Chapter 4 Work and Heat 4 1 WORK























- Slides: 43

Chapter 4 Work and Heat

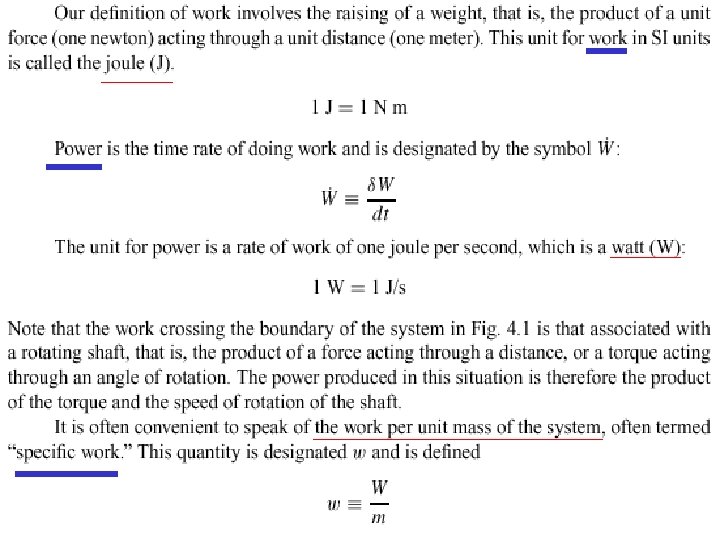
4. 1 WORK n n From a macroscopic point of view, Work is done by a system if the sole effect on the surroundings (everything external to the system) could be the raising of a weight. Work is a form of energy in transit.

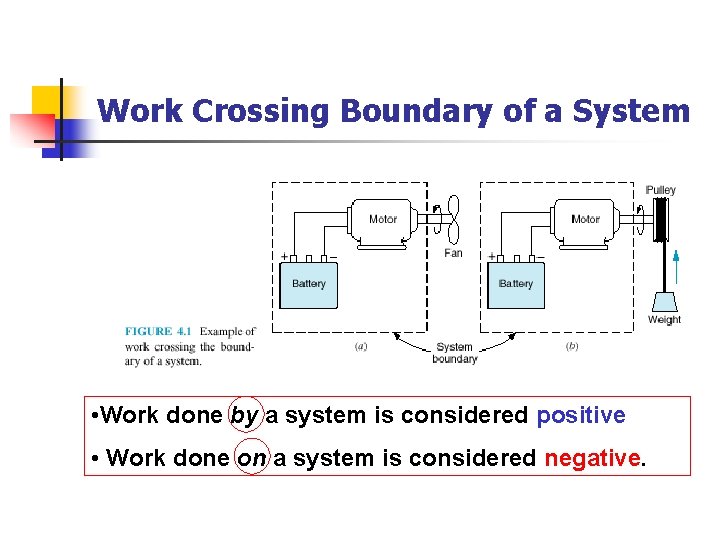
Work Crossing Boundary of a System • Work done by a system is considered positive • Work done on a system is considered negative.


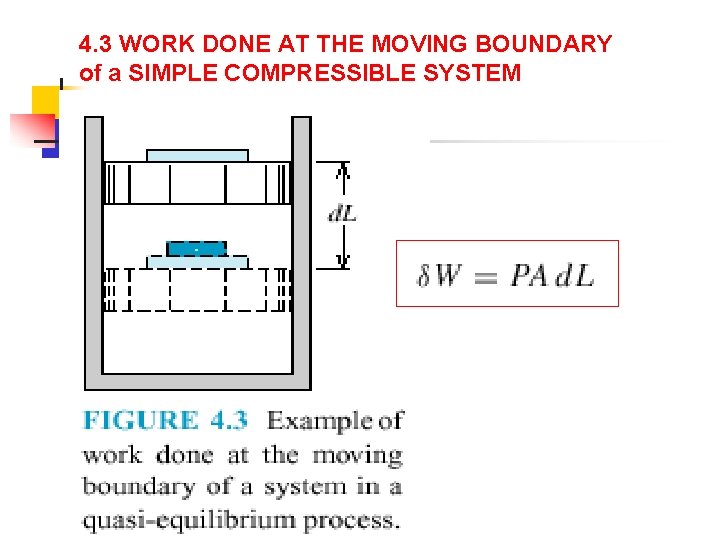
4. 3 WORK DONE AT THE MOVING BOUNDARY of a SIMPLE COMPRESSIBLE SYSTEM

