Net Ionic Equations Net Ionic Equation defined Shows






- Slides: 11

Net Ionic Equations

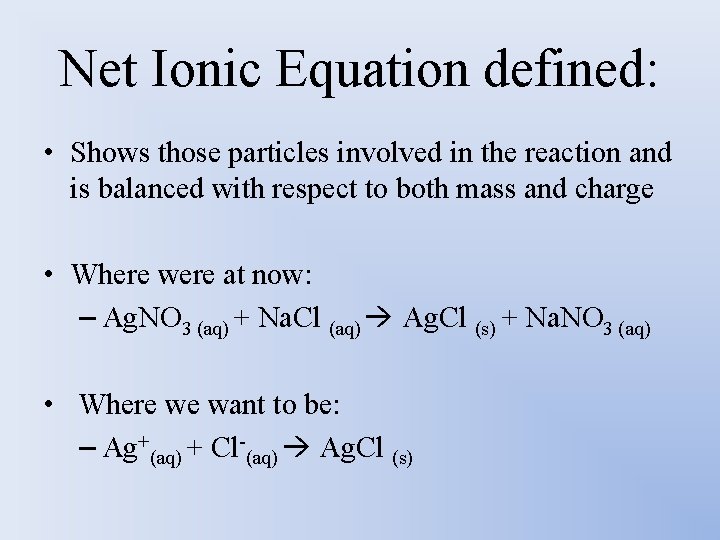
Net Ionic Equation defined: • Shows those particles involved in the reaction and is balanced with respect to both mass and charge • Where were at now: – Ag. NO 3 (aq) + Na. Cl (aq) Ag. Cl (s) + Na. NO 3 (aq) • Where we want to be: – Ag+(aq) + Cl-(aq) Ag. Cl (s)

How do we get there? Write a Complete Ionic Equation: - An equation that shows dissolved ionic compounds - Step 1: Write the complete ionic equation - Ag. NO 3 (aq) + Na. Cl (aq) Ag. Cl (s) + Na. NO 3 (aq) - Step 2: Separate the aqueous ionic substances (ONLY) - Ag+(aq) + NO 3 -(aq) + Na+(aq) + Cl-(aq) Ag. Cl (s) + Na+(aq) + NO 3 -(aq)

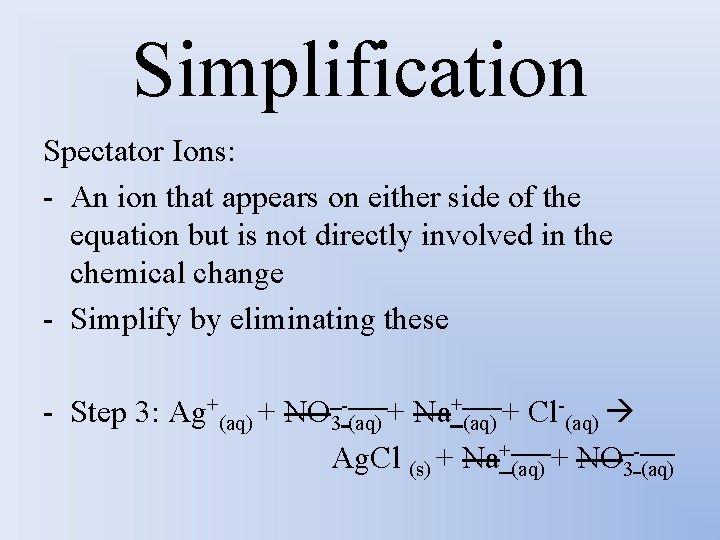
Simplification Spectator Ions: - An ion that appears on either side of the equation but is not directly involved in the chemical change - Simplify by eliminating these - Step 3: Ag+(aq) + NO 3 -(aq) + Na+(aq) + Cl-(aq) Ag. Cl (s) + Na+(aq) + NO 3 -(aq)

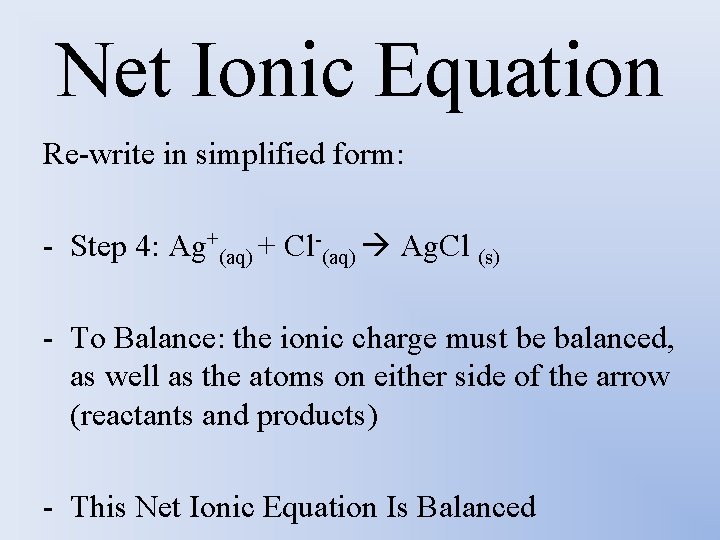
Net Ionic Equation Re-write in simplified form: - Step 4: Ag+(aq) + Cl-(aq) Ag. Cl (s) - To Balance: the ionic charge must be balanced, as well as the atoms on either side of the arrow (reactants and products) - This Net Ionic Equation Is Balanced

