Colligative Properties Colligative Properties Why does salt melt




![A. Equal salt content in and out of cell B. [Salt]cell > [Salt]fluid C. A. Equal salt content in and out of cell B. [Salt]cell > [Salt]fluid C.](https://slidetodoc.com/presentation_image/20aadbd01825883ba1e758df3a69971f/image-19.jpg)
- Slides: 20

Colligative Properties

Colligative Properties • Why does salt melt ice? • Why does antifreeze prevent freezing in car radiators? • These are examples of the colligative properties of solutions. • Colligative properties of solutions are those properties that result from interactions between solute and solvent particles • They depend only on the ratio of solute to solvent molecules

Expressing Solute Concentration • Colligative properties are physical properties of solutions that vary depending on ratio of solute to solvent • There are 4 methods for expressing these ratios: �Molarity �Percent mass �Mole fraction �Molality

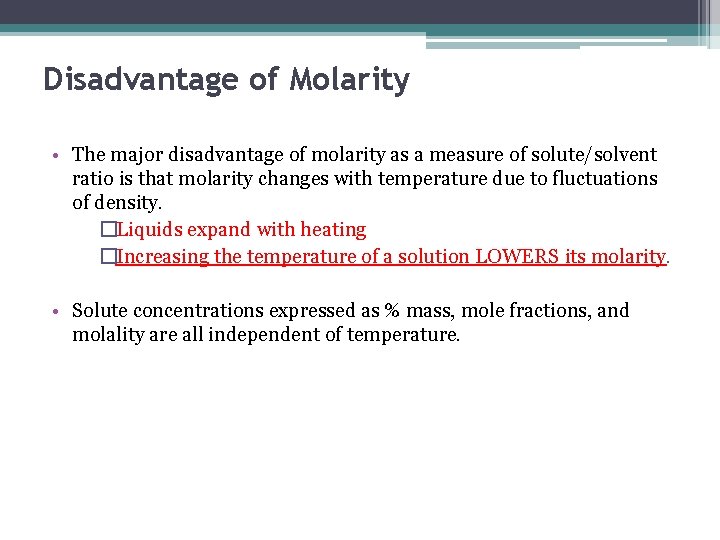
Disadvantage of Molarity • The major disadvantage of molarity as a measure of solute/solvent ratio is that molarity changes with temperature due to fluctuations of density. �Liquids expand with heating �Increasing the temperature of a solution LOWERS its molarity. • Solute concentrations expressed as % mass, mole fractions, and molality are all independent of temperature.

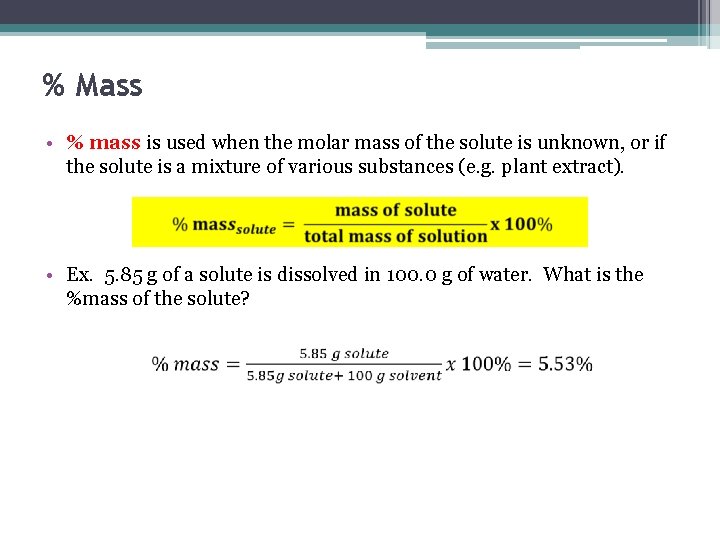
% Mass • % mass is used when the molar mass of the solute is unknown, or if the solute is a mixture of various substances (e. g. plant extract). • Ex. 5. 85 g of a solute is dissolved in 100. 0 g of water. What is the %mass of the solute?