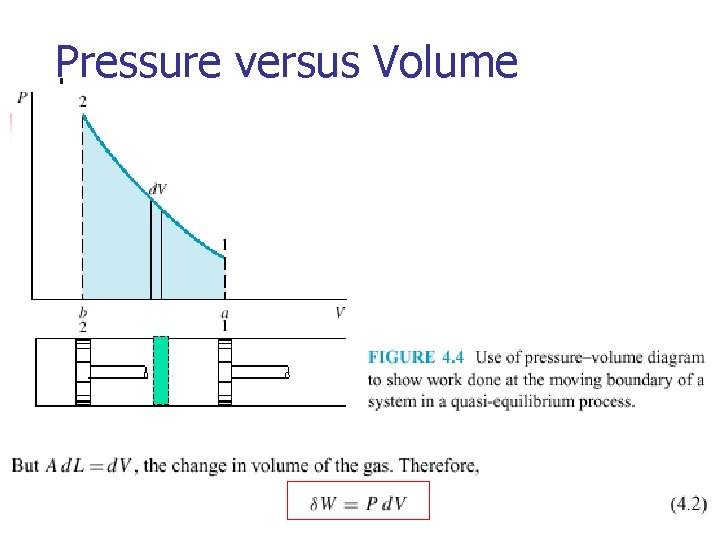
Pressure versus Volume


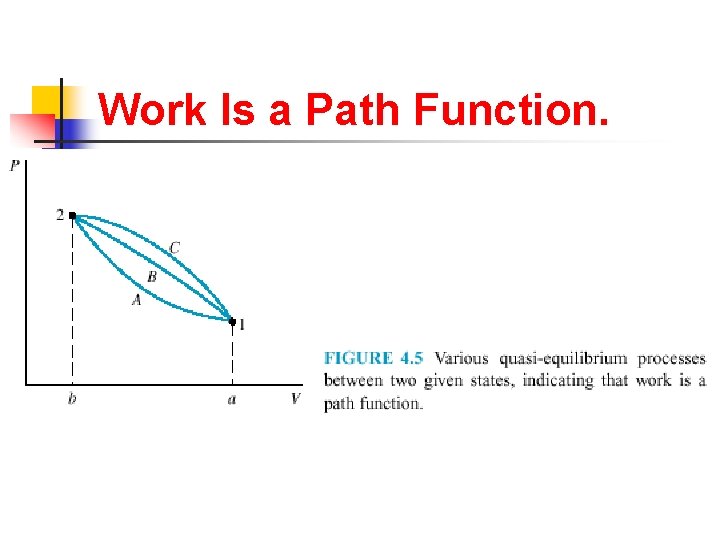
Work Is a Path Function.

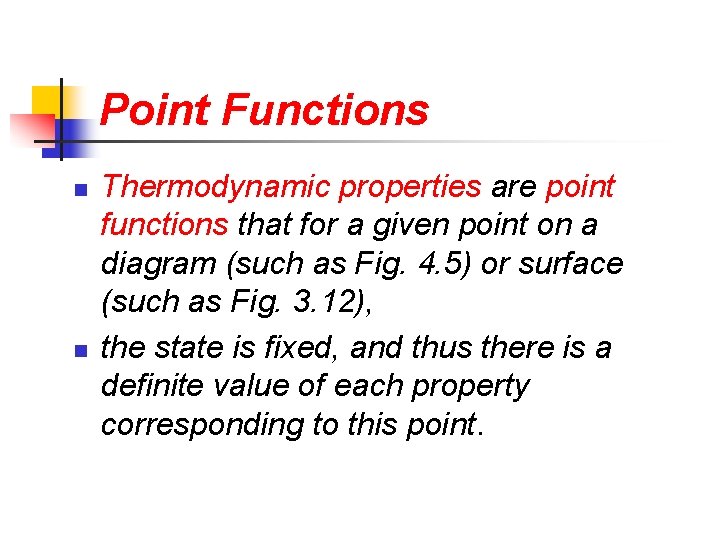
Point Functions n n Thermodynamic properties are point functions that for a given point on a diagram (such as Fig. 4. 5) or surface (such as Fig. 3. 12), the state is fixed, and thus there is a definite value of each property corresponding to this point.

