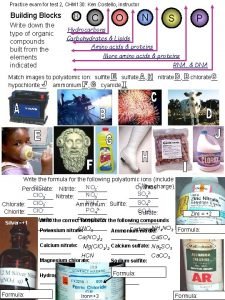
CHM 585 Chapter 20 a Zeolites Zeolites Hundreds


















- Slides: 22

CHM 585 Chapter 20 a Zeolites

Zeolites • Hundreds of thousands of tons of zeolites are used every year, as water softeners in detergents, as catalysts, as adsorbents or desiccants, or even as soil improvers, to control soil p. H, moisture, and manure malodour

What are zeolites? • They are a group of crystalline aluminosilicates, with group I or II elements as counterions. They consist of a framework of [Si. O 4]4 - and [Al. O 4]5 - tetrahedra, linked to each other at the corners by sharing their oxygens

• This leads to the following empirical formula for zeolites: M 2/n. O·Al 2 O 3·x. Si. O 2·y. H 2 O • n is the cation valence. • The tetrahedra make up a three-dimensional network, with lots of voids and open spaces. It is these voids that define the many special properties of zeolites, such as the adsorption of molecules on the huge internal surface (between 500 and 1000 m 2/g

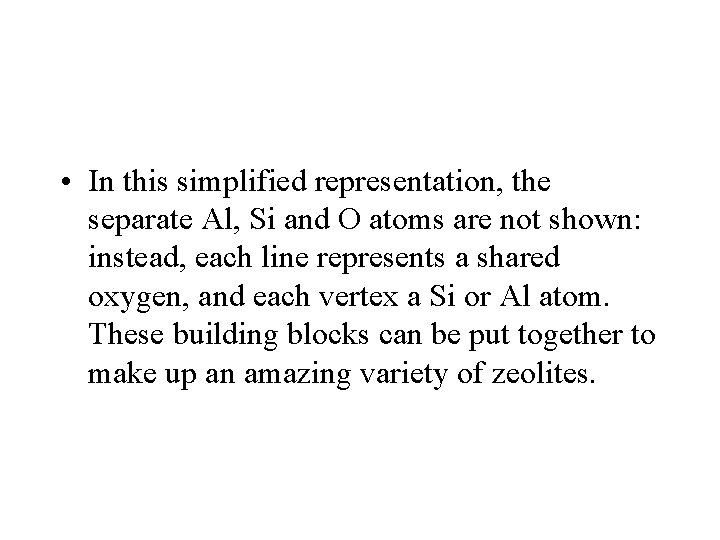
• In this simplified representation, the separate Al, Si and O atoms are not shown: instead, each line represents a shared oxygen, and each vertex a Si or Al atom. These building blocks can be put together to make up an amazing variety of zeolites.


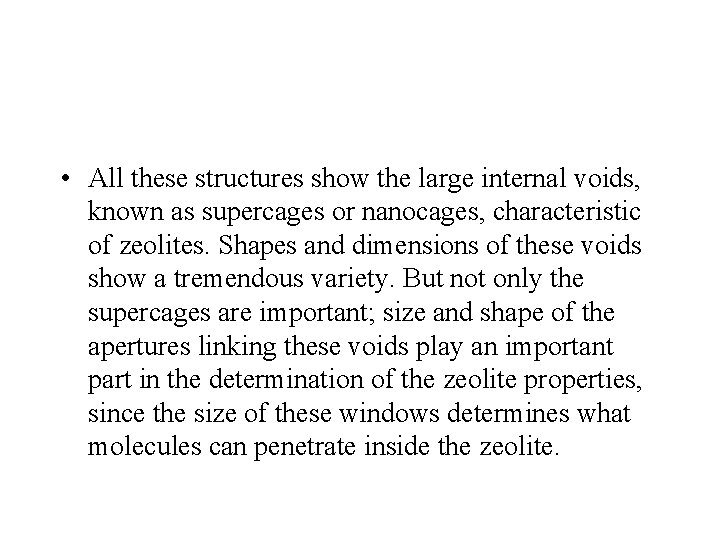
• All these structures show the large internal voids, known as supercages or nanocages, characteristic of zeolites. Shapes and dimensions of these voids show a tremendous variety. But not only the supercages are important; size and shape of the apertures linking these voids play an important part in the determination of the zeolite properties, since the size of these windows determines what molecules can penetrate inside the zeolite.

• In sodalite, the internal cage is rather small; only 6. 6 Å. The windows linking these cages are even smaller: 2. 3 Å. • Zeolite Y has a much larger supercage, 13 Å across, with windows of 7. 4 Å. • The supercage of zeolite A is only slightly smaller, 11. 4 Å, but the windows are much smaller: 4. 2 Å

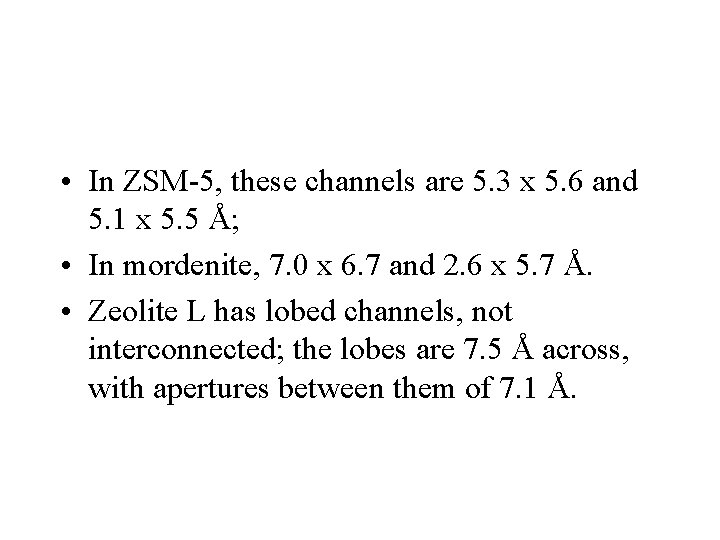
• In ZSM-5, these channels are 5. 3 x 5. 6 and 5. 1 x 5. 5 Å; • In mordenite, 7. 0 x 6. 7 and 2. 6 x 5. 7 Å. • Zeolite L has lobed channels, not interconnected; the lobes are 7. 5 Å across, with apertures between them of 7. 1 Å.



