Complete Ionic Equation Net Ionic Equations Electrolytes from




- Slides: 25

Complete Ionic Equation Net Ionic Equations

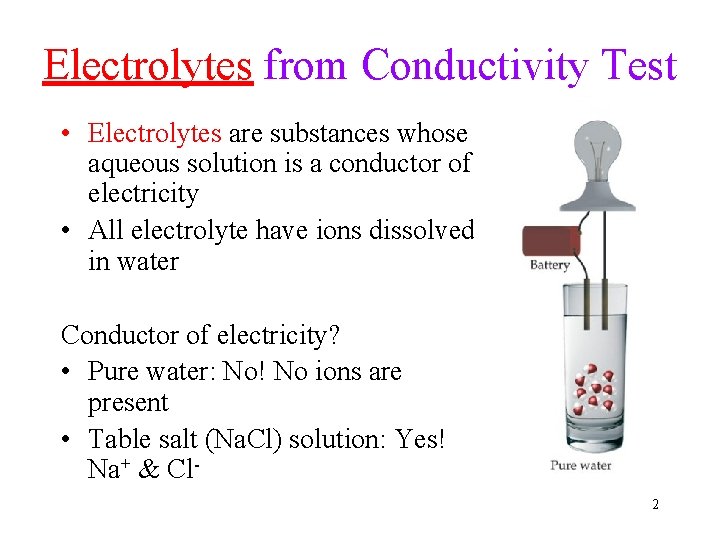
Electrolytes from Conductivity Test • Electrolytes are substances whose aqueous solution is a conductor of electricity • All electrolyte have ions dissolved in water Conductor of electricity? • Pure water: No! No ions are present • Table salt (Na. Cl) solution: Yes! Na+ & Cl 2

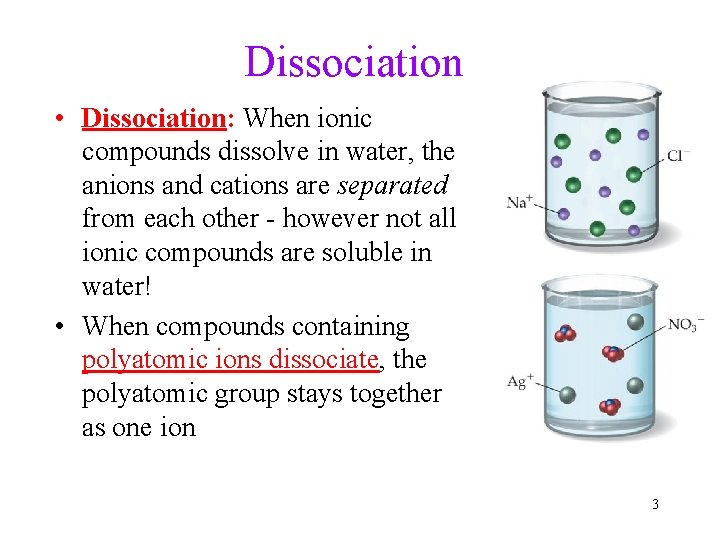
Dissociation • Dissociation: When ionic compounds dissolve in water, the anions and cations are separated from each other - however not all ionic compounds are soluble in water! • When compounds containing polyatomic ions dissociate, the polyatomic group stays together as one ion 3

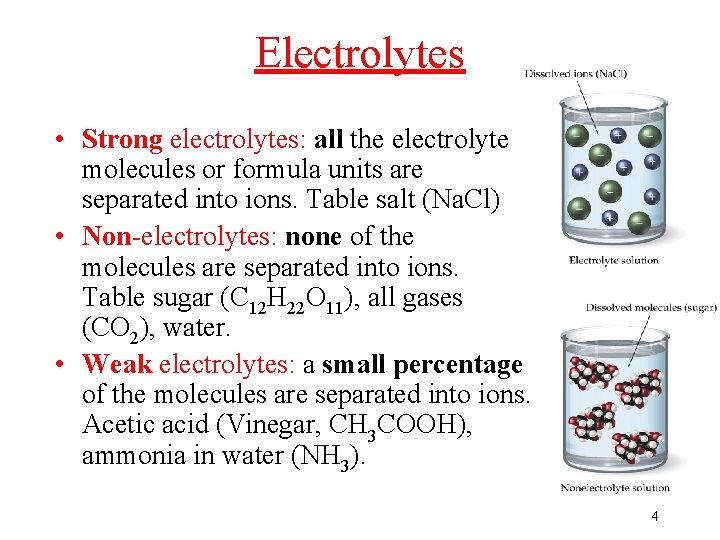
Electrolytes • Strong electrolytes: all the electrolyte molecules or formula units are separated into ions. Table salt (Na. Cl) • Non-electrolytes: none of the molecules are separated into ions. Table sugar (C 12 H 22 O 11), all gases (CO 2), water. • Weak electrolytes: a small percentage of the molecules are separated into ions. Acetic acid (Vinegar, CH 3 COOH), ammonia in water (NH 3). 4

