Chapter Thirteen Chemistry of Some Polymeric Materials Or







































- Slides: 56

Chapter Thirteen Chemistry of Some Polymeric Materials Or Synthetic Polymers & Plastics

Timeline

Definition • Plastic is broadly defined as – Any inherently formless material that can be molded or modeled under heat or pressure

In the beginning… • Greek word plastikos • First natural plastics – Tortoise shell – Tree resins – Shellac • Insect secretion

Observations About Plastics • • Some plastics are clear, others translucent Some plastics are stiff, others are flexible Some plastics stretch, others don’t Some plastics melt, others don’t Some plastics smell, particularly when hot Some glues dry, others harden without drying Most burn and generate toxic gases upon combustion Involved in virtually all common fires

What Are Polymers? • Molecules are larger than most, and chemists call them macromolecules. • Some polymers soften when exposed to heat but then return to their original condition when cooled. – Thermoplastics • Other polymers solidify or set irreversibly when heated. – Thermosetting polymers

What Are Polymers? • Polymers are often classified according to the ways they are used. – Plastics are polymers that can be shaped. – Some are used as threads or yarns called fiber. • Fibers are usually woven or knitted to produce textiles. – Elastomers are a type of polymer characterized by molecules that can elongate when strained and reversibly assume the original shape when the tension is released.

Polymers • Chemists often place the prefix polybefore the name of the substances used to produce specific polymers.

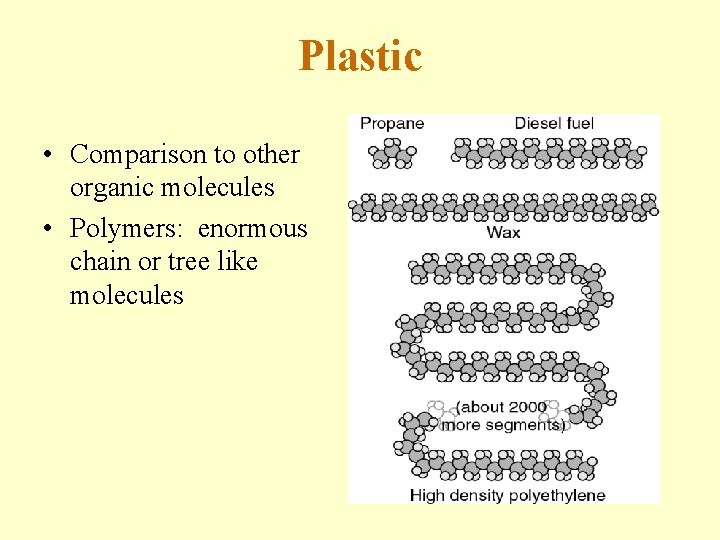
Plastic • Comparison to other organic molecules • Polymers: enormous chain or tree like molecules

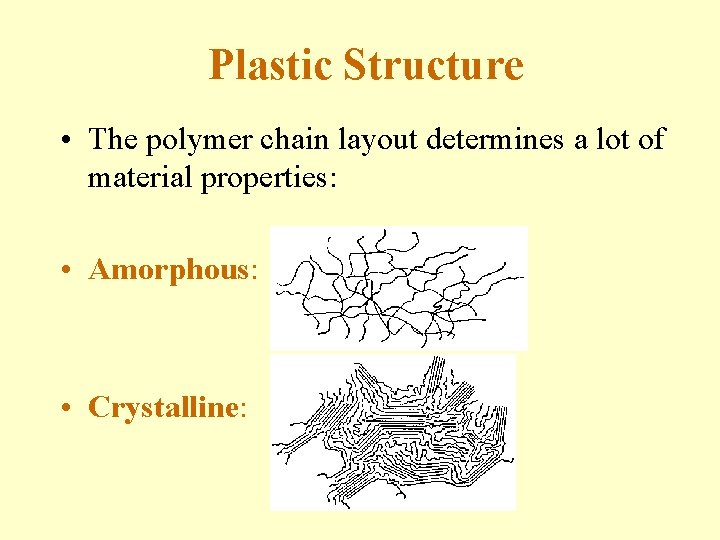
Plastic Structure • The polymer chain layout determines a lot of material properties: • Amorphous: • Crystalline:

