Classifying Matter C 3 Matter is Anything that



- Slides: 10

Classifying Matter (C 3) Matter is Anything that has a mass and takes up space Anything else is Energy (well…. ) Matter is Made of tiny particles (atoms these can combine to make molecules) Can exist in 3 physical states (solid; liquid; gas) Can undergo physical or chemical change

States of Matter o One way to classify matter is as a: n SOLID n LIQUID n GAS Eureka! Episode 16 - Molecules in Solids https: //www. youtube. com/watch? v=5 jp. A 1 H 1 ad. II Eureka! Episode 17 - Molecules in Liquid http: //www. youtube. com/watch? v=w_m. Ovnv 1 ns 4 Eureka! Episode 18 - Evaporation and Condensation http: //www. youtube. com/watch? v=M 9 gs. OEF 71 b. Y The behaviour of the 3 states of matter can be explained using the: Particle Theory of Matter

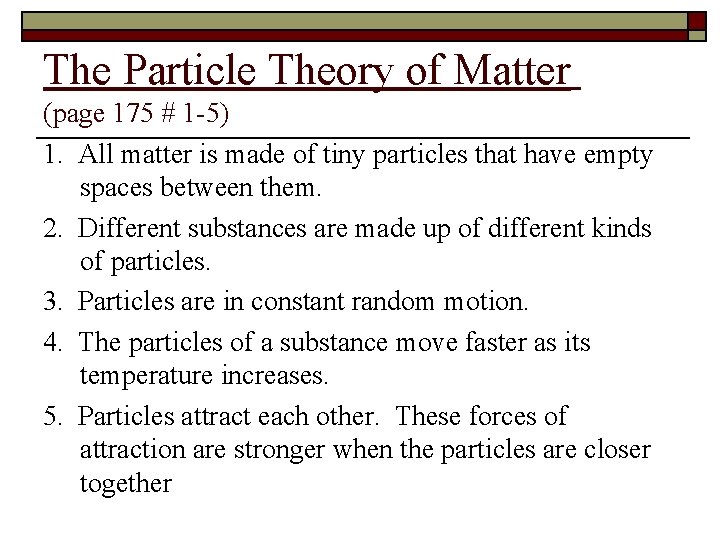
The Particle Theory of Matter (page 175 # 1 -5) 1. All matter is made of tiny particles that have empty spaces between them. 2. Different substances are made up of different kinds of particles. 3. Particles are in constant random motion. 4. The particles of a substance move faster as its temperature increases. 5. Particles attract each other. These forces of attraction are stronger when the particles are closer together

Solid Particle Arrangement Shape of Material Closeness of Particles Material Density Liquid Gas

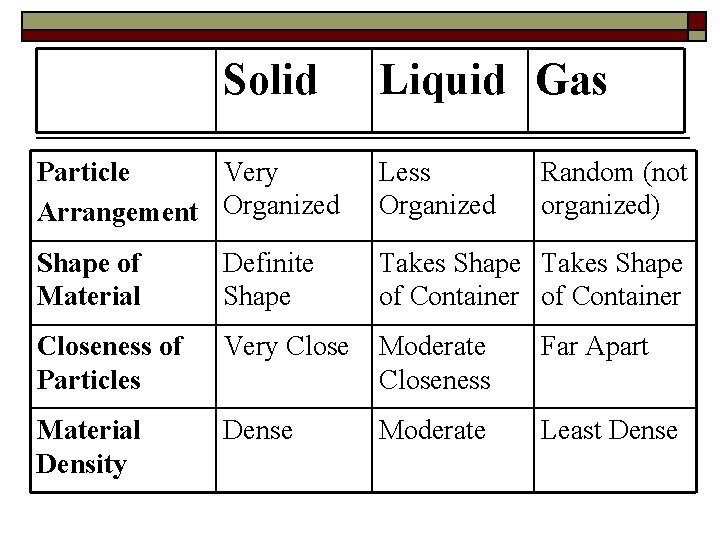
Solid Liquid Gas Particle Very Arrangement Organized Less Organized Random (not organized) Shape of Material Definite Shape Takes Shape of Container Closeness of Particles Very Close Moderate Closeness Far Apart Material Density Dense Moderate Least Dense

