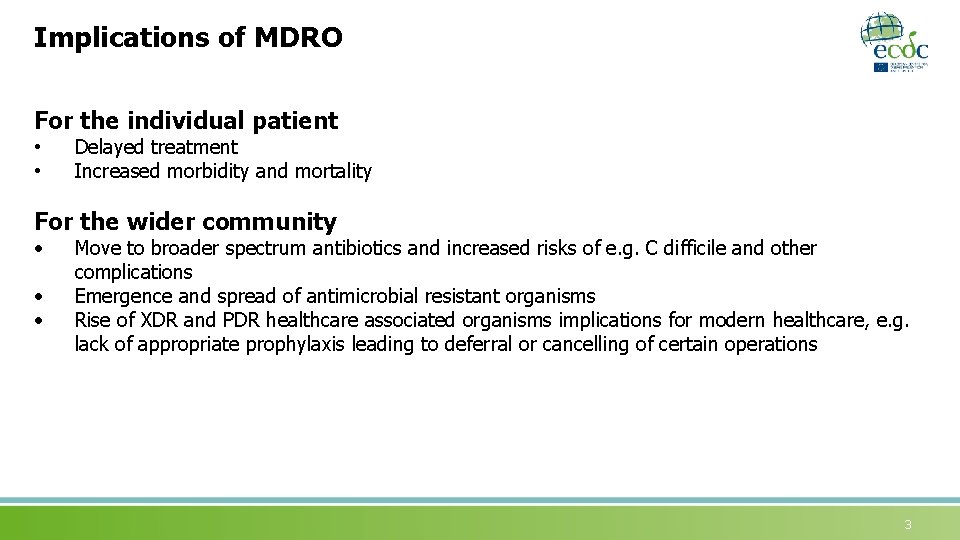
Implications of MDRO For the individual patient • • Delayed treatment Increased morbidity and mortality For the wider community • • • Move to broader spectrum antibiotics and increased risks of e. g. C difficile and other complications Emergence and spread of antimicrobial resistant organisms Rise of XDR and PDR healthcare associated organisms implications for modern healthcare, e. g. lack of appropriate prophylaxis leading to deferral or cancelling of certain operations 3

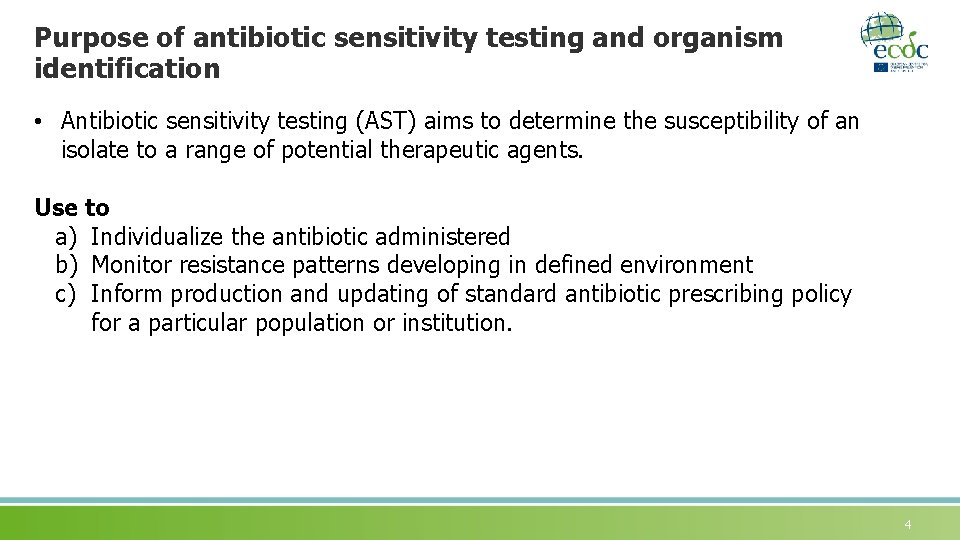
Purpose of antibiotic sensitivity testing and organism identification • Antibiotic sensitivity testing (AST) aims to determine the susceptibility of an isolate to a range of potential therapeutic agents. Use to a) Individualize the antibiotic administered b) Monitor resistance patterns developing in defined environment c) Inform production and updating of standard antibiotic prescribing policy for a particular population or institution. 4