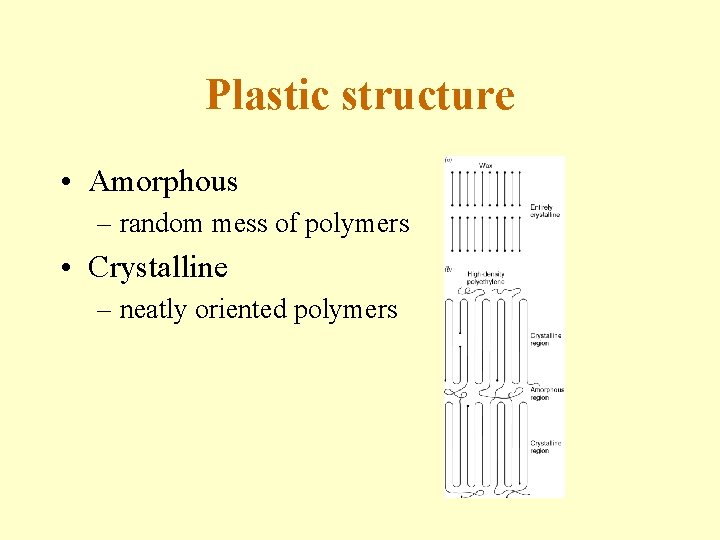
Plastic structure • Amorphous – random mess of polymers • Crystalline – neatly oriented polymers

Amorphous vs. Crystalline • Amorphous (a-morphous = without shape) - The polymer chains are in random arrangement. Molecular structure is incapable of forming regular order (crystallizing) with molecules or portions of molecules regularly stacked in crystal-like fashion. Molecular arrangement is randomly twisted, kinked and coiled. • Crystalline - The polymer chains form a regular pattern. Molecular structure forms regular order (crystals) with molecules or portions of molecules regularly stacked in crystal-like fashion. Molecular arrangement is arranged in an ordered fashion.

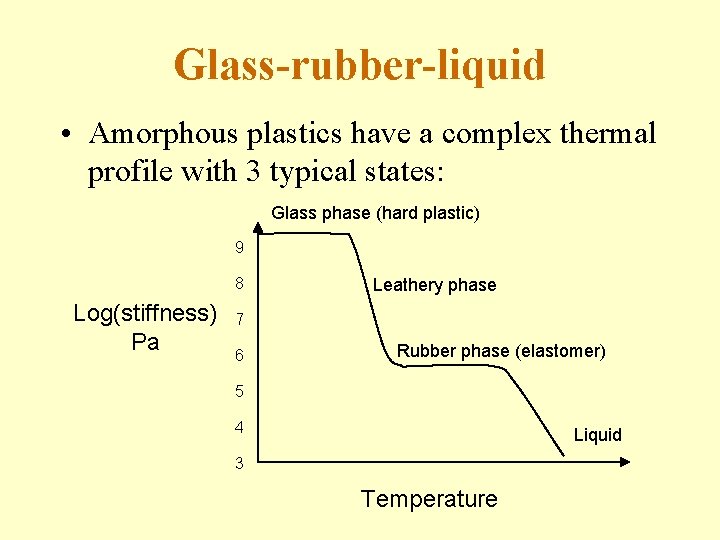
Glass-rubber-liquid • Amorphous plastics have a complex thermal profile with 3 typical states: Glass phase (hard plastic) 9 8 Log(stiffness) Pa Leathery phase 7 6 Rubber phase (elastomer) 5 4 Liquid 3 Temperature