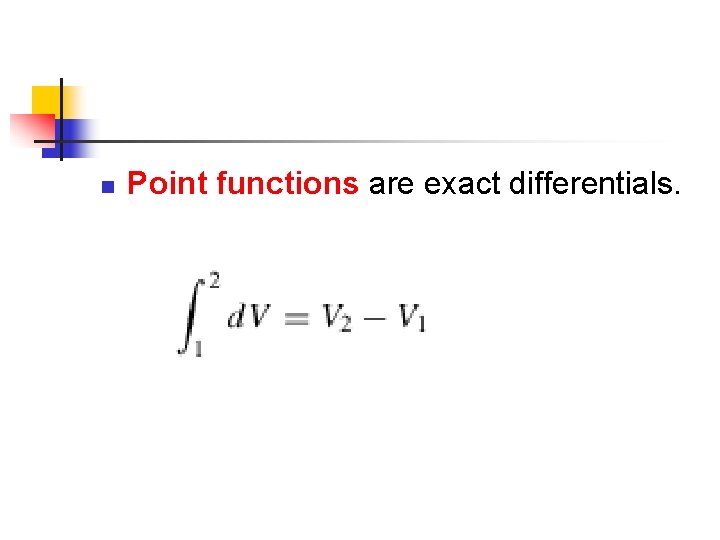
n Point functions are exact differentials.

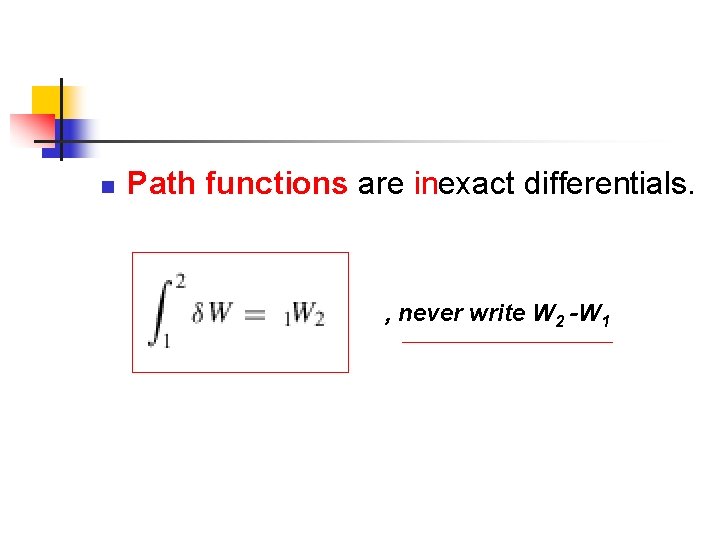
n Path functions are inexact differentials. , never write W 2 -W 1

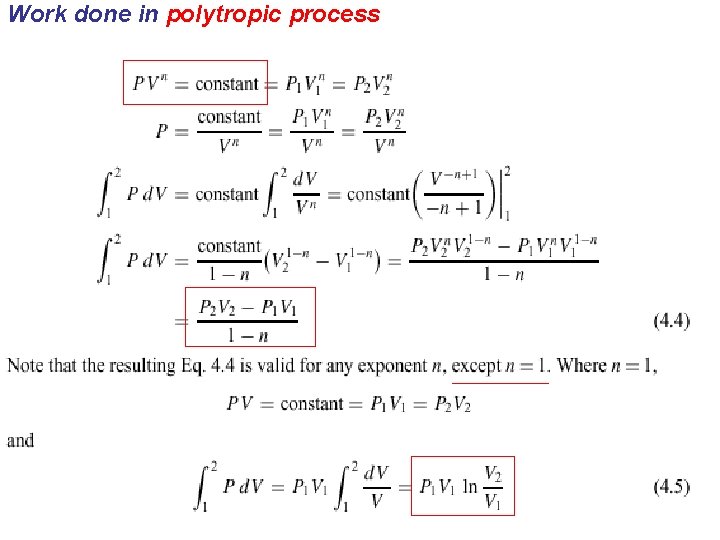
Polytropic Process n n Path-functional relationship is a process called a polytropic process. PV n = constant