Purpose of antibiotic sensitivity testing and organism identification (continued. . ) • Identification of an organism is required alongside the Sensitivity test, knowing what organism you have isolated together with knowledge of the isolation site, will give an indication of what type of antibiotics should be considered. • The sensitivity of an isolate to a particular antibiotic is measured by establishing the Minimum Inhibitory Concentration (MIC) or breakpoint, this is the lowest concentration (conventionally tested in doubling dilutions) of antibiotic at which an isolate cannot produce visible growth after overnight incubation. • Results may be reported as Sensitive, Intermediate sensitive ie requiring a higher dose of antibiotic or Resistant 5

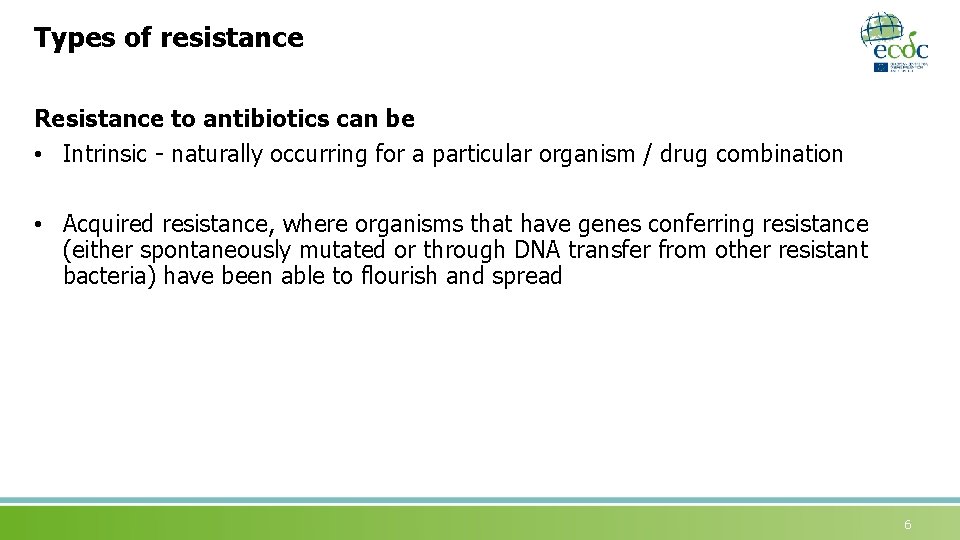
Types of resistance Resistance to antibiotics can be • Intrinsic - naturally occurring for a particular organism / drug combination • Acquired resistance, where organisms that have genes conferring resistance (either spontaneously mutated or through DNA transfer from other resistant bacteria) have been able to flourish and spread 6

ECDC Definitions of MDRO Magiorakos A P, Srinivasan A, Carey R et al Multidrug-resistant MDR • • Extremely drug-Resistant XDR • • non-susceptible to at least one agent in all but 2 or fewer antimicrobial categories Pandrug-Resistant PDR • • • non-susceptible to at least one agent in >3 antimicrobial categories non-susceptible to all agents in all antimicrobial categories The categories vary for each species and exclude known intrinsic resistance Clin Microbiol Infect. 2011 May 7. doi: 10. 1111/j. 1469 -0691. 2011. 03570. x. 7