Strong Electrolytes • Salts = water soluble ionic compounds üNa. Cl, NH 4 C 2 H 3 O 2, Ba(NO 3)2, Fe. Br 3, Cu. SO 4 • Strong Acids = completely dissociate to form H+ ions in water solution. üHCl, HBr, HI, HNO 3, H 2 SO 4, HCl. O 4 • Strong Bases = water soluble metal hydroxides (OH-) ü Metal hydroxide of ALL GIA metals such as Na. OH, KOH, etc. üLower GIIA hydroxides: Ca(OH)2), Sr(OH)2, Ba(OH)2 5

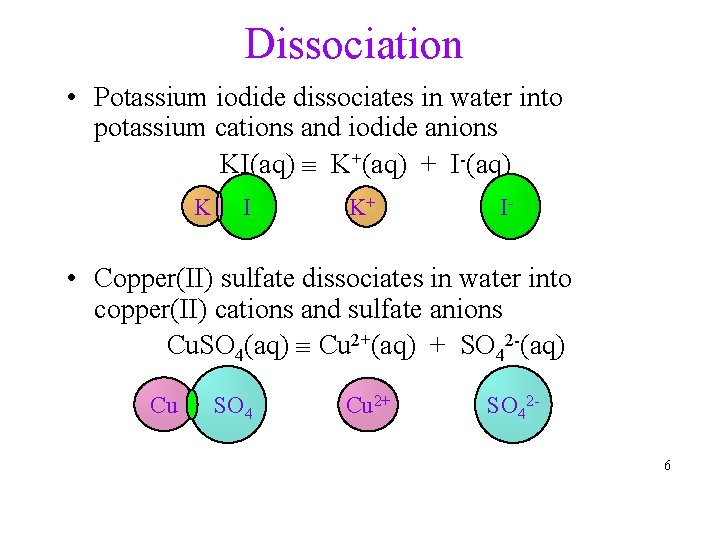
Dissociation • Potassium iodide dissociates in water into potassium cations and iodide anions KI(aq) K+(aq) + I-(aq) K I K+ I- • Copper(II) sulfate dissociates in water into copper(II) cations and sulfate anions Cu. SO 4(aq) Cu 2+(aq) + SO 42 -(aq) Cu SO 4 Cu 2+ SO 426

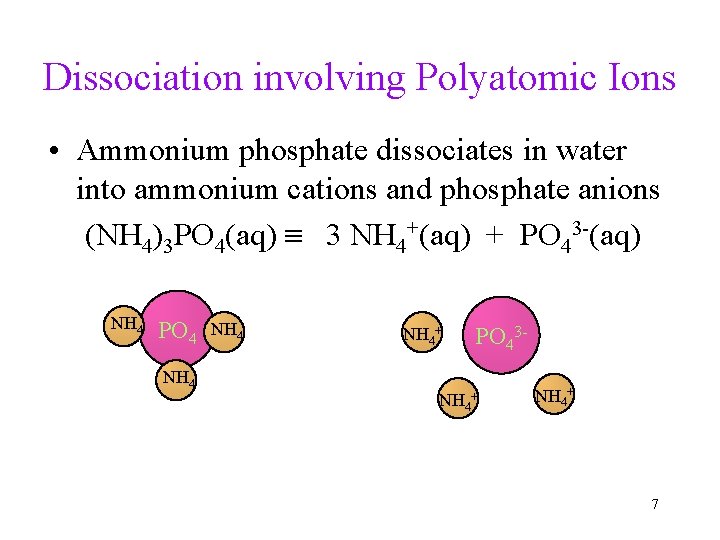
Dissociation involving Polyatomic Ions • Ammonium phosphate dissociates in water into ammonium cations and phosphate anions (NH 4)3 PO 4(aq) 3 NH 4+(aq) + PO 43 -(aq) NH 4 PO 4 NH 4+ PO 43 - NH 4+ 7