Work done in polytropic process

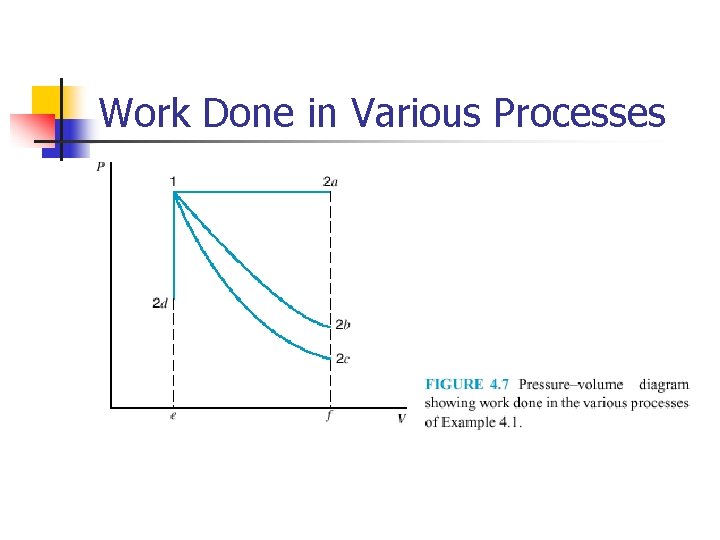
Example 4. 1





Work Done in Various Processes

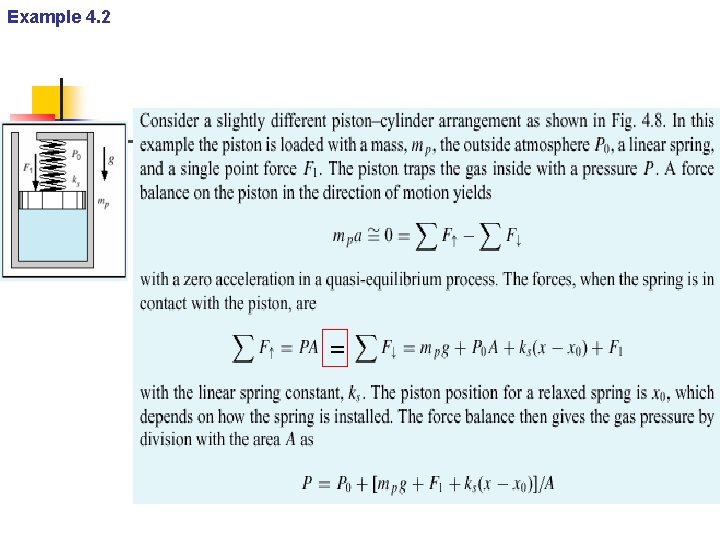
Example 4. 2 =


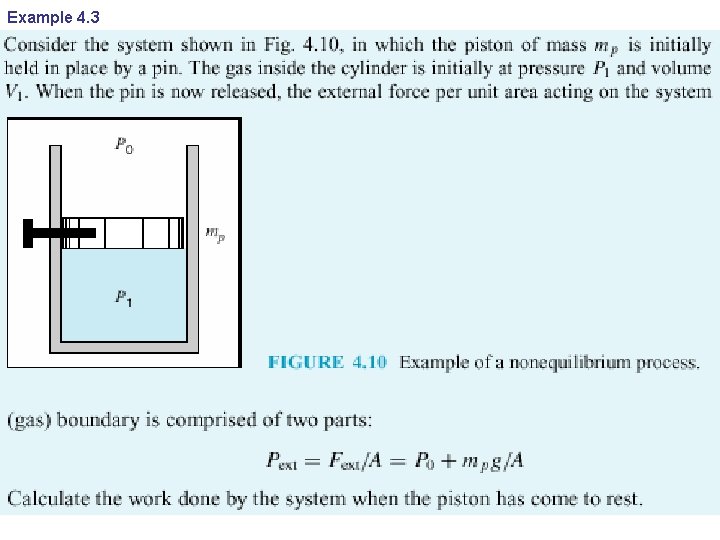
Example 4. 3

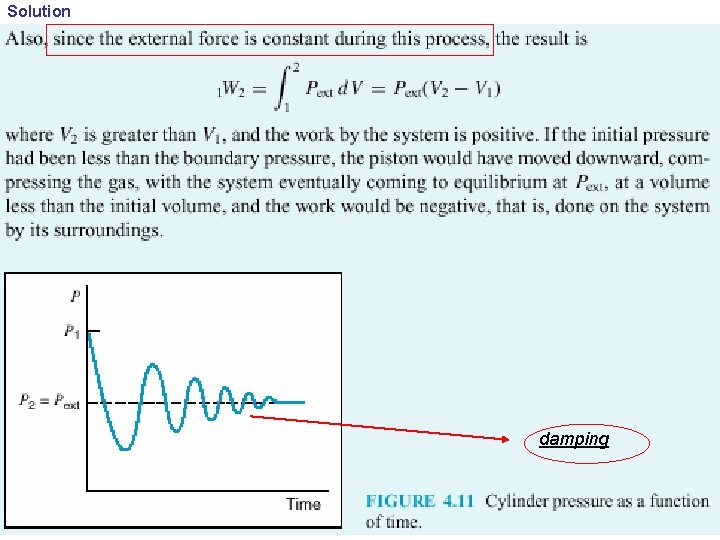
Solution damping

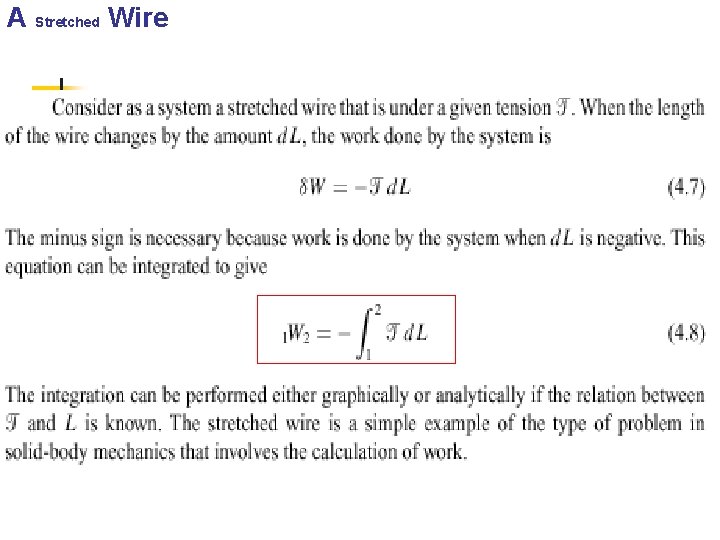