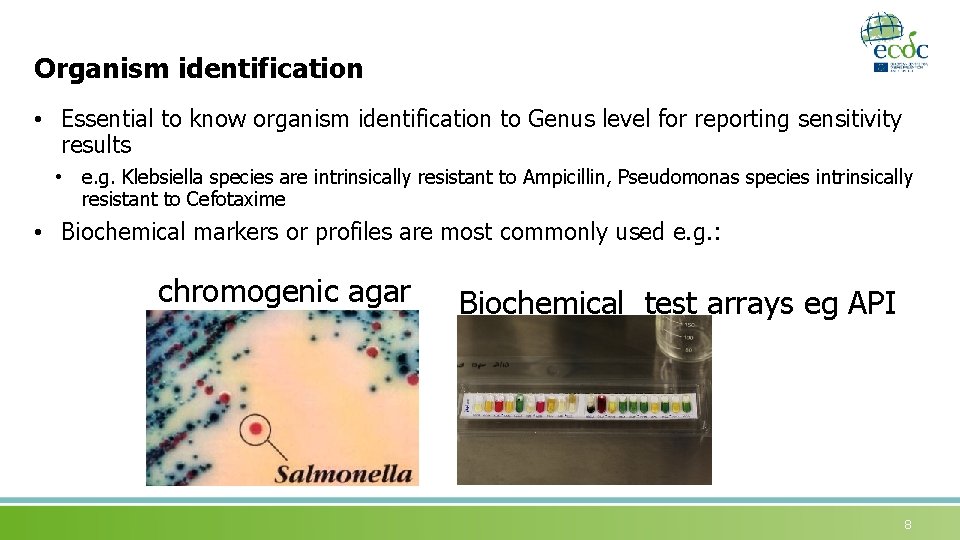
Organism identification • Essential to know organism identification to Genus level for reporting sensitivity results • e. g. Klebsiella species are intrinsically resistant to Ampicillin, Pseudomonas species intrinsically resistant to Cefotaxime • Biochemical markers or profiles are most commonly used e. g. : chromogenic agar Biochemical test arrays eg API 8

Key characteristics of Antimicrobial susceptibility testing Aim is to measure susceptibility of an isolate to range of antibiotics at the individual patient level for effective prescribing, but also to assess emerging bacterial resistance patterns data used to revise standard prescribing policies. 9

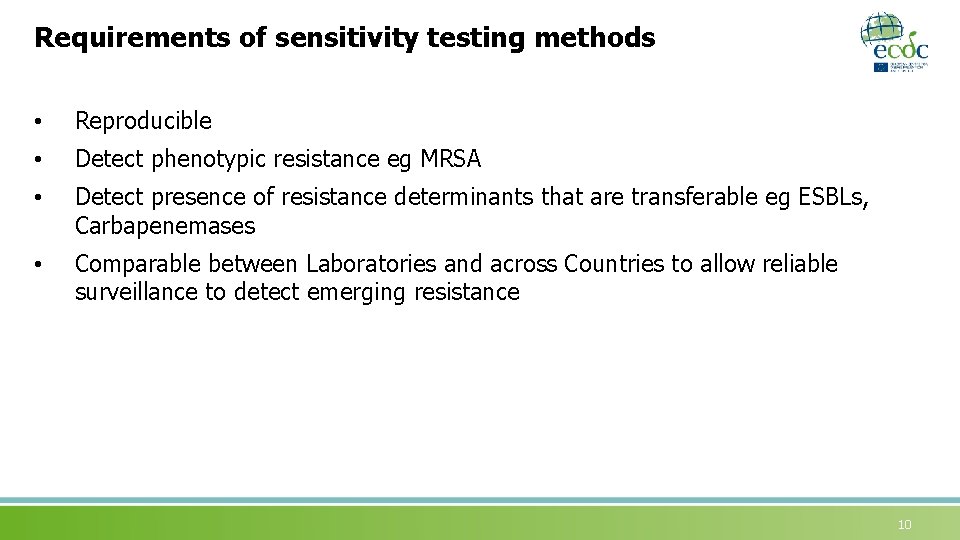
Requirements of sensitivity testing methods • Reproducible • Detect phenotypic resistance eg MRSA • Detect presence of resistance determinants that are transferable eg ESBLs, Carbapenemases • Comparable between Laboratories and across Countries to allow reliable surveillance to detect emerging resistance 10

Recognised Sensitivity testing Methods • USA - CLSI • Europe - BSAC, CA-SFM, CRG, DIN, NWGA, SRGA • EUCAST – is harmonising standards for breakpoints from all European National breakpoint committees. • EUCAST - is being adopted across the ECDC as the preferred method • it will allow consistency of reporting and comparison between member states. Clinical breakpoints are for everyday use in the clinical laboratory to advise on patient therapy. http: //www. eucast. org/ 11