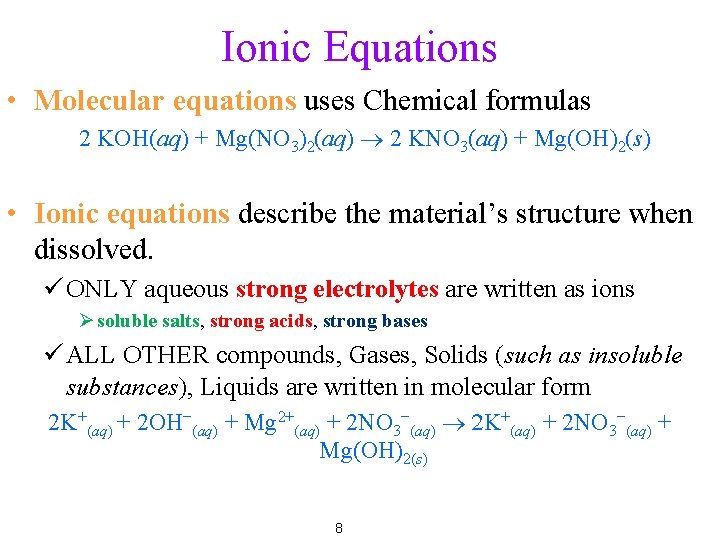
Ionic Equations • Molecular equations uses Chemical formulas 2 KOH(aq) + Mg(NO 3)2(aq) ® 2 KNO 3(aq) + Mg(OH)2(s) • Ionic equations describe the material’s structure when dissolved. ü ONLY aqueous strong electrolytes are written as ions Ø soluble salts, strong acids, strong bases ü ALL OTHER compounds, Gases, Solids (such as insoluble substances), Liquids are written in molecular form 2 K+(aq) + 2 OH−(aq) + Mg 2+(aq) + 2 NO 3−(aq) ® 2 K+(aq) + 2 NO 3−(aq) + Mg(OH)2(s) 8

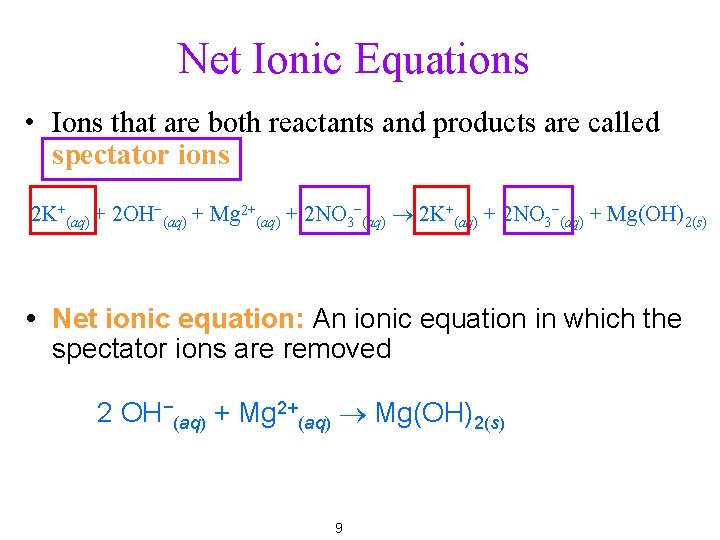
Net Ionic Equations • Ions that are both reactants and products are called spectator ions 2 K+(aq) + 2 OH−(aq) + Mg 2+(aq) + 2 NO 3−(aq) ® 2 K+(aq) + 2 NO 3−(aq) + Mg(OH)2(s) Net ionic equation: An ionic equation in which the spectator ions are removed 2 OH−(aq) + Mg 2+(aq) ® Mg(OH)2(s) 9

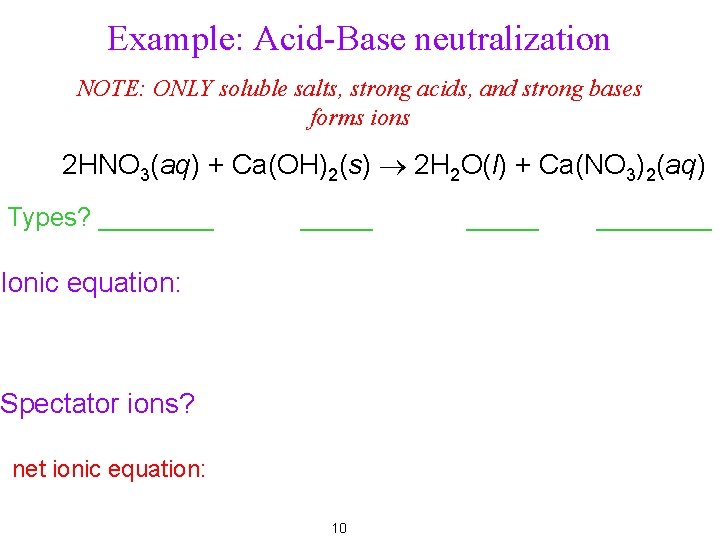
Example: Acid-Base neutralization NOTE: ONLY soluble salts, strong acids, and strong bases forms ions 2 HNO 3(aq) + Ca(OH)2(s) ® 2 H 2 O(l) + Ca(NO 3)2(aq) Types? _____ Ionic equation: Spectator ions? net ionic equation: 10 ________