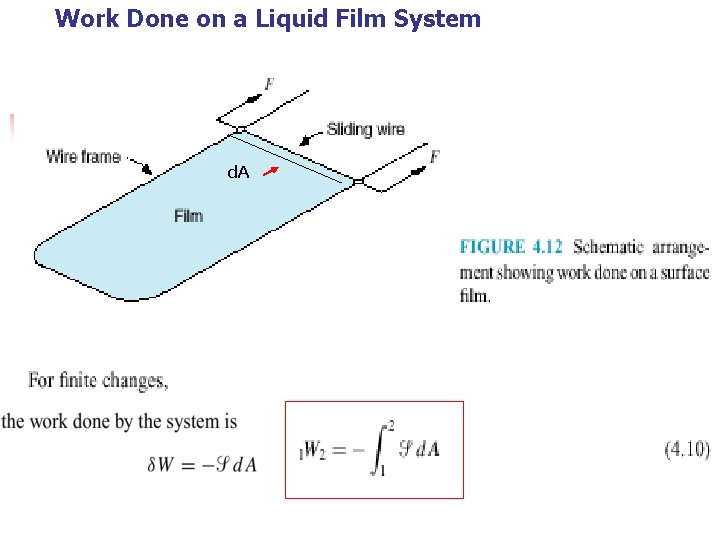
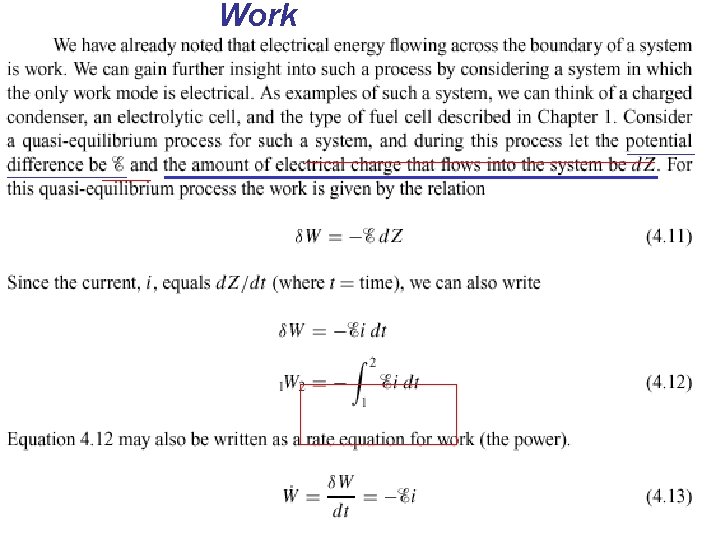
4. 4 OTHER SYSTEMS THAT INVOLVE WORK n n Other types of systems in which work is done at a moving boundary. In this section we briefly consider three such systems, a stretched wire, a surface film, and electrical work.