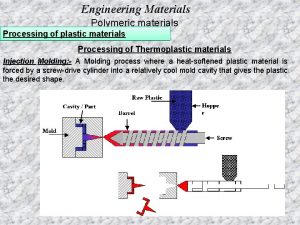
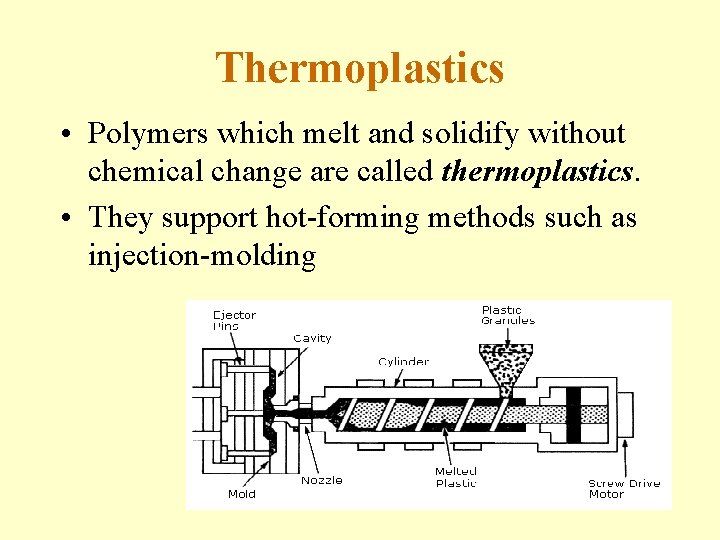
Thermoplastics • Polymers which melt and solidify without chemical change are called thermoplastics. • They support hot-forming methods such as injection-molding

Thermoset plastics • Polymers which irreversibly change when heated are called thermosets. • Most often, the change involves cross-linking which strengthens the polymer (setting). • Thermosets will not melt, and have good heat resistance. • They are often made from multi-part compounds and formed before setting (e. g. epoxy resin). • Setting accelerates with heat, or for some polymers with UV light.

Polymerization • Plastics employ covalent bonds • Individual monomer molecules are joined • This polymerization forms giant molecules – Some molecules are linear chains – Some are branched tree-like structures – Some are networked together completely

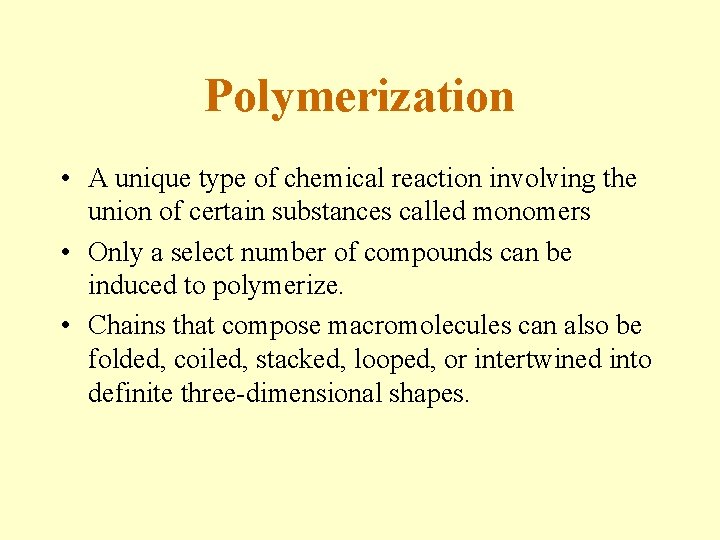
Polymerization • A unique type of chemical reaction involving the union of certain substances called monomers • Only a select number of compounds can be induced to polymerize. • Chains that compose macromolecules can also be folded, coiled, stacked, looped, or intertwined into definite three-dimensional shapes.

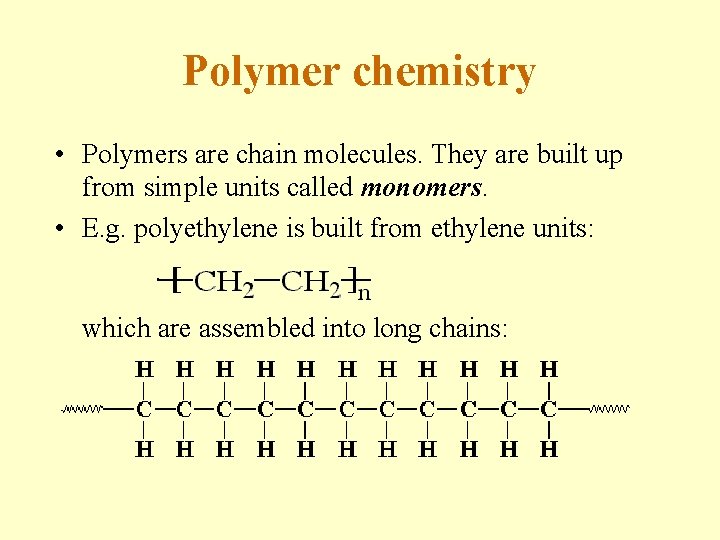
Polymer chemistry • Polymers are chain molecules. They are built up from simple units called monomers. • E. g. polyethylene is built from ethylene units: which are assembled into long chains:

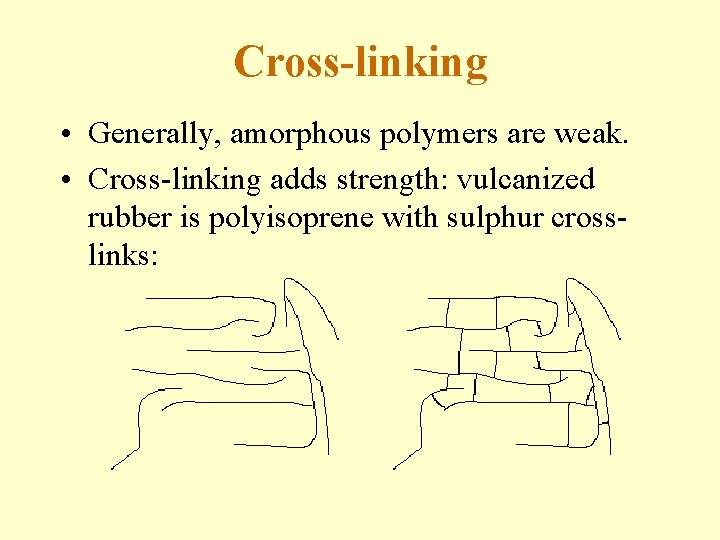
Cross-linking • Generally, amorphous polymers are weak. • Cross-linking adds strength: vulcanized rubber is polyisoprene with sulphur crosslinks:

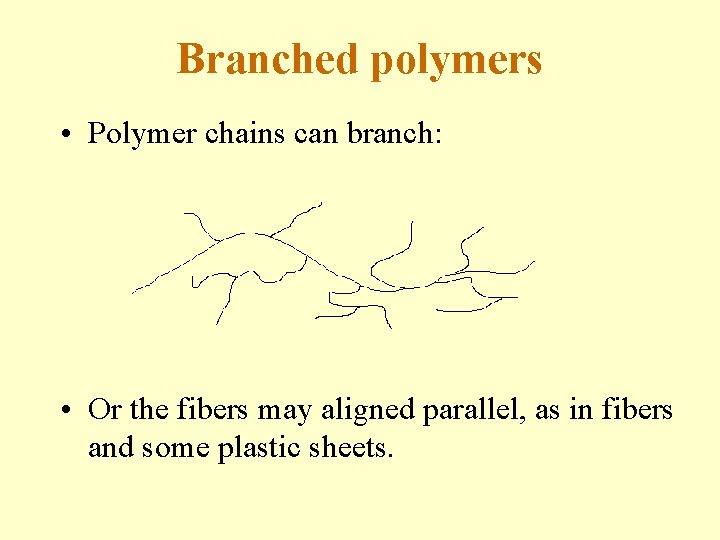
Branched polymers • Polymer chains can branch: • Or the fibers may aligned parallel, as in fibers and some plastic sheets.

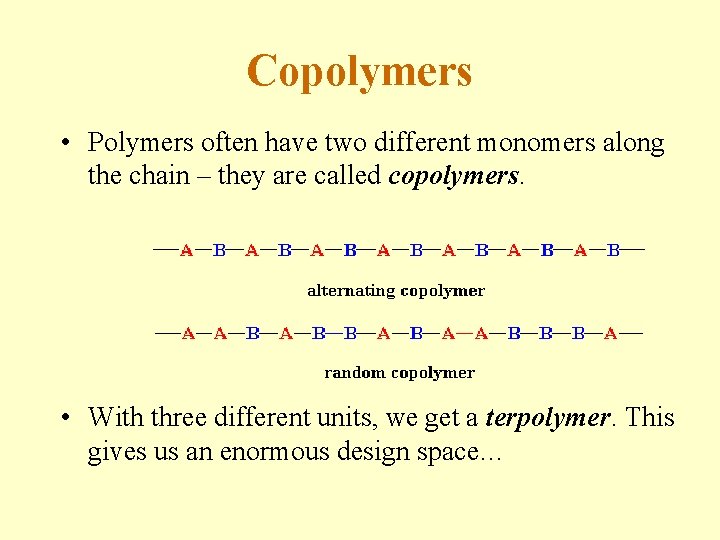
Copolymers • Polymers often have two different monomers along the chain – they are called copolymers. • With three different units, we get a terpolymer. This gives us an enormous design space…