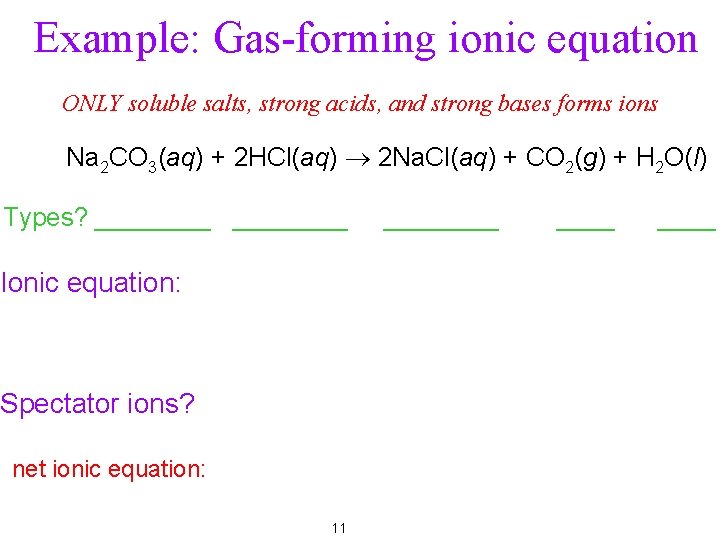
Example: Gas-forming ionic equation ONLY soluble salts, strong acids, and strong bases forms ions Na 2 CO 3(aq) + 2 HCl(aq) ® 2 Na. Cl(aq) + CO 2(g) + H 2 O(l) Types? ________ Ionic equation: Spectator ions? net ionic equation: 11 ____

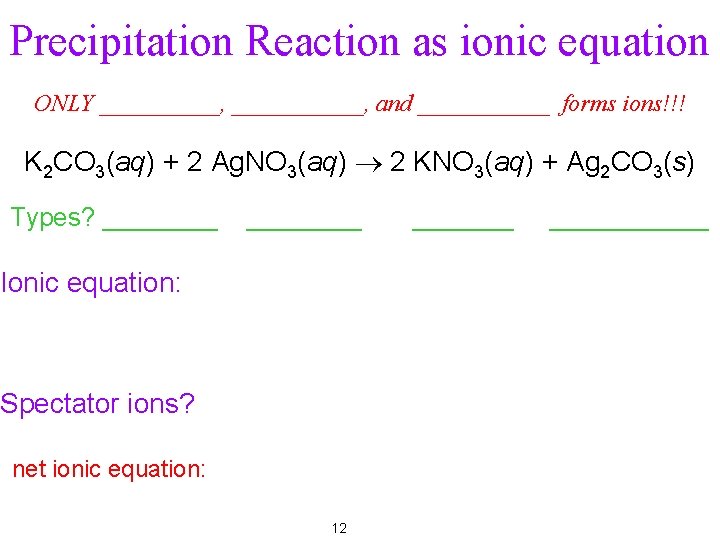
Precipitation Reaction as ionic equation ONLY _____, ______, and ______ forms ions!!! K 2 CO 3(aq) + 2 Ag. NO 3(aq) ® 2 KNO 3(aq) + Ag 2 CO 3(s) Types? ________ Ionic equation: Spectator ions? net ionic equation: 12 ___________

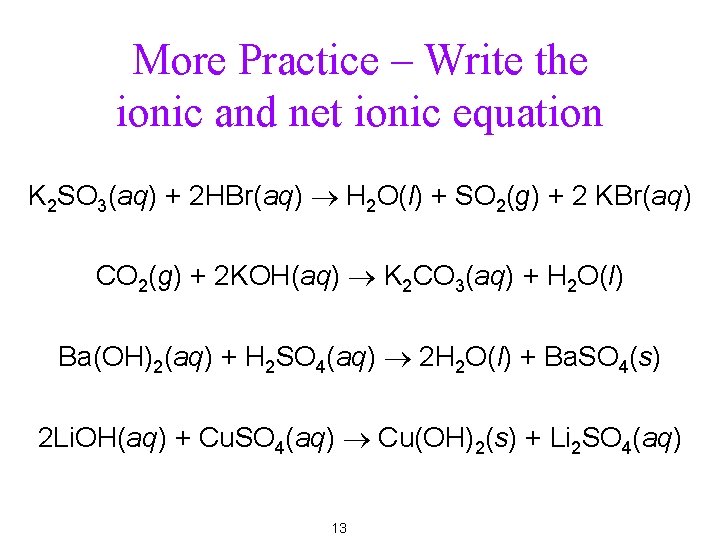
More Practice – Write the ionic and net ionic equation K 2 SO 3(aq) + 2 HBr(aq) ® H 2 O(l) + SO 2(g) + 2 KBr(aq) CO 2(g) + 2 KOH(aq) ® K 2 CO 3(aq) + H 2 O(l) Ba(OH)2(aq) + H 2 SO 4(aq) ® 2 H 2 O(l) + Ba. SO 4(s) 2 Li. OH(aq) + Cu. SO 4(aq) ® Cu(OH)2(s) + Li 2 SO 4(aq) 13