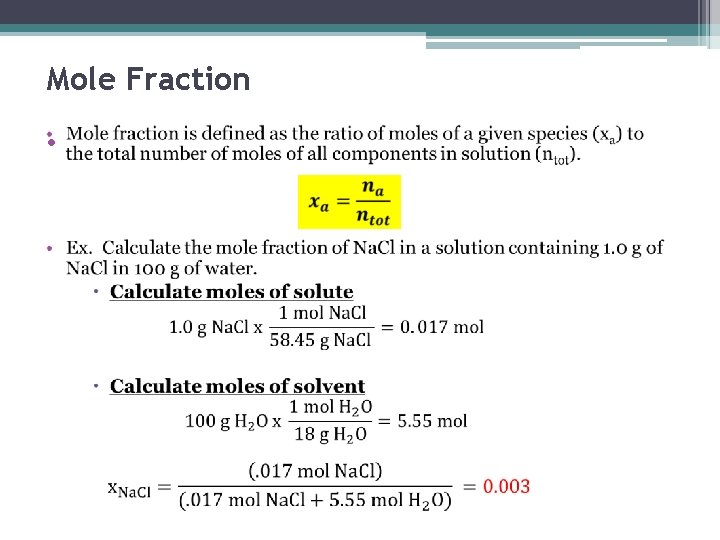
Mole Fraction •

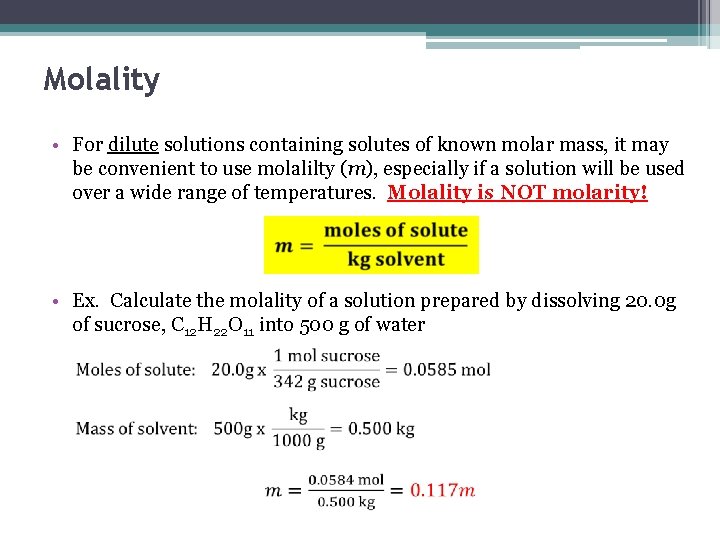
Molality • For dilute solutions containing solutes of known molar mass, it may be convenient to use molalilty (m), especially if a solution will be used over a wide range of temperatures. Molality is NOT molarity! • Ex. Calculate the molality of a solution prepared by dissolving 20. 0 g of sucrose, C 12 H 22 O 11 into 500 g of water

Examples • If 2. 8 g of lithium sulfide is dissolved in 50 g of water, determine the following: ▫ Mole fraction of the solute? ▫ Mass % of the solute? ▫ Molality of the solution? • An aqueous solution is 4. 2% ammonium chloride by mass. Calculate a mole fraction and a molality of a solution? ▫ Hint: For such a problem, it helps to make an assumption of the total mass of the solution. The most logical assumption is 100 g (4. 2 g NH 4 Cl, 95. 8 g H 2 O)

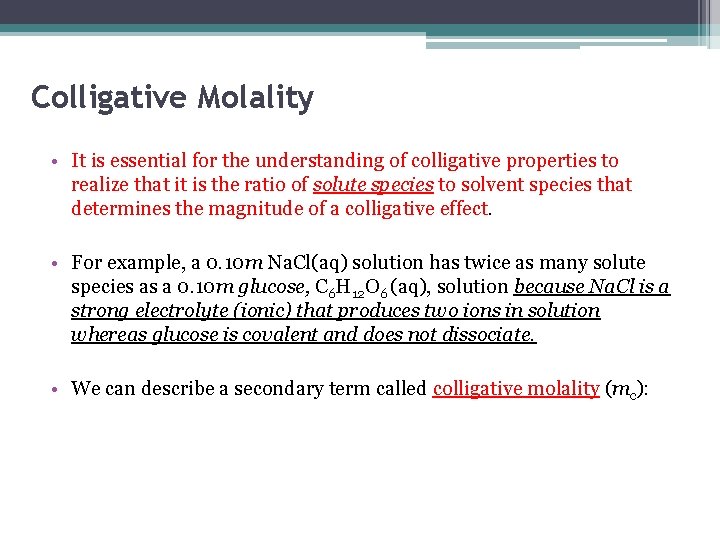
Colligative Molality • It is essential for the understanding of colligative properties to realize that it is the ratio of solute species to solvent species that determines the magnitude of a colligative effect. • For example, a 0. 10 m Na. Cl(aq) solution has twice as many solute species as a 0. 10 m glucose, C 6 H 12 O 6 (aq), solution because Na. Cl is a strong electrolyte (ionic) that produces two ions in solution whereas glucose is covalent and does not dissociate. • We can describe a secondary term called colligative molality (mc):