Autopolymerization • Some monomers are capable of undergoing spontaneous polymerization. • While inert compounds may be selected to dilute liquids that are prone to autopolymerization, the common practice used is to add an inhibitor. • To warn fire fighters, the NFPA recommends the insertion of a “P” in the bottom quadrant of the NFPA diamond posted in the storage area.

Polymer Decomposition and Combustion • Most products from natural and synthetic polymers are combustible when exposed to an ignition source. • Polymeric products often melt and thermally decompose into the monomers from which they were made or a mixture of simpler substances. • The surfaces of some polymeric products tend to char as they burn.

Polymer Decomposition and Combustion • Burning polymeric products releases considerable heat. • Burning polymeric products can evolve voluminous amounts of smoke, carbon monoxide, and other hazardous gases, vapors, and fumes.

Polymer Decomposition and Combustion • The generation of flammable vapors, a result of thermal decomposition, at a location isolated from the source of heat gives rise to flashover, the phenomenon responsible in part for the spread of fire from one room into an adjacent room. • Most common polymers evolve nearly twice as much heat when they burn as an equivalent amount of wood.

Polymer Decomposition and Combustion • Carbon monoxide is the most prevalent gas at a fire. • Other gases include hydrogen chloride, ammonia, hydrogen cyanide, sulfur dioxide, and nitrogen dioxide. • More smoke is typically produced in fires involving polymers—usually heavy, thick, noxious smoke

Vegetable and Animal Fibers • Cotton and linen are examples of vegetable fibers. • Wool and silk are examples of animal fibers. • If all natural, burning does not produce toxic gases • Both can be chemically altered to produce synthetic fibers.

Cellulose and Cellulosic Derivatives • When the natural binding agent lignin is removed from wood, the principle substance that remains is a polymer called cellulose. • Primary structural component of the cell walls in plants • Cellulose is the raw material of the paper, wood, cotton textiles, etc. • All natural cellulose does not produce toxic gases when it burns • Cellulose plastics industries—synthetic adaption

Parkes Invents Celluloid • The first man-made plastic was unveiled by Alexander Parkes at the 1862 Great International Exhibition in London. – Parkesine- organic material derived from cellulose that could be molded in heat and retain its shaped when cooled • Buttons • Combs • Pens

Wool and Silk • Common animal fabrics • Composed of proteins, which are biological substances whose molecules possess recurring amino groups • Ignition temperature >1058°F

Glues are Plastics • • • White Glue (Water-Soluble Plastic) Model Cement (Solvent-Soluble Plastic) Heat Melting Glue (Glue Gun Glue) UV-Hardening Acrylic Glues (Acrylates) Superglues (Cyanoacrylates) Mix-Hardening Glues (Epoxies)

Bakelite • Dr. Leo Baekeland – First totally synthetic plastic (1907) – Didn’t throw away his foul glassware • Patented in 1909 • Thermoset resin • Replaced rubber for insulation in electrics

Bakelite

Bakelite • Phenol-formaldehyde resins which he called Bakelite.