More Practice – Convert and Complete, then Write the ionic and net ionic equation chloric acid + ammonium sulfite silver acetate + iron(III) bromide zinc hydroxide + sulfuric acid lithium sulfite + manganese(II) chloride 14

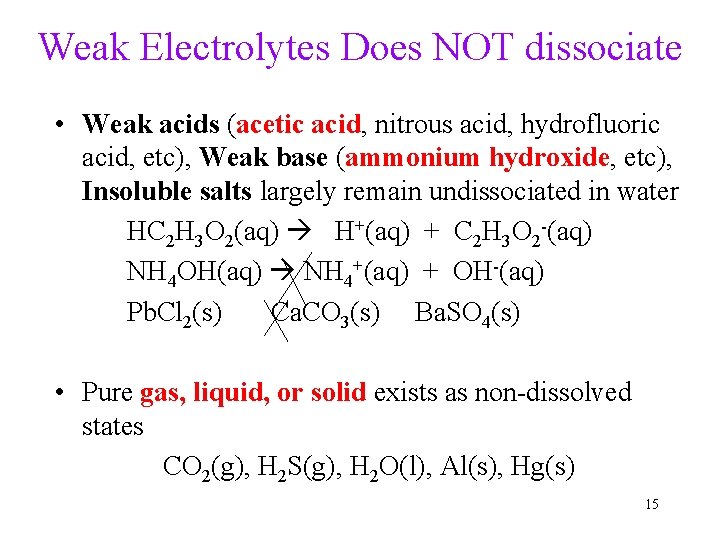
Weak Electrolytes Does NOT dissociate • Weak acids (acetic acid, nitrous acid, hydrofluoric acid, etc), Weak base (ammonium hydroxide, etc), Insoluble salts largely remain undissociated in water HC 2 H 3 O 2(aq) H+(aq) + C 2 H 3 O 2 -(aq) NH 4 OH(aq) NH 4+(aq) + OH-(aq) Pb. Cl 2(s) Ca. CO 3(s) Ba. SO 4(s) • Pure gas, liquid, or solid exists as non-dissolved states CO 2(g), H 2 S(g), H 2 O(l), Al(s), Hg(s) 15

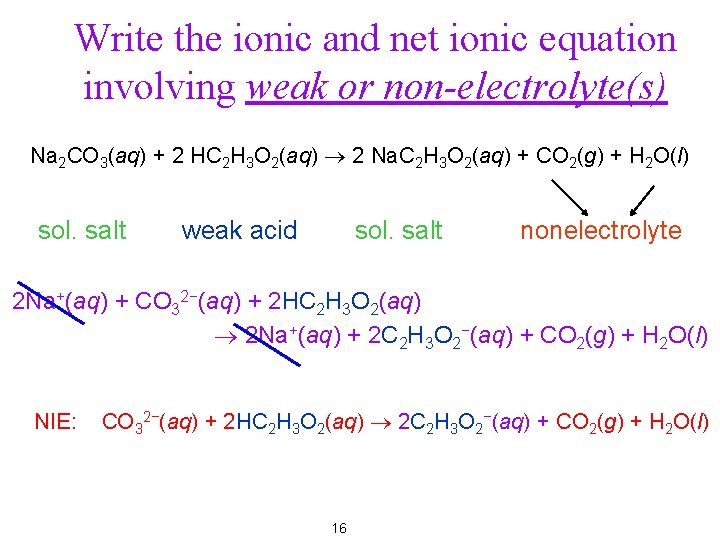
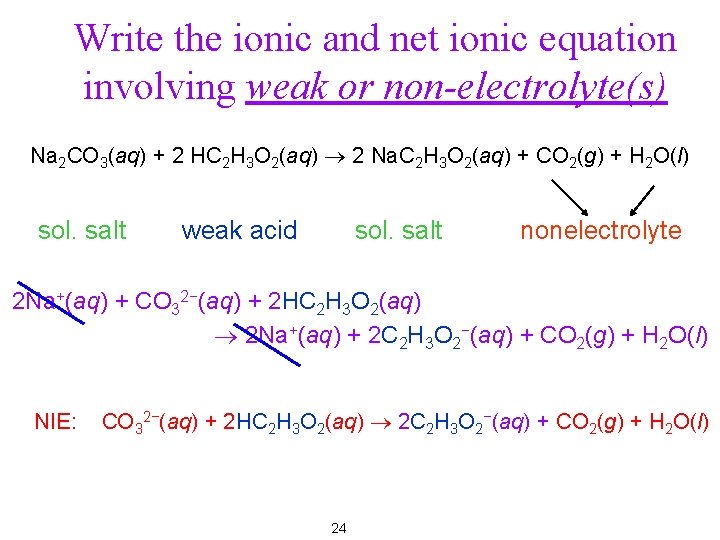
Write the ionic and net ionic equation involving weak or non-electrolyte(s) Na 2 CO 3(aq) + 2 HC 2 H 3 O 2(aq) ® 2 Na. C 2 H 3 O 2(aq) + CO 2(g) + H 2 O(l) sol. salt weak acid sol. salt nonelectrolyte 2 Na+(aq) + CO 32−(aq) + 2 HC 2 H 3 O 2(aq) ® 2 Na+(aq) + 2 C 2 H 3 O 2−(aq) + CO 2(g) + H 2 O(l) NIE: CO 32−(aq) + 2 HC 2 H 3 O 2(aq) ® 2 C 2 H 3 O 2−(aq) + CO 2(g) + H 2 O(l) 16

