Colligative Properties Changes in colligative properties depend only









- Slides: 23

Colligative Properties • Changes in colligative properties depend only on the number of solute particles present, not on the identity of the solute particles. • Among colligative properties are – Vapor pressure lowering – Boiling point elevation – Melting point depression – Osmotic pressure © 2009, Prentice-Hall, Inc.

Colligative Properties: • Vapor Pressure • Freezing Point Depression • Boiling Point Elevation • Osmotic Pressure

Vapor Pressure Because of solutesolvent intermolecular attraction, higher concentrations of nonvolatile solutes make it harder for solvent to escape to the vapor phase. © 2009, Prentice-Hall, Inc.

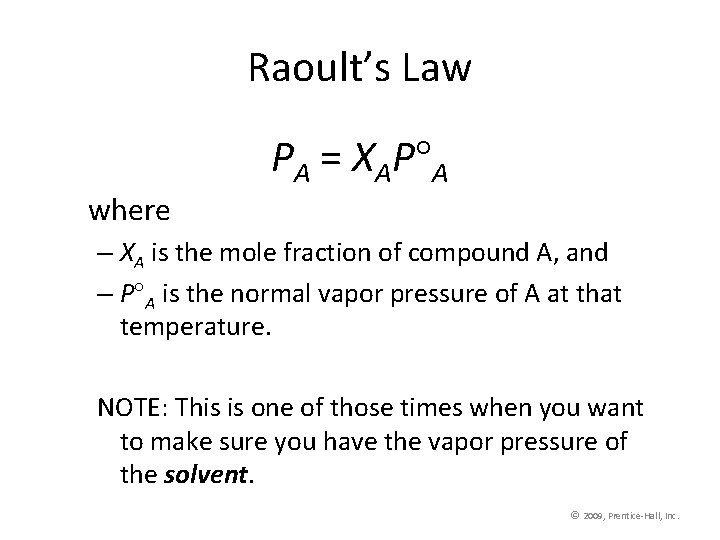
Raoult’s Law PA = XAP A where – XA is the mole fraction of compound A, and – P A is the normal vapor pressure of A at that temperature. NOTE: This is one of those times when you want to make sure you have the vapor pressure of the solvent. © 2009, Prentice-Hall, Inc.

Vapor Pressure: • Raoult’s Law: Psolution = Xsolvent. Po. H 2 O 12 g Sucrose (C 12 H 22 O 11)is dissolved in 250. 0 g water at 90 °C. What is the vapor pressure of water over this solution? (Po. H 2 O = 525. 8 mm. Hg- from data table) • Ans. 524 mm. Hg

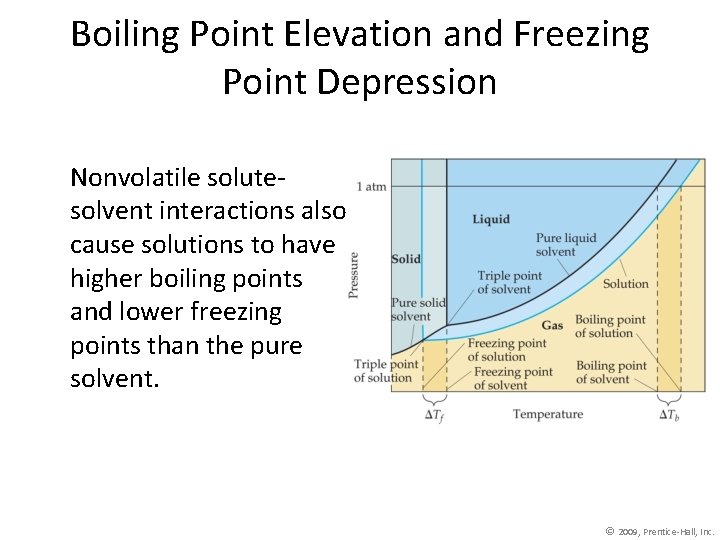
Boiling Point Elevation and Freezing Point Depression Nonvolatile solutesolvent interactions also cause solutions to have higher boiling points and lower freezing points than the pure solvent. © 2009, Prentice-Hall, Inc.