Vinyl Polymers • A synthetic polymer made from one or more vinyl compounds • Used in the production of textiles, including carpets, curtains, and upholstery fabrics

Polyethylene • Probably most common plastic – food bags, packing material, children’s toys • Simple formula: • Not quite amorphous! • Melting point 130 C

Polyethylene • High density or HDPE • Low Density or LDPE – Thermosetting – Products like toys • Both can be recycled. • Releases more heat per unit mass than any other commercially popular polymer when it burns

Polypropylene • Used to manufacture silky fibers to rigid containers • Recyclable polymer • Does not ignite easily

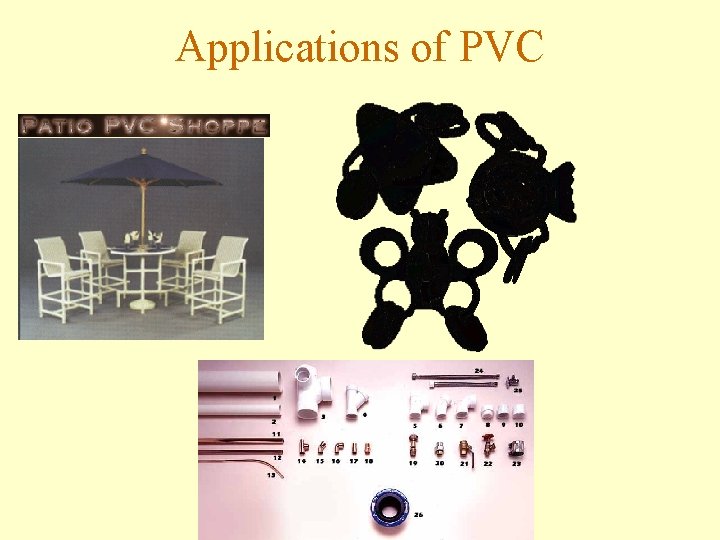
Poly(vinyl chloride) or PVC • Flammable gas subject to autopolymerization • Recognized as a human carcinogen • Used in building construction materials • PVC is recyclable

Applications of PVC

Poly(vinyl chloride) or PVC • Hydrogen chloride is produced when PVC burns. • During incomplete combustion, dioxins are produced which cause a variety of illnesses.

Polyacrylonitrile • Very volatile, toxic, and flammable liquid • Acrylonitrile is the monomer from which polyacrylonitrile is made. • Acrylic fibers are those fibers composed of at least 85% by mass of acrylonitrile units. • Orlon and Acrilan are popular trade names.

Poly(methyl methacrylate) • Several polyvinyl polymers are produced from the esters of acrylic acid and methacrylic acid. • Very volatile and flammable liquid • Used in the production of Plexiglas and Lucite

Polyurethane • A substance produced by the condensation of a glycol and an organic diisocyante • Polyurethane foam results when the frothy mixture solidifies. – Used as insulation, sound-deadening boards, and wall panels – Flexible foams are used in carpet padding and bedding, furniture, and automobile seat cushioning.

Polyurethane – It is essential to check for total fire extinguishment within the foam to prevent reigniting. – Products in today's market have been formulated with fire retardants or treated with additives to reduce their ease of combustion. – Note: fire retardants are toxic to the human body & can be absorbed through the skin

Heat- and Fire-Resistant Polymers • Poly(tetrafluoroethylene) or Teflon • Used to produce virtually non-destructible tubing, gaskets, and valves • Heat resistance is extraordinary. • Extraordinarily unreactive with hot corrosive acids • Teflon coated cooking utensils—teflon leaches into food & is toxic

Teflon • Teflon – Polytetrafluoroethylene (PTFE) – Dupont Chemical Department – First used for artillery shell covers

Heat- and Fire-Resistant Polymers • Nomex • Heat-resistant fabric used in the fire service • Carbonizes and thickens when exposed to intense heat

Kevlar • Kevlar is flameproof and can be drawn into fibers having a strength five times stronger than steel.

Natural and Synthetic Rubber • Rubber has two main properties: – Deformation under strain and elastic recovery after vulcanization • Vulcanization – Produced by heating natural rubber with elemental sulfur • Vulcanization permits the casting of rubber into shapes.

Natural Rubber • Natural rubber: mainly polyisopropene • Tends to be sticky when hot, brittle when warm • Does not reform when stretched

Natural Rubber • Natural rubber: mainly polyisopropene • Tends to be sticky when hot, brittle when warm • Does not reform when stretched Charles Goodyear, 1839 Oven cleaning

Ebonite bracelet from 1880 1851: Hard Rubber— 20 -30% Sulfur

Natural and Synthetic Rubber • The amount of sulfur used determines how soft and elastic the product will be. • Natural rubber accounts for 35% of the demand. • Chemists have synthesized elastomers having the properties similar to those of natural rubber. • Styrene Butadiene Rubber or SBR – Raw material used to produce tire treads • Neoprene rubber is chemically resistant to petroleum products and ozone.