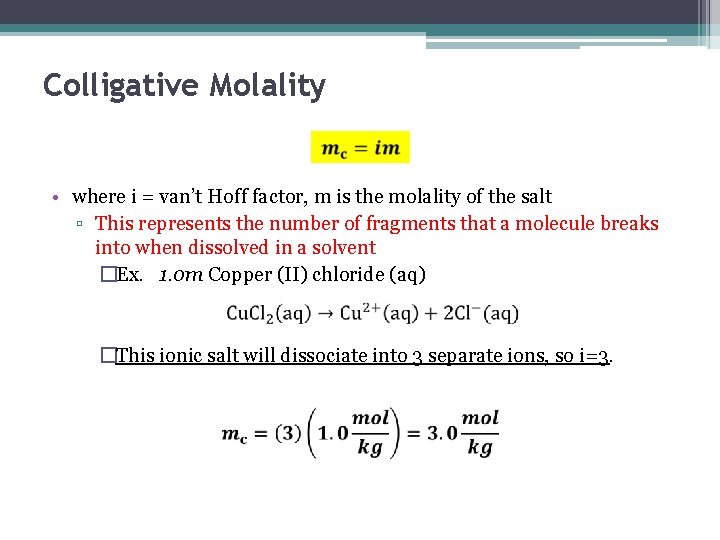
Colligative Molality • where i = van’t Hoff factor, m is the molality of the salt ▫ This represents the number of fragments that a molecule breaks into when dissolved in a solvent �Ex. 1. 0 m Copper (II) chloride (aq) �This ionic salt will dissociate into 3 separate ions, so i=3.

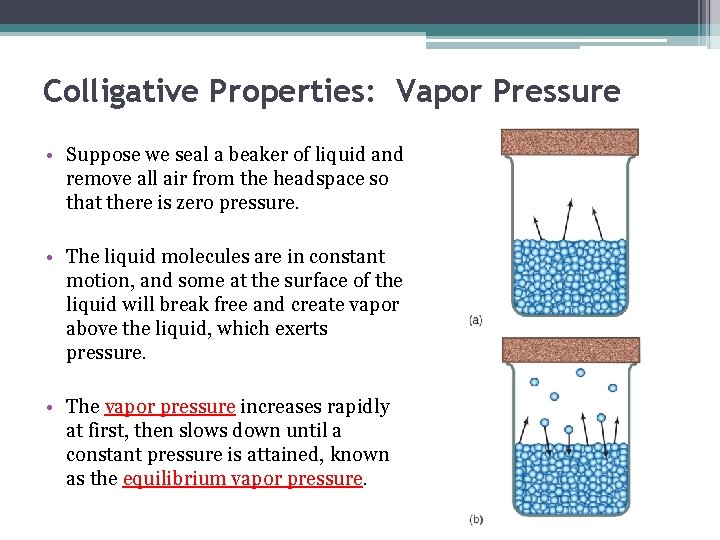
Colligative Properties: Vapor Pressure • Suppose we seal a beaker of liquid and remove all air from the headspace so that there is zero pressure. • The liquid molecules are in constant motion, and some at the surface of the liquid will break free and create vapor above the liquid, which exerts pressure. • The vapor pressure increases rapidly at first, then slows down until a constant pressure is attained, known as the equilibrium vapor pressure.

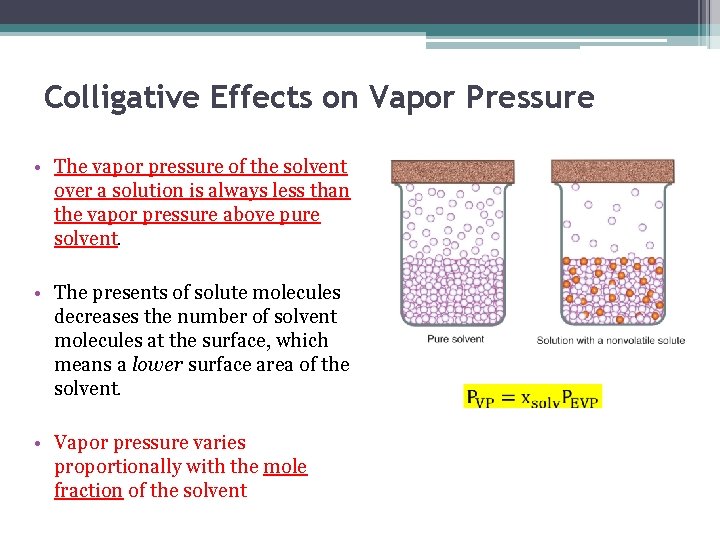
Colligative Effects on Vapor Pressure • The vapor pressure of the solvent over a solution is always less than the vapor pressure above pure solvent. • The presents of solute molecules decreases the number of solvent molecules at the surface, which means a lower surface area of the solvent. • Vapor pressure varies proportionally with the mole fraction of the solvent