A Stretched Wire

Example 4. 4

Work Done on a Liquid Film System d. A

Work

4. 5 CONCLUDING REMARKS REGARDING WORK

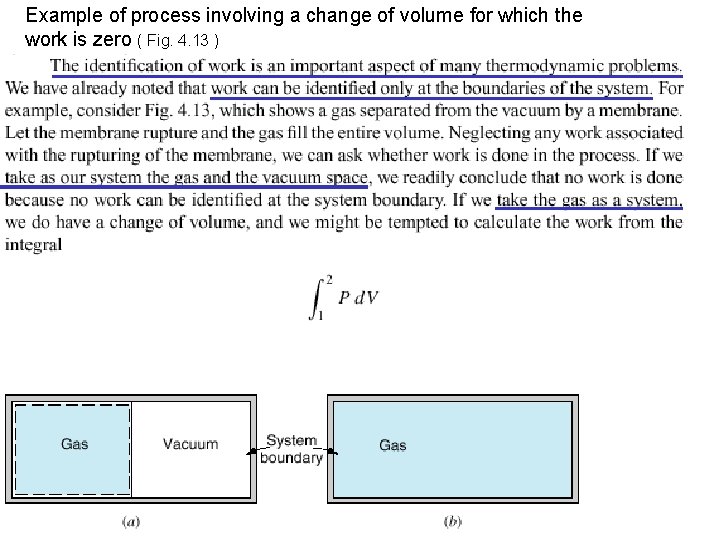
Example of process involving a change of volume for which the work is zero ( Fig. 4. 13 )

n n However, this is not a quasi-equilibrium process, and therefore the work cannot be calculated from this relation. Because there is no resistance at the system boundary as the volume increases, we conclude that for this system no work is done in this process of filling the vacuum.