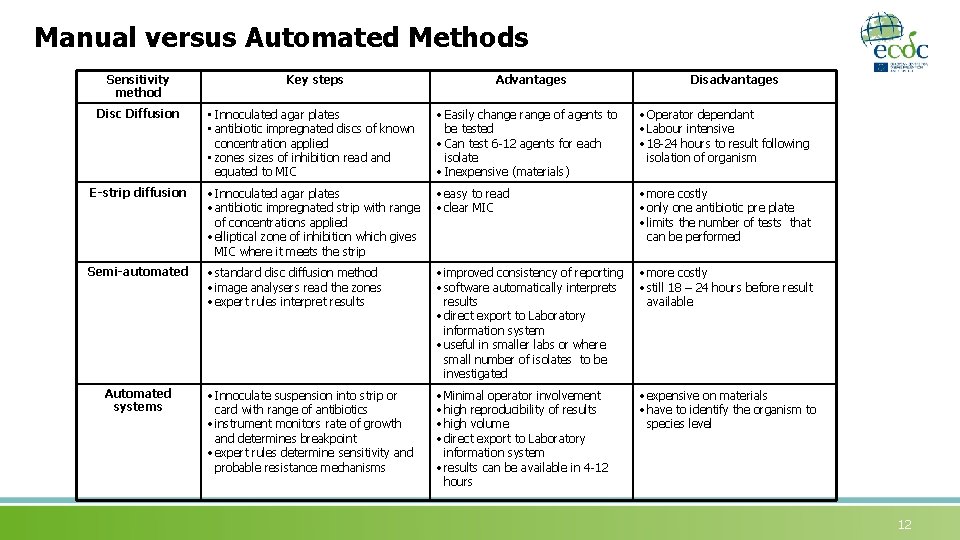
Manual versus Automated Methods Sensitivity method Key steps Advantages Disadvantages Disc Diffusion • Innoculated agar plates • antibiotic impregnated discs of known concentration applied • zones sizes of inhibition read and equated to MIC • Easily change range of agents to be tested • Can test 6 -12 agents for each isolate • Inexpensive (materials) • Operator dependant • Labour intensive • 18 -24 hours to result following isolation of organism E-strip diffusion • Innoculated agar plates • antibiotic impregnated strip with range of concentrations applied • elliptical zone of inhibition which gives MIC where it meets the strip • easy to read • clear MIC • more costly • only one antibiotic pre plate • limits the number of tests that can be performed Semi-automated • standard disc diffusion method • image analysers read the zones • expert rules interpret results • improved consistency of reporting • software automatically interprets results • direct export to Laboratory information system • useful in smaller labs or where small number of isolates to be investigated • more costly • still 18 – 24 hours before result available • Innoculate suspension into strip or card with range of antibiotics • instrument monitors rate of growth and determines breakpoint • expert rules determine sensitivity and probable resistance mechanisms • Minimal operator involvement • high reproducibility of results • high volume • direct export to Laboratory information system • results can be available in 4 -12 hours • expensive on materials • have to identify the organism to species level Automated systems 12

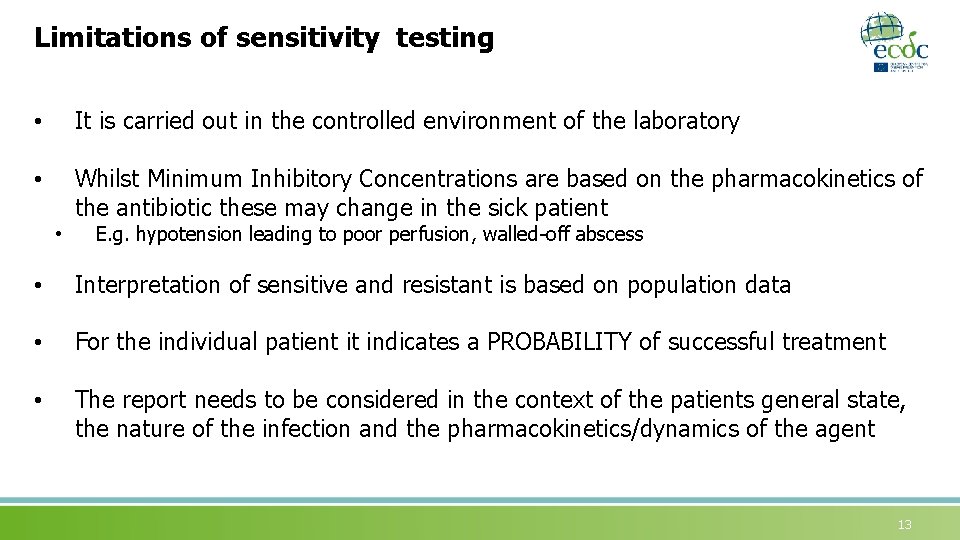
Limitations of sensitivity testing • It is carried out in the controlled environment of the laboratory • Whilst Minimum Inhibitory Concentrations are based on the pharmacokinetics of the antibiotic these may change in the sick patient • E. g. hypotension leading to poor perfusion, walled-off abscess • Interpretation of sensitive and resistant is based on population data • For the individual patient it indicates a PROBABILITY of successful treatment • The report needs to be considered in the context of the patients general state, the nature of the infection and the pharmacokinetics/dynamics of the agent 13