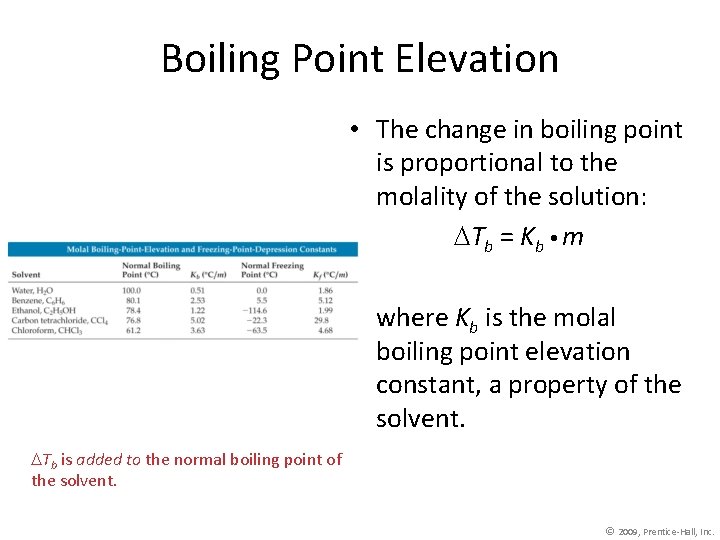
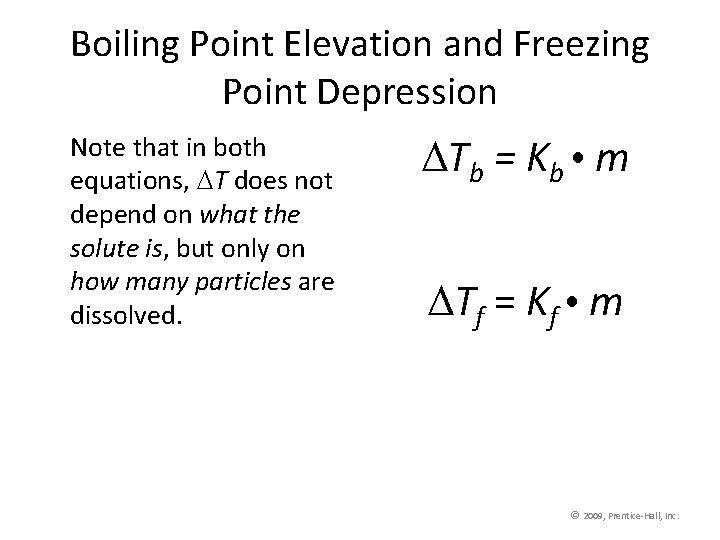
Boiling Point Elevation • The change in boiling point is proportional to the molality of the solution: Tb = Kb m where Kb is the molal boiling point elevation constant, a property of the solvent. Tb is added to the normal boiling point of the solvent. © 2009, Prentice-Hall, Inc.

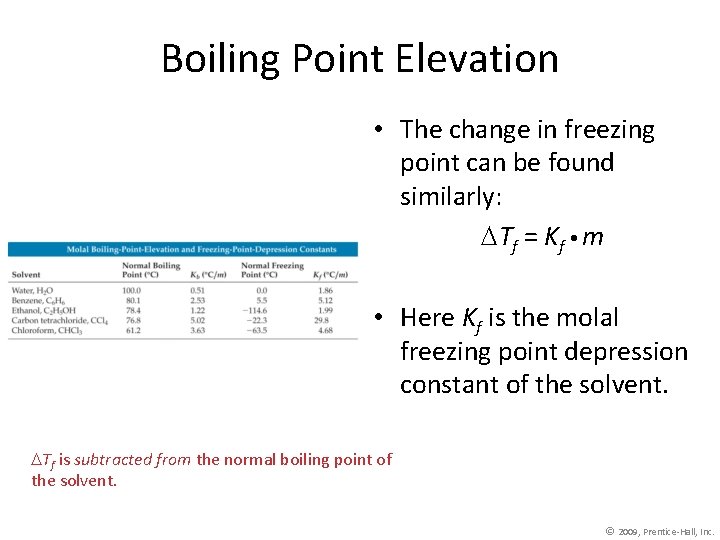
Boiling Point Elevation • The change in freezing point can be found similarly: Tf = Kf m • Here Kf is the molal freezing point depression constant of the solvent. Tf is subtracted from the normal boiling point of the solvent. © 2009, Prentice-Hall, Inc.