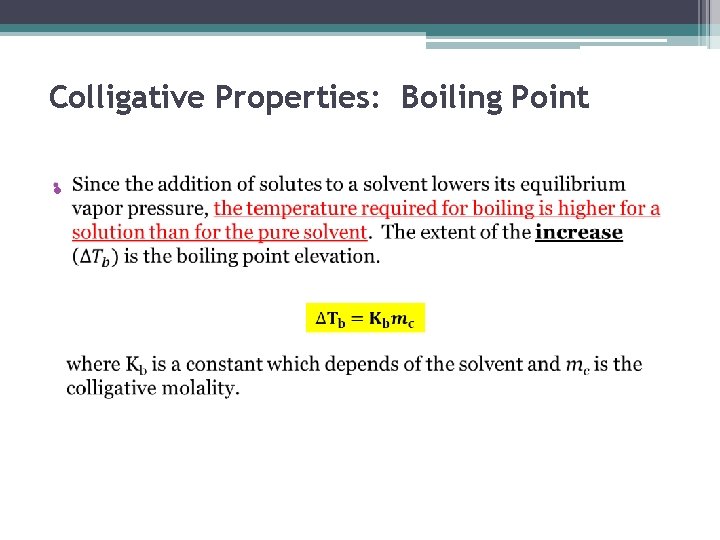
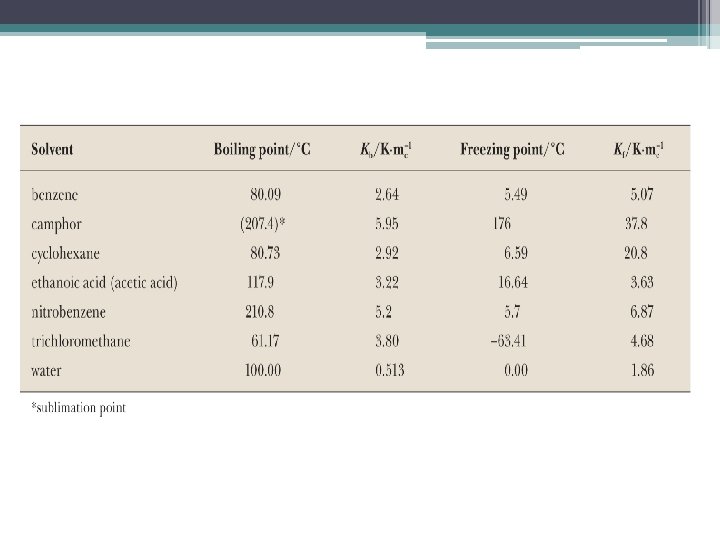
Colligative Properties: Boiling Point •


Colligative Properties: Freezing Point •

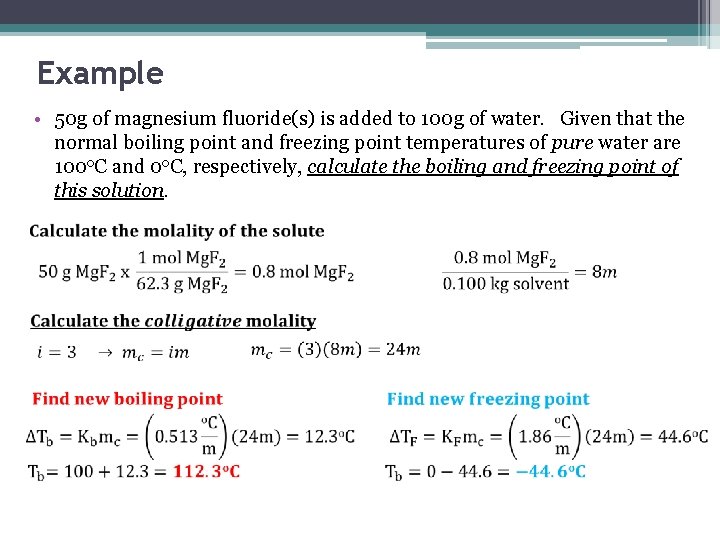
Example • 50 g of magnesium fluoride(s) is added to 100 g of water. Given that the normal boiling point and freezing point temperatures of pure water are 100 o. C and 0 o. C, respectively, calculate the boiling and freezing point of this solution.