Investigation of emerging MDR, XDR, PDR Need to identify if this is clonal spread (i. e. a specific strain of a species e. g. MRSA E-15) or the resistance is present in a range of organisms Need to identify the resistance mechanism • • • e. g. Carbapenemase NDM 1 which is highly transmissible between organisms or Carbapenem resistance due to a permeability defect which is not transmissible Need to identify resistance mechanism • • e. g. ESBL / Carbapenemase because this will impact on choice of antibiotic (particularly enzyme inhibitors) Reasons for emergence • • e. g. foreign travel with acquisition, establishment within hospital population, nursing home or other institutions, general population - this has implications for control measures Need to review control measures • • e. g. infection control, antimicrobial prescribing policies 14

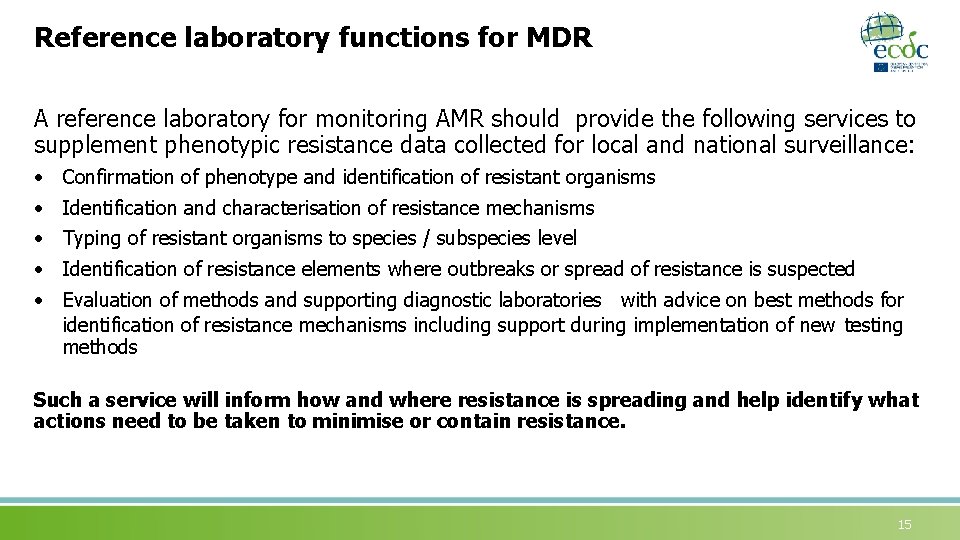
Reference laboratory functions for MDR A reference laboratory for monitoring AMR should provide the following services to supplement phenotypic resistance data collected for local and national surveillance: • • • Confirmation of phenotype and identification of resistant organisms Identification and characterisation of resistance mechanisms Typing of resistant organisms to species / subspecies level Identification of resistance elements where outbreaks or spread of resistance is suspected Evaluation of methods and supporting diagnostic laboratories with advice on best methods for identification of resistance mechanisms including support during implementation of new testing methods Such a service will inform how and where resistance is spreading and help identify what actions need to be taken to minimise or contain resistance. 15