Boiling Point Elevation and Freezing Point Depression Note that in both equations, T does not depend on what the solute is, but only on how many particles are dissolved. Tb = Kb m Tf = Kf m © 2009, Prentice-Hall, Inc.

Freezing Point Depression: • Freezing Point Depression: ΔTfp = Kfpmsolution • A solution is prepared by adding 0. 50 g of caffeine (C 8 H 10 O 2 N 4) to 100 g of benzene (C 6 H 6). • Calculate the freezing point of this solution. • The freezing point of pure benzene is 5. 50°C • Kfp for benzene = 5. 23 °C/m) Ans. : 0. 132 ° C Tfreezing= 5. 37 C

Boiling Point Elevation: • Boiling point elevation: ΔTbp = Kbpmsolute • A glycerol solution (C 3 H 8 O 3) in water is prepared by dissolving glycerol is 500 g water. The boiling point of the solution is 100. 42°C at 760 mm. Hg. What mass of glycerol was dissolved to make this solution? • Kbp = 0. 5121 °C/m Ans: 38 g glycerol

Colligative Properties of Electrolytes Since these properties depend on the number of particles dissolved, solutions of electrolytes (which dissociate in solution) should show greater changes than those of nonelectrolytes. © 2009, Prentice-Hall, Inc.

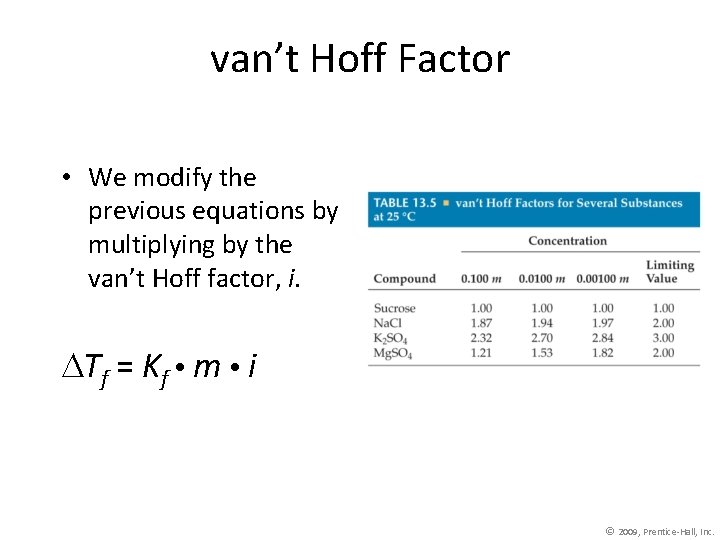
van’t Hoff Factor • We modify the previous equations by multiplying by the van’t Hoff factor, i. Tf = Kf m i © 2009, Prentice-Hall, Inc.

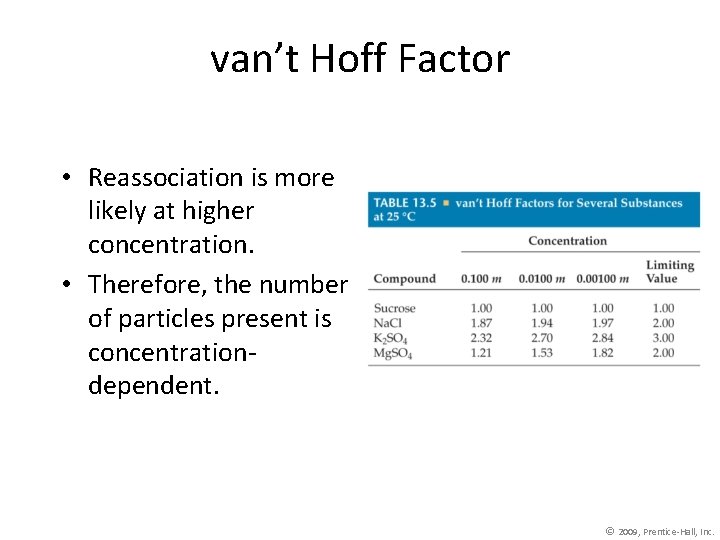
van’t Hoff Factor • Reassociation is more likely at higher concentration. • Therefore, the number of particles present is concentrationdependent. © 2009, Prentice-Hall, Inc.