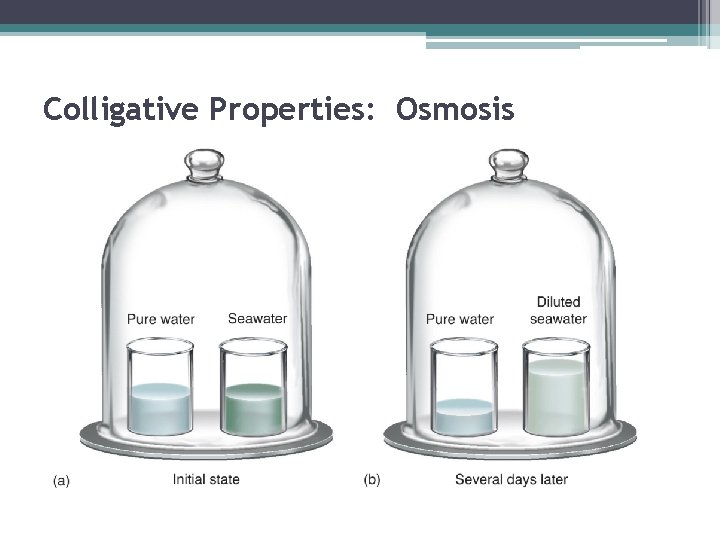
Colligative Properties: Osmosis

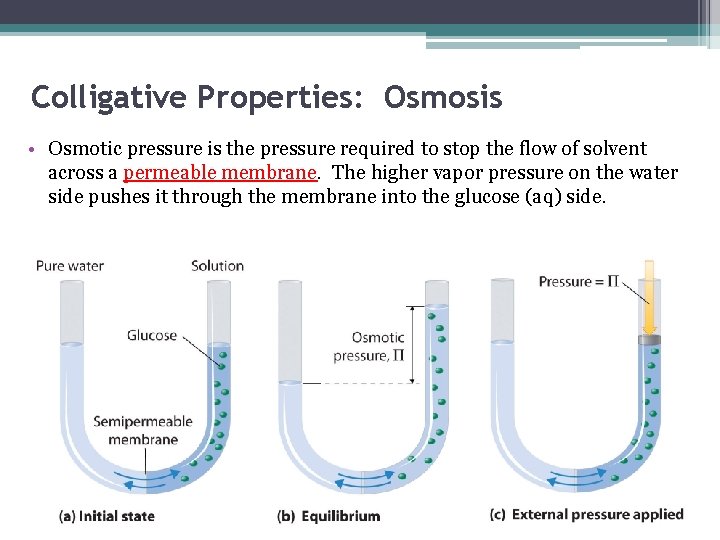
Colligative Properties: Osmosis • Osmotic pressure is the pressure required to stop the flow of solvent across a permeable membrane. The higher vapor pressure on the water side pushes it through the membrane into the glucose (aq) side.
![A Equal salt content in and out of cell B Saltcell Saltfluid C A. Equal salt content in and out of cell B. [Salt]cell > [Salt]fluid C.](https://slidetodoc.com/presentation_image/20aadbd01825883ba1e758df3a69971f/image-19.jpg)
A. Equal salt content in and out of cell B. [Salt]cell > [Salt]fluid C. [Salt]cell < [Salt]fluid Water flows toward the domain of higher solute concentration.

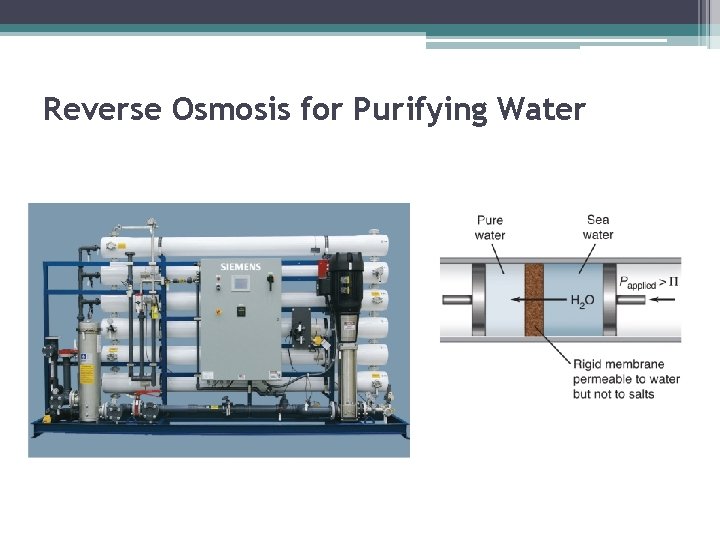
Reverse Osmosis for Purifying Water