Role of screening for MDR carriage (public health principles for screening) • The purpose of screening is to detect early those patients colonised with MDR who are at risk of developing infection or where there is a risk of onward transmission. • There must be a clear intervention for screening to be introduced with a sound evidence base • • e. g. infection control measures, alteration of antibiotic prophylaxis regime, eradication measure (such as mupirocin for MRSA) The general principles for screening for disease apply equally to screening for carriage of MDR 16

Wilson and Jungner Classic Screening Criteria (WHO, 1968) • • • The condition sought should be an important health problem There should be an accepted treatment for patients with recognized disease. Facilities for diagnosis and treatment should be available. There should be a recognisable latent or early symptomatic stage. There should be a suitable test or examination. The test should be acceptable to the population. The natural history of the condition, including development from latent to declared disease, should be adequately understood. There should be an agreed policy on whom to treat as patients. The cost of case-finding (including diagnosis and treatment of patients diagnosed) should be economically balanced in relation to possible expenditure on medical care as a whole. Case-finding should be a continuing process and not a 'once and for all' project. Wilson. JMG, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. Available from: http: //www. who. int/bulletin/volumes/86/4/07 -050112 BP. pdf 17

Methods of screening for carriage of MDR organisms • Screening sites are dependant on the natural ecology of the organism e. g. : • MRSA - nose, skin, • VRE - rectal swabs • KPC producing Klebsiella sp – Rectal swabs • Screening methods • Chromogenic agar - detects the target organisms by colour of the colony, based on biochemical markers - may take up to 72 hours to confirm • PCR - near patient testing, Laboratory testing. Rapid turnaround (4 hours) good at confirming negatives, require culture confirmation for positives Always a danger that we may miss new, mutant strains 18

Non-prescribing measures for MDR containment • Infection control measures • Hand hygiene • Transmission based precautions • Protective clothing • Screening for carriage if appropriate • Eradication methods if appropriate eg Mupirocin for MRSA 19

Prescribing measures for MDR containment • Antimicrobial prescribing policies • Audit of compliance with prescribing • Ongoing surveillance of local resistance profiles of common pathogens. • Regular review and modification of prescribing policies based on surveillance data 20

Monitoring unintended consequences of antimicrobial Stewardship • Unintended consequences may be anticipated or unanticipated events which occur as a result of changes in antibiotic prescribing practice. • Clinicians need to be reassured that changing prescribing practise will not have adverse effects on their patients. 21

Three categories of ‘Unintended consequences’ Selection of Antimicrobial resistance • Changes in antibacterial prescribing patterns (including loss of diversity in prescribing) will alter selection pressures on certain microbes Efficacy of antimicrobial treatment regimens • Substitution of an established clinically effective antimicrobial for the treatment of bacterial infection with another may be associated with, or raise concerns about the risk of an inferior clinical response, increase frequency of treatment failure and increase in infection-related mortality Antimicrobial related toxicity • Increased use of a certain medicine with a known toxicity profile can be anticipated to result in an increase in toxicity. Examples include: renal and oto-vestibular (VIII nerve) toxicity with gentamicin; Rash or Stevens-Johnson Syndrome with co-trimoxazole 22

Summary • MDRO have potential serious implications for the individual patient • MDRO have implications for public health and delivery of health care • Surveillance of MDRO is necessary to inform prescribing policies and other control measures • For effective sensitivity testing organisms have to be identified to genus level • For surveillance to be effective there has to be a standardised approach to sensitivity testing and categorising of MDR, XDR and PDR bacteria • In Europe the EUCAST system for antimicrobial susceptibility is recommended 23

Summary (continued. . ) • Sensitivity testing can be carried out in various ways all of which have advantages and limitations • Controlling the spread of MDR organisms depends on the nature of the organism, where it is carried and what interventions are effective • Screening for carriage may be of value e. g. MRSA where there is a clear intervention • Decisions to screen for carriage must be informed by the public health principles for screening • This should include monitoring unintended consequences of altered prescribing such as increased admission rates to hospital, increased mortality, etc. to ensure prescribing policies are appropriate 24