Role of Electrolytes on Colligative Properties: • Van’t Hoff Factor: ΔTfp = Kfpmsolutei • A 0. 0711 m aqueous solution of Sodium sulfate freezes at -0. 32 C. What is the actual value (i) of the van’t Hoff factor? Kfp = 1. 86 °C/m Ans: i = 2. 42

Rank for following: • Increasing boiling point (ΔTb) – 0. 25 m C 6 H 12 O 11 – 0. 40 m Na. Cl – 0. 15 m Mg. Cl 2 C 6 H 12 O 11 Mg. Cl 2 Na. Cl

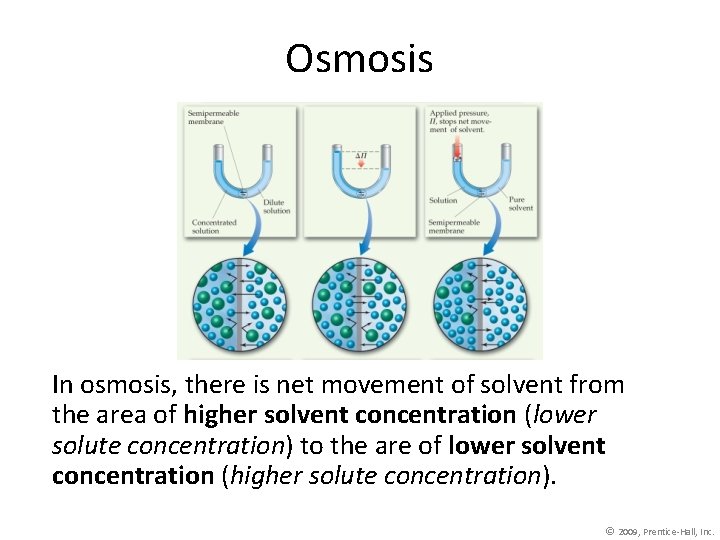
Osmosis In osmosis, there is net movement of solvent from the area of higher solvent concentration (lower solute concentration) to the are of lower solvent concentration (higher solute concentration). © 2009, Prentice-Hall, Inc.

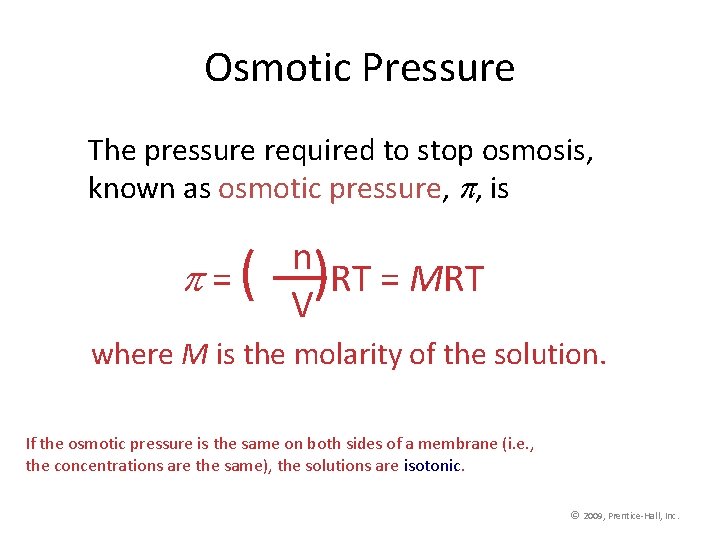
Osmotic Pressure The pressure required to stop osmosis, known as osmotic pressure, , is =( n ) RT = MRT V where M is the molarity of the solution. If the osmotic pressure is the same on both sides of a membrane (i. e. , the concentrations are the same), the solutions are isotonic. © 2009, Prentice-Hall, Inc.