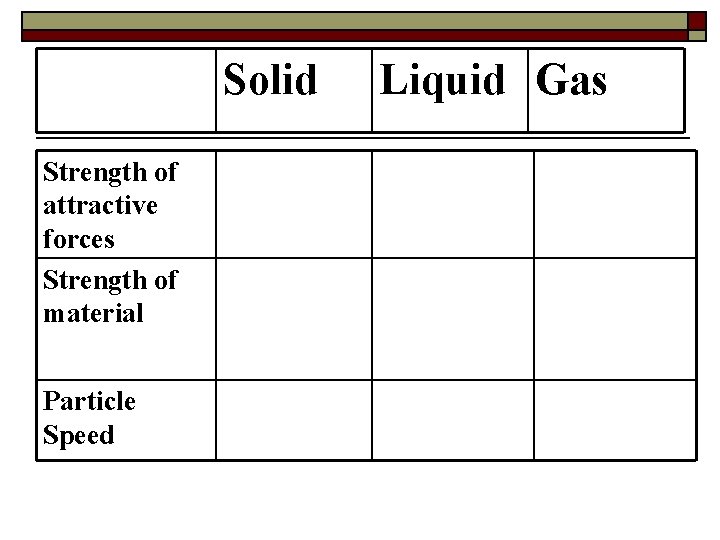
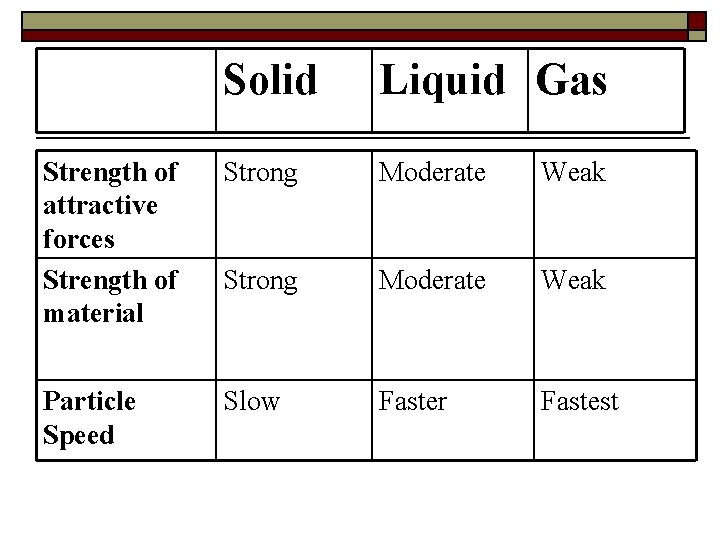
Solid Strength of attractive forces Strength of material Particle Speed Liquid Gas

Solid Liquid Gas Strength of attractive forces Strength of material Strong Moderate Weak Particle Speed Slow Faster Fastest

A second way to classify matter is to: Determine whether a substance is: n n Ex: HOMOGENEOUS Contains one uniform phase; HETEROGENEOUS Contains more than one phase Salad dressing ____ Sugar ____ Aluminum foil _______ Beach sand _____

A Third way to classify matter is: Determine the composition of the substance. Particles can be made of the same or different material. o o If the particles are the same, the substance is a pure substance. If the particles are different, the substance is a mixture. If the material consists of different particles it can be broken down into two or more pure substances. Example water vs. sugar water Water Oxygen Soil Soup All pure substances are homogeneous, whereas a mixture could be homogeneous or heterogeneous.

Homework: Ø Ø Ø Ø Check wiki & send email; Complete wksheets (Matter- PS vs M; Diagrams etc) Read page 8 - 15 Copy into notes: Vocabulary in margins & « In Summary» p 15 Read page 176 - 177 Page 178 # 2, 7, 8, 9, 10 (Alloys) Work on Text Book Scavenger Hunt (Quiz soon)