Ebonite bracelet from 1880 1851: Hard Rubber— 20 -30% Sulfur

Responding to Incidents • As they burn, rubber products produce sulfur dioxide, carbon monoxide, and water vapor. • Smoke is extraordinarily dense. • Neoprene, when it burns, also produces hydrogen chloride.
 Polymerization in dentistry
Polymerization in dentistry Nature of bonding in phosphazenes
Nature of bonding in phosphazenes Gene interaction example
Gene interaction example Thirteen colonies
Thirteen colonies Eleven twelve thirteen fourteen fifteen
Eleven twelve thirteen fourteen fifteen 13 ways of seeing nature in la
13 ways of seeing nature in la Rule of thirteen mass spectrometry
Rule of thirteen mass spectrometry Freddy the thirteen
Freddy the thirteen 13 colonies quizlet
13 colonies quizlet How many dogs can you see
How many dogs can you see 50 nifty united states from 13 original colonies
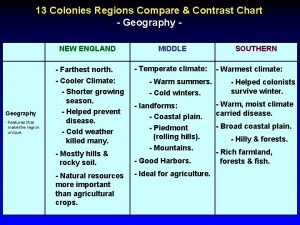
50 nifty united states from 13 original colonies 13 colonies regions chart
13 colonies regions chart Brainpop 13 colonies
Brainpop 13 colonies The 13 apostles
The 13 apostles Thirteen equals one
Thirteen equals one Sometimes you win some
Sometimes you win some God when you choose to leave mountains unmovable
God when you choose to leave mountains unmovable Ice cream count or noncount
Ice cream count or noncount Contact force
Contact force Some say the world will end in fire some say in ice
Some say the world will end in fire some say in ice Some say the world will end in fire some say in ice
Some say the world will end in fire some say in ice Some trust in chariots and some in horses song
Some trust in chariots and some in horses song Examples of materials that are useful and harmful
Examples of materials that are useful and harmful Favourite cars
Favourite cars Man made map
Man made map Adapting and adopting materials
Adapting and adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Mechanics of materials chapter 10
Mechanics of materials chapter 10 Mechanics of materials chapter 9
Mechanics of materials chapter 9 Mechanics of materials 7th edition solutions chapter 7
Mechanics of materials 7th edition solutions chapter 7 Mechanics of materials 7th edition solutions chapter 6
Mechanics of materials 7th edition solutions chapter 6 Mechanics of materials chapter 5
Mechanics of materials chapter 5 Mechanics of materials
Mechanics of materials Mechanics of materials chapter 3 solutions
Mechanics of materials chapter 3 solutions Mechanics of materials
Mechanics of materials Chapter 47 laboratory materials and procedures
Chapter 47 laboratory materials and procedures Chapter 46 impression materials fill in the blank
Chapter 46 impression materials fill in the blank Introduction to materials science for engineers chapter 10
Introduction to materials science for engineers chapter 10 Mechanics of materials 6th edition beer solution chapter 3
Mechanics of materials 6th edition beer solution chapter 3 Mechanics of materials
Mechanics of materials Mechanics of materials chapter 3 solutions
Mechanics of materials chapter 3 solutions Mechanics of materials 6th edition solutions chapter 8
Mechanics of materials 6th edition solutions chapter 8 Introduction to materials science for engineers chapter 10
Introduction to materials science for engineers chapter 10 Strength of materials chapter 1
Strength of materials chapter 1 Chapter 4 metals and nonmetals
Chapter 4 metals and nonmetals Beer john
Beer john Mucostatic impression material examples
Mucostatic impression material examples Approach chemistry chalk chapter
Approach chemistry chalk chapter Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Organic chemistry chapter 1
Organic chemistry chapter 1 Modern chemistry chapter 9 review answers
Modern chemistry chapter 9 review answers Chemical formulas and chemical compounds chapter 7
Chemical formulas and chemical compounds chapter 7 Modern chemistry chapter 15 review answers
Modern chemistry chapter 15 review answers Chapter 14 review acids and bases
Chapter 14 review acids and bases