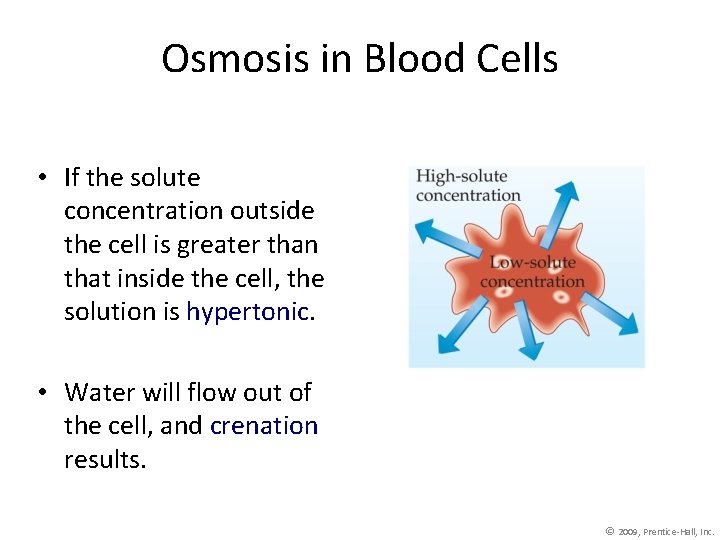
Osmosis in Blood Cells • If the solute concentration outside the cell is greater than that inside the cell, the solution is hypertonic. • Water will flow out of the cell, and crenation results. © 2009, Prentice-Hall, Inc.

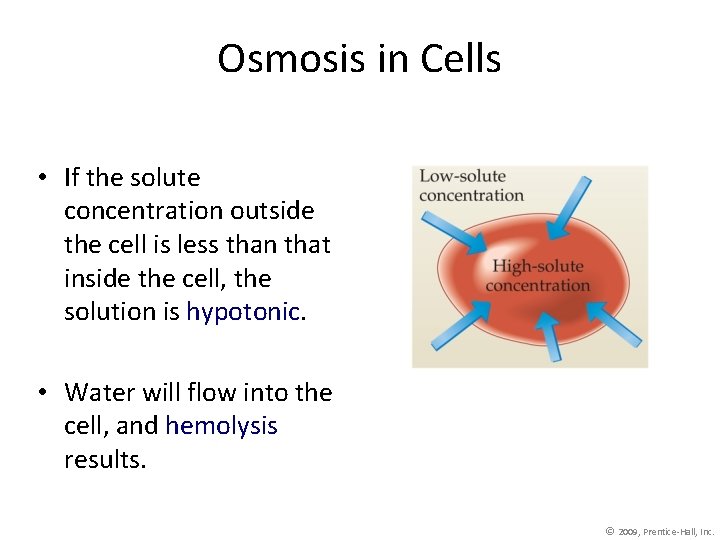
Osmosis in Cells • If the solute concentration outside the cell is less than that inside the cell, the solution is hypotonic. • Water will flow into the cell, and hemolysis results. © 2009, Prentice-Hall, Inc.

Osmotic Pressure • Osmotic Pressure: π= MRT • What is the osmotic pressure of a 0. 1 M solution of sucrose at 25 C? (remember: R=. 08206 L-atm/mol-K) Ans: 2. 45 atm

Another Osmotic pressure problem. • 100. mg of a protein are dissolved in enough water to make 10. 0 m. L of a solution. • If this solution has an osmotic pressure of 13. 3 mm. Hg at 25°C, what is the molar mass of the protein? Ans: 1. 4 x 104 g/mole

VP problem • How would you prepare 1. 00 L of an aqueous oxalic acid, H 2 C 2 O 4, solution (d= 1. 05 g/m. L) with a vapor pressure of 21. 97 mm. Hg at 24°C (H 20 vp = 22. 38 mm. Hg)? Ans. Dissolve 89 g in 1. 0 L solution