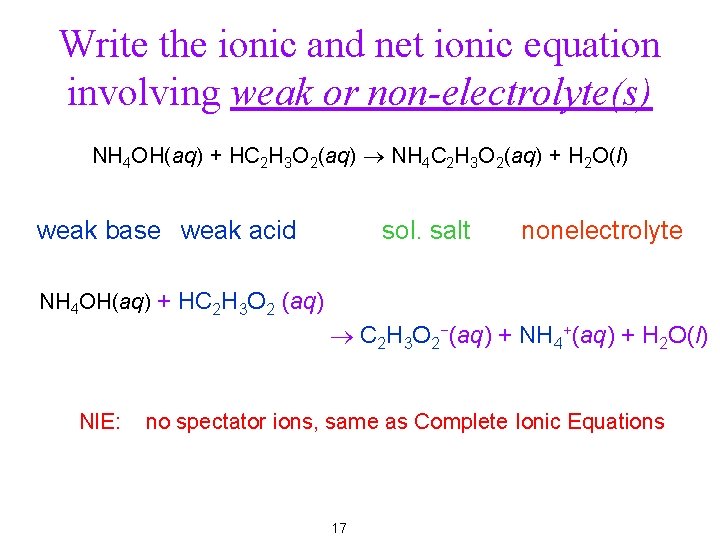
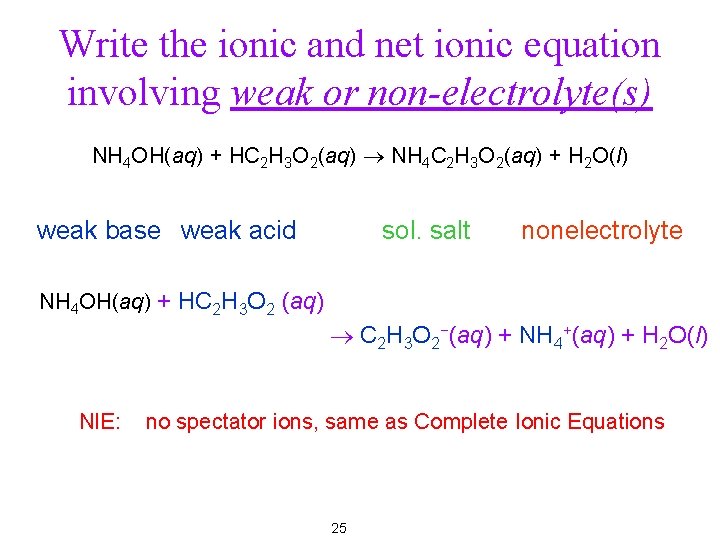
Write the ionic and net ionic equation involving weak or non-electrolyte(s) NH 4 OH(aq) + HC 2 H 3 O 2(aq) ® NH 4 C 2 H 3 O 2(aq) + H 2 O(l) weak base weak acid sol. salt nonelectrolyte NH 4 OH(aq) + HC 2 H 3 O 2 (aq) ® C 2 H 3 O 2−(aq) + NH 4+(aq) + H 2 O(l) NIE: no spectator ions, same as Complete Ionic Equations 17