4. 6 DEFINITION OF HEAT n n Definition of heat is out of a transfer of energy. Heat is defined as the form of energy n n that is transferred across the boundary of a system at a given temperature to another system (or the surroundings) at a lower temperature by virtue of the temperature difference between the two systems. Heat can be identified only as it crosses the boundary. Thus, heat is a transient phenomenon.

n n A body never contains heat. If we consider the hot block of copper as one system and the cold water in the beaker as another system, we recognize that originally neither system contains any heat (they do contain energy, of course).

n n The units for heat are the same as the units for work. In the International System the unit for heat (energy) is the joule. Heat transferred to a system is considered positive, and heat transferred from a system is negative. A process in which there is no heat transfer (Q =0) is called an adiabatic process.

n From a mathematical perspective, heat, like work, is a path function and is recognized as an inexact differential.


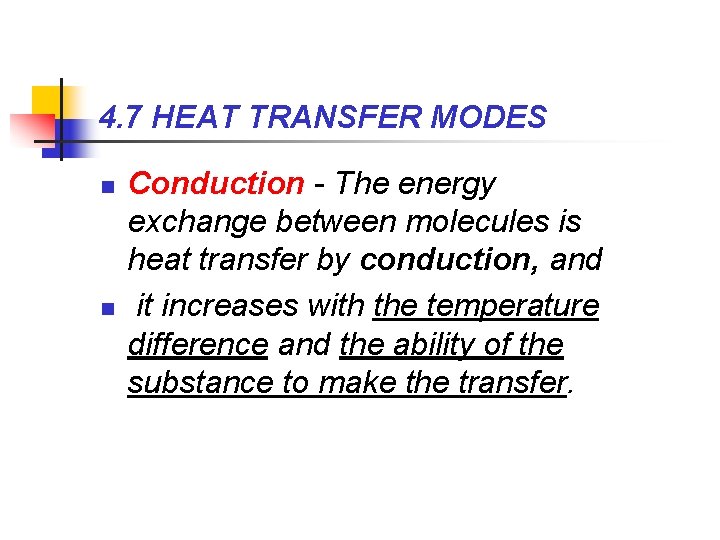
4. 7 HEAT TRANSFER MODES n n Conduction - The energy exchange between molecules is heat transfer by conduction, and it increases with the temperature difference and the ability of the substance to make the transfer.

Convection n n Convection takes place when a media is flowing. In this mode, the bulk motion of a substance moves matter with a certain temperature over or near a surface with a different temperature.

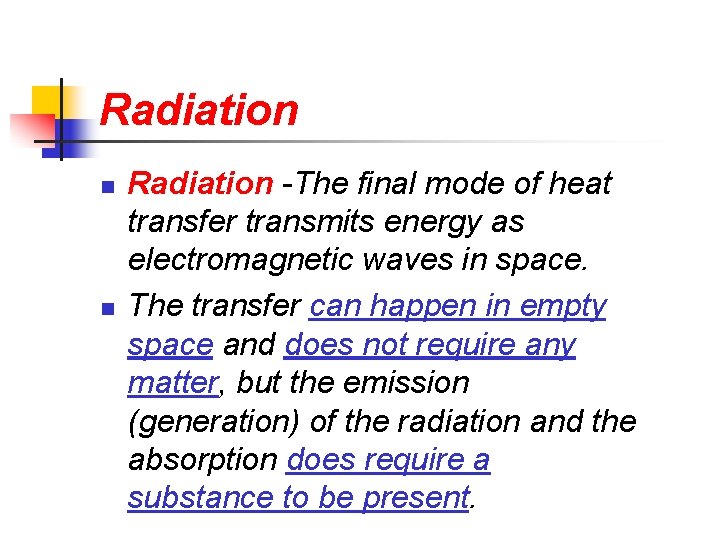
Radiation n n Radiation -The final mode of heat transfer transmits energy as electromagnetic waves in space. The transfer can happen in empty space and does not require any matter, but the emission (generation) of the radiation and the absorption does require a substance to be present.

4. 8 COMPARISON OF HEAT AND WORK n 1. 2. 3. Similarities between heat and work Both are transient phenomena. Both are boundary phenomena. Both are path functions and inexact differentials.

n Difference between heat and work Which crosses the boundary of the system, heat or work?

Which crosses the boundary of the system, heat or work? Fig. 4. 16