Group Activity Group discussion around the following key areas: 1. Different mechanisms of reporting to public health 2. Current laboratory practices in member states, identifying common practice 3. Discuss the implementation of a standardised approach towards sensitivity testing in european member states 25

References 1. Clin Microbiol Infect. 2011 May 7. doi: 10. 1111/j. 1469 -0691. 2011. 03570. x. 2. Wilson. JMG, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. Available from: http: //www. who. int/bulletin/volumes/86/4/07 -050112 BP. pdf 26

Acknowledgements The creation of this training material was commissioned in 2011 by ECDC to Health Protection of Scotland, National Services Scotland, University of Chester and University of Dundee with the direct involvement of Dr Anne Eastaway, C Wiuff, A Seaton, J Reilly, Miss Michelle Rivett, P Davey, A Bryan, D Robertson (2011) The revision and update of this training material was commissioned in 2017 by ECDC to Transmissible (Netherlands) with the direct involvement of Rita Szabo, Remco Schrijver and Arnold Bosman 27

This work is licensed under the Creative Commons (http: //creativecommons. org) Attribution 2. 0 Generic license (http: //creativecommons. org/licenses/by/2. 0/deed. en). ---- This last slide or page must always be included with this work ---Author: Dr Anne Eastaway, C Wiuff, A Seaton, J Reilly, Miss Michelle Rivett, P Davey, A Bryan, D Robertson (2011) Original creator of the document. {Author, affiliation, date, event} May be “Unknown” or “Various. ” ATTENTION AUTHORS & ADAPTERS: All external sources should be mentioned on individual pages, diagrams, tables, photos within the work. Adapted / Modified by: S. Rosales Klintz, Karolinska Institutet, Stockholm, Sweden, 2015, ECDC MDRO course Diamantis Plachouras, ECDC, 2015, ECDC MDRO course Oliver Kacelnik, Norwegian Institute of Public Health, 2016, ECDC MDRO course Rita Szabo, Remco Schrijver in collaboration with Transmissible (Netherlands), 2018 If you make any modifications to this work, enter your name, organization, date, and event here. ATTENTION READER: The original author may not have endorsed modifications. You are free: to share – to copy, distribute and transmit the work to remix – to adapt the work Under the following conditions: attribution – You must attribute the work in the manner specified by the author or licensor but not : • in any way that suggests that they endorse you or your use of the work. • In any way that suggests you are author of the work The authors of this slide/page encourage educators, trainers, and professionals to include this slide within their documents and presentations for rightful attribution of their works and thus also allow it to be easily shared. (ECV 1 -29/9/11) 28
 Primary, secondary, tertiary care
Primary, secondary, tertiary care Health and social care unit 2
Health and social care unit 2 Spill resistant vacuum breaker
Spill resistant vacuum breaker Cyanide-resistant respiration slideshare
Cyanide-resistant respiration slideshare Resistant materials tools
Resistant materials tools Collision resistant hash function
Collision resistant hash function Cryptographic
Cryptographic Fiber optic cable rodent protection
Fiber optic cable rodent protection Kyloc usmc
Kyloc usmc Ebf
Ebf Design of seismic-resistant steel building structures
Design of seismic-resistant steel building structures Inhibition of nucleic acid synthesis
Inhibition of nucleic acid synthesis Define temper evident packaging
Define temper evident packaging Insect-resistant packaging solutions
Insect-resistant packaging solutions Fire resistant cable thai yazaki
Fire resistant cable thai yazaki Flame resistant lab coat amazon
Flame resistant lab coat amazon Savage syndrome
Savage syndrome Tuned mass damper
Tuned mass damper Puncture resistant container
Puncture resistant container Arc duct switchgear
Arc duct switchgear Health and social component 3
Health and social component 3 Learning objectives of microorganisms
Learning objectives of microorganisms Classification schemes of a fungus and a bacterium
Classification schemes of a fungus and a bacterium Are microorganisms helpful or harmful
Are microorganisms helpful or harmful Harmful microorganisms
Harmful microorganisms The five i's of studying microorganisms
The five i's of studying microorganisms Flora ir fauna
Flora ir fauna What are intrinsic factors for microbial growth
What are intrinsic factors for microbial growth Observing microorganisms through a microscope
Observing microorganisms through a microscope