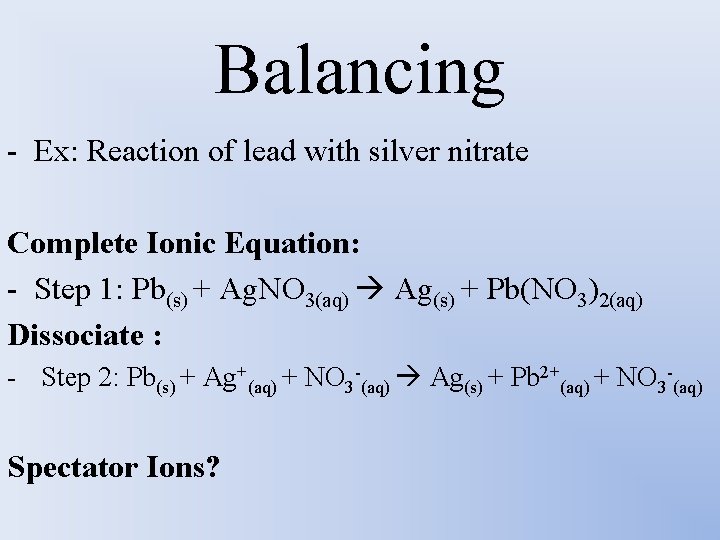
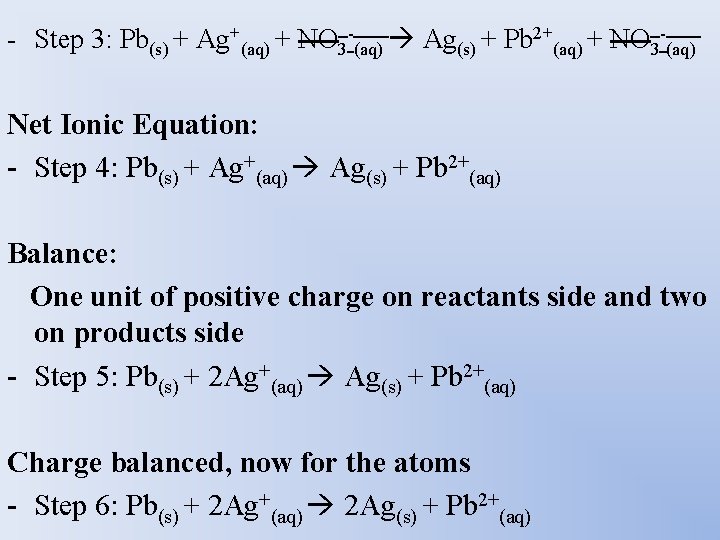
Balancing - Ex: Reaction of lead with silver nitrate Complete Ionic Equation: - Step 1: Pb(s) + Ag. NO 3(aq) Ag(s) + Pb(NO 3)2(aq) Dissociate : - Step 2: Pb(s) + Ag+(aq) + NO 3 -(aq) Ag(s) + Pb 2+(aq) + NO 3 -(aq) Spectator Ions?

- Step 3: Pb(s) + Ag+(aq) + NO 3 -(aq) Ag(s) + Pb 2+(aq) + NO 3 -(aq) Net Ionic Equation: - Step 4: Pb(s) + Ag+(aq) Ag(s) + Pb 2+(aq) Balance: One unit of positive charge on reactants side and two on products side - Step 5: Pb(s) + 2 Ag+(aq) Ag(s) + Pb 2+(aq) Charge balanced, now for the atoms - Step 6: Pb(s) + 2 Ag+(aq) 2 Ag(s) + Pb 2+(aq)

Practice • Determine type of reaction, predict products, write the complete ionic equation, eliminate spectator ions, write the net ionic equation, balance charges and atoms: • Na. OH(aq) + HCl(aq) • Zn(s) + Cu. SO 4(aq)

Video • https: //www. youtube. com/watch? v=vbx 5 nf. Ai. Bl. M • Na. OH(aq) + HCl(aq) Na. Cl(aq) + H 2 O(aq) • Zn(s) + Cu. SO 4(aq) Zn. SO 4(aq) + Cu(s) • We will only write net Ionic Equations for single and double replacement reactions • Synthesis, decomposition, and combustion are typically not performed in solution (no water therefore no ions)

References • • Wilbraham, A. Staley, D. Matta, M. Waterman, E. (2008). Prentice Hall chemistry. Boston, MA: Pearson Prentice Hall. https: //www. youtube. com/watch? v=vbx 5 nf. Ai. Bl. M

Thursday April 2, 15 Name: Ashley Vautour Subject Area: Chemistry Grade: 11 1 Lesson Title: Net Ionic Equations Time Required: 60 Minutes Lesson Plan Curriculum Outcomes: • Explain solubility, using the concept of equilibrium. (323 -4) • Identify different types of solutions (acids, bases, neutral, ionic and molecular) and their properties • Identify dissociation and ionization equations. • Conduct a precipitate lab and include recording, observing and collecting data, writing ionic and net ionic equations , and analyzing results. Learning Objectives: • Foster learning on determining states of matter for compounds • Learn how to write and balance net ionic equations • Practice for upcoming midterms Materials and Resources: • Slide Show • Youtube video Technology Used: • Lap top • Projector Learning Cycle: • Welcome class. Tell them we will be building off previous lesson on solubility to be able to write and balance net ionic equations • Show students where we are now and where we want to be • Tell them what a complete ionic equation is and how to get to a net ionic equation – Dissociate – Eliminate spectator ions – Re-write equation – Balance charges and atoms • Example • Practice • Video • Practice Sheets Accommodations: • Understand how to re-explain something in another way • Notes will be posted on ed-line so students can have a hard copy of the lesson Evaluation: • Continue to ask students to hand in their labs that were due • Practice on problem sheets for the upcoming midterm • Answer questions students had from the test on Wednesday