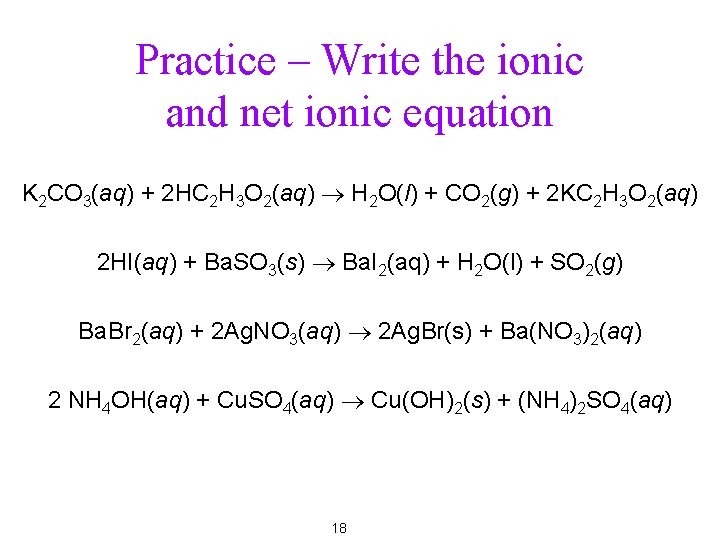
Practice – Write the ionic and net ionic equation K 2 CO 3(aq) + 2 HC 2 H 3 O 2(aq) ® H 2 O(l) + CO 2(g) + 2 KC 2 H 3 O 2(aq) 2 HI(aq) + Ba. SO 3(s) ® Ba. I 2(aq) + H 2 O(l) + SO 2(g) Ba. Br 2(aq) + 2 Ag. NO 3(aq) ® 2 Ag. Br(s) + Ba(NO 3)2(aq) 2 NH 4 OH(aq) + Cu. SO 4(aq) ® Cu(OH)2(s) + (NH 4)2 SO 4(aq) 18

More Practice – Convert and Complete, then Write the ionic and net ionic equation sulfuric acid + ammonium hydroxide aluminum acetate + ammonium hydroxide iron(II) sulfite + acetic acid scandium(III) hydroxide + acetic acid 19

More Practice: Write and Balance the Ionic and Net Ionic Equation in aqueous solution from Word Equation • hydrobromic acid + zinc metal zinc bromide + hydrogen gas • chlorine gas + water hydrochloric acid + hypochlorous acid (as a weak acid) • copper + iron(III) nitrate iron(II) nitrate + copper(II) nitrate • acetic acid + magnesium metal magnesium acetate + hydrogen gas 20

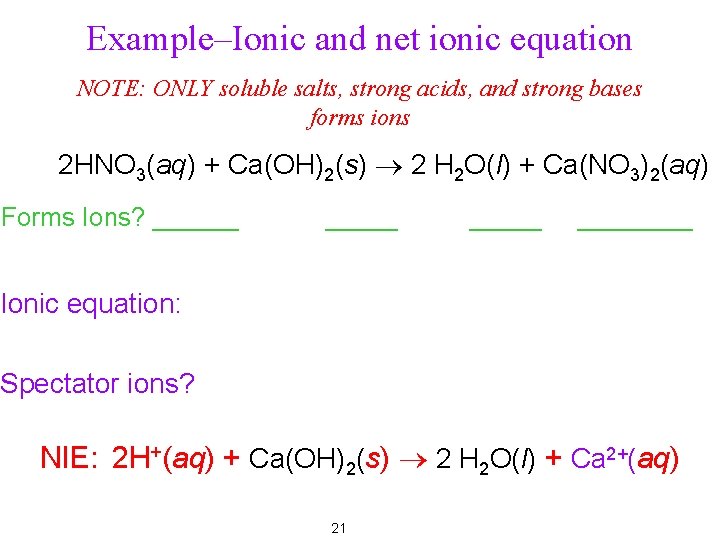
Example–Ionic and net ionic equation NOTE: ONLY soluble salts, strong acids, and strong bases forms ions 2 HNO 3(aq) + Ca(OH)2(s) ® 2 H 2 O(l) + Ca(NO 3)2(aq) Forms Ions? ______ ____ Ionic equation: Spectator ions? NIE: 2 H+(aq) + Ca(OH)2(s) ® 2 H 2 O(l) + Ca 2+(aq) 21

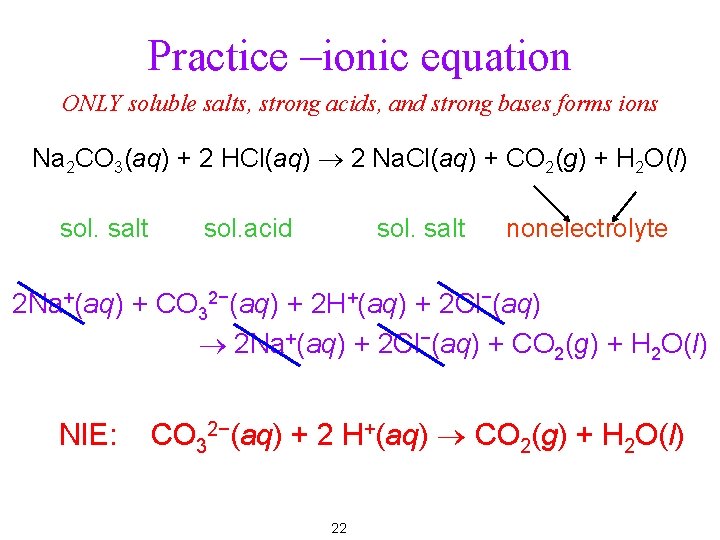
Practice –ionic equation ONLY soluble salts, strong acids, and strong bases forms ions Na 2 CO 3(aq) + 2 HCl(aq) ® 2 Na. Cl(aq) + CO 2(g) + H 2 O(l) sol. salt sol. acid sol. salt nonelectrolyte 2 Na+(aq) + CO 32−(aq) + 2 H+(aq) + 2 Cl−(aq) ® 2 Na+(aq) + 2 Cl−(aq) + CO 2(g) + H 2 O(l) NIE: CO 32−(aq) + 2 H+(aq) ® CO 2(g) + H 2 O(l) 22

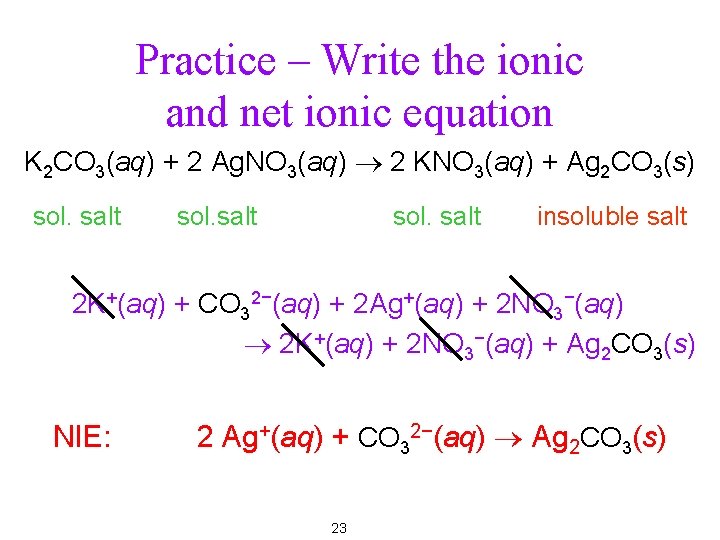
Practice – Write the ionic and net ionic equation K 2 CO 3(aq) + 2 Ag. NO 3(aq) ® 2 KNO 3(aq) + Ag 2 CO 3(s) sol. salt insoluble salt 2 K+(aq) + CO 32−(aq) + 2 Ag+(aq) + 2 NO 3−(aq) ® 2 K+(aq) + 2 NO 3−(aq) + Ag 2 CO 3(s) NIE: 2 Ag+(aq) + CO 32−(aq) ® Ag 2 CO 3(s) 23

Write the ionic and net ionic equation involving weak or non-electrolyte(s) Na 2 CO 3(aq) + 2 HC 2 H 3 O 2(aq) ® 2 Na. C 2 H 3 O 2(aq) + CO 2(g) + H 2 O(l) sol. salt weak acid sol. salt nonelectrolyte 2 Na+(aq) + CO 32−(aq) + 2 HC 2 H 3 O 2(aq) ® 2 Na+(aq) + 2 C 2 H 3 O 2−(aq) + CO 2(g) + H 2 O(l) NIE: CO 32−(aq) + 2 HC 2 H 3 O 2(aq) ® 2 C 2 H 3 O 2−(aq) + CO 2(g) + H 2 O(l) 24

Write the ionic and net ionic equation involving weak or non-electrolyte(s) NH 4 OH(aq) + HC 2 H 3 O 2(aq) ® NH 4 C 2 H 3 O 2(aq) + H 2 O(l) weak base weak acid sol. salt nonelectrolyte NH 4 OH(aq) + HC 2 H 3 O 2 (aq) ® C 2 H 3 O 2−(aq) + NH 4+(aq) + H 2 O(l) NIE: no spectator ions, same as Complete Ionic Equations 25