• The presence of the trivalent aluminium ions gives rise to a surplus of negative charge; hence the need for counterions. The number of counterions present in the unit cell thus depends on the Si/Al ratio.

• In natural zeolites, the most common counterions are Na+ and Ca+ • These counterions reside within the threedimensional network. They are easily traded in for other ions by ion exchange

• When zeolites are ion-exchanged, the new cation is usually put in front of the name: thus, Na. Y denotes zeolite Y with sodium counterions

• Zeolite A has replaced the phosphates in household detergents. Phosphates can cause eutrophication of lakes and rivers by stimulating the growth of algae and upsetting the natural balance.

• Sodium Aluminate liquor is dissolved in calculated quantity of water and required quantity of caustic soda is added to obtain required Na 2 O: Al 2 O 3 ratio. Sodium silicate is dissolved in calculated quantity water and here also caustic soda is added to attain the required Na 2 O : Si. O 2 ratio

• Sodium aluminate and sodium silicate solutions are then reacted under controlled conditions to obtain a Gel. The Gel is heated for the formation of complex- composition known as Zeolite. A.

• Zeolite A is a aluminosilicate. The crystal lattice carries a negative charge which is balanced by positive sodium ions (cations) in the interstices. The cations are loosely bound and can easily be replaced by other cations such as calcium. This is the principle of ion exchange.

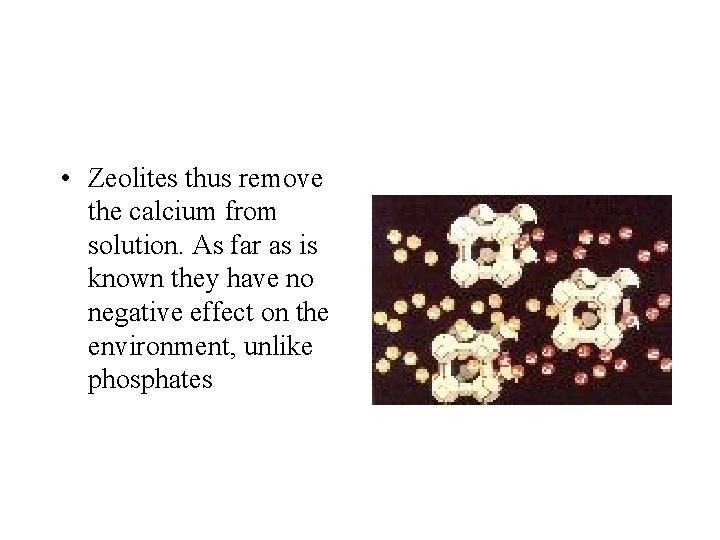
• Zeolites thus remove the calcium from solution. As far as is known they have no negative effect on the environment, unlike phosphates

• Builders in detergents are basically water softeners, which soften the wash water by extracting and binding the calcium and magnesium ions. Depending on the type of builder, other properties and benefits accrue. The builders used in powdered laundry detergents have drastically changed in the last two decades. Traditionally sodium tripolyphosphate (STPP) was the preferred builder.

• The current (2001) global consumption of zeolite A is around 1. 3 million metric tons per annum

• The overall consumption of zeolites is expected to rise from the current level of 5. 34 million metric tons per annum to 6. 19 million metric tons per annum by 2005 and to 7. 36 million metric tons per annum by 2010. • The value of this market is estimated to be $2. 15 billion at present, rising to $2. 52 billion by 2005 and to $2. 94 billion by 2010.
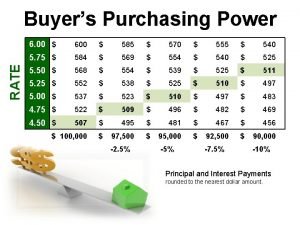
 Chm 130 chapter 12 practice problems answer key
Chm 130 chapter 12 practice problems answer key 888-585-9008
888-585-9008 Comp 585
Comp 585 600-585
600-585 Opwekking 694 tekst
Opwekking 694 tekst Cpsc 585
Cpsc 585 Opwekking 349 tekst
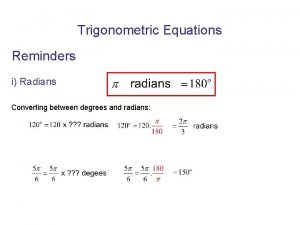
Opwekking 349 tekst Satc trigonometry
Satc trigonometry Cpsc 329
Cpsc 329 Opwekking 585
Opwekking 585 Opwekking 585
Opwekking 585 Chemistry 151 final exam
Chemistry 151 final exam Chm 1045
Chm 1045 Chemical blanking
Chemical blanking Chm 241
Chm 241 Chm 201
Chm 201 Chm 1045
Chm 1045 Chm 111
Chm 111 Chm machining
Chm machining Chmportal
Chmportal Chm logo
Chm logo Rounding key words
Rounding key words A windstorm blows in hundreds of seeds from a nearby meadow
A windstorm blows in hundreds of seeds from